Chemistry (from Egyptian kēme (chem), meaning “earth”[1]) is the science concerned with the composition, structure, and properties of matter, as well as the changes it undergoes during chemical reactions.
Chemists and chemical engineers have the tools to design essential molecules, and impart particular properties to these molecules so they play their expected role in an efficient and standalone manner. Chemicals are used throughout industry, research laboratories, and also in our own homes. Discoveries and development of fundamental chemical transformations contribute to longer, healthier, and happier lives. We need chemistry and chemicals to live.
However, chemophobia and the unnatural perception that all chemicals are bad have origins in the remote past, but are still in people’s minds today. The following historical background sheds some light on the evolution of the environmental movement.
Fermentation, an original chemical process that was discovered in ancient times, led to the production of wine and beer. With relatively crude techniques, a simple enzyme contained in yeast was found to catalyze the conversion of sugar into alcohol. Control of the ingredients in the fermentation broth would impact the flavor of the alcohol, and the effectiveness of the conversion was controlled by the length of time the fermentation was allowed to proceed and the temperature of the reaction.
Today, ethyl alcohol, acetic acid, and penicillin are produced through fermentation processes. Separation of the product (which is usually a dilute species in an aqueous solvent) and recycle of the enzyme is required to make these processes operate economically.
In the 19th century chemistry was viewed as the “central discipline” around which physics and biology gravitated. The medical revolution with the synthesis of drugs and antibiotics coupled with the development of chemicals protecting crops and the expansion of organic chemistry in every aspect of life increased the life expectancy from 47 years in 1900 to 75 years in the 1990s and to over 80 years in 2007.
Chemistry has contributed greatly to improve the quality of human life. For many years, manufacturers took the approach that the world is big and chemical production is relatively small, so chemicals could be absorbed by the environment without effect. The high value of the chemicals produced created an atmosphere in which the manufacturers believed that successful production was the only concern, and control of their waste stream was irrelevant to success. Eventually, the public developed concerns about the impact of chemicals on health and the environment.
In the 20th century the growth of chemical and allied industries was unprecedented and represented the major source of exports in the most powerful nations in the world. Among some of the major exports were chemicals derived from the petrochemical, agricultural, and pharmaceutical industries.
In the 19th century, oil was discovered. Originally extracted and refined to produce paraffin for lamps and heating, oil was rapidly adopted as a source of energy in motor cars. Eventually, techniques were developed that allowed oil to be converted to chemicals, and its availability and financial accessibility allowed the petrochemical industry to grow at a tremendous rate. Developments in the modern plastics, rubbers, and fibers industries led to significant demand growth for synthetic materials.
TABLE 1.1. End Products Made from Common Hydrocarbons
| Hydrocarbons | Trade Names | Consumer Products |
| Ethylene (C2H4) | Polyethylene (Polythene) | Plastic bags, wire and cable, packaging containers, plastic kitchen items, toys |
| Propylene (C3H6) | Polypropylene (Vectra, Herculon) | Carpets, yogurt pots, household cleaners’ bottles, electrical appliances, rope |
| Butadiene (C4H6) | Copolymers with butadiene named Nipol, Kyrnac, Europrene | Synthetic rubber for automobile tires, footwear, golf balls |
| Benzene (C6H6) | Polystyrene | Insulation, cups, packaging for carry-out foods |
| Toluene (C7H8) | Polyurethanes | Furniture, bedding, footwear, varnishes, adhesives |
| Paraxylene (C8H10) | Polyesters | Clothes, tapes, water and soft drink bottles |
Fossil resources, which include oil, natural gas, and coal, are the major sources of chemical products impacting our modern lives. Hydrocarbons, the principal components of fossil resources, can be transformed through a number of refining processes to more valuable products. One of these processes is called cracking, in which the long carbon chains are cracked (broken down) into smaller and more useful fractions. After these fractions are sorted out, they become the building blocks of the petrochemical industry such as olefins (ethylene, propylene, and butadiene) and aromatics (benzene, toluene, and xylenes). These new hydrocarbon products are then transformed into the final consumer products. Table 1.1 gives examples of some end products made from hydrocarbons.
More than 10 million metric tons of oil is used in the world every day. The increasing world population (expected to reach 10 billion people in a few decades) puts increasing pressure on this nonrenewable resource to provide the raw material for a growing consumer demand. Fossil resources also produce 85% of the world’s energy supply, and the growing population and increasing energy consumption puts even greater demand on their use. Because society is increasing its consumption of this nonrenewable resource, identification of alternative, renewable sources of energy and raw materials for chemicals is emerging as one of the biggest challenges for the 21st century.
As the rate of population grew in the 20th century, the demand for food increased dramatically. Production kept up with demand through the use of new technologies such as the synthesis of fertilizers, pesticides, new crop varieties, and extensive irrigation [2]. To provide the necessary cropland, forests were destroyed and prairies and similar types of rangelands were converted.
As new lands were made available for farming, it was discovered that most soils lacked sufficient nitrogen to permit maximum plant growth. Through the nitrogen cycle, bacteria convert atmospheric nitrogen to ammonia and nitrates, which are then absorbed by the plants through their roots. In a natural environment, nitrogen-containing compounds are eventually returned to the soil when plants die and decompose. A natural balance is achieved between the amount of nitrogen removed from the soil through plant growth and the amount returned to the soil through decay. In order to boost the amount of nitrogen required for plant growth, synthetic inorganic fertilizers containing ammonia and nitrates were often applied by farmers. The excessive addition of fertilizers led to runoff of the extra nitrogen-containing compounds in the rivers and lakes and damage to the environment.
More damage to the environment and human health resulted from the development of pesticides to control the impact of insects and other pests. Health issues associated with pesticides were substantial, especially in less developed countries where farmers and employees of the pesticide industries did not take adequate precautions when spraying pesticides. The worst insecticide accident happened in 1984 in Bhopal, India (see Highlight 1.4). One well-known pesticide based on inorganic arsenic salts was used extensively to destroy rodents, insects, and fungi. However, arsenic was recognized as a carcinogen, increasing the risk of bladder cancer. Pesticides based on organophosphates (organic compounds containing phosphorus) were also developed but are especially toxic to human health. A further problem arose when some pests and insects developed resistance to pesticides following repeated uses. In order to overcome the resistance, a more potent pesticide would be applied until resistance was gained, and the cycle repeats. The farmers found themselves on a “pesticide treadmill” [3, p. 451].
A third factor contributing to the increase of grain production was the development of new varieties of crop plants. To produce high-yielding crops, selective cross-breeding was introduced into India, South America, Africa, and other developing countries. Genetically engineered crops started to appear on grocery store shelves in the late 20th century. Through enzymatic transformations, the structure of DNA in living organisms can be modified. Molecular biologists are able to incorporate wanted genes into the DNA of living organisms. For example, in 1994, the first genetically engineered tomato was marketed. Tomatoes are known to be sensitive to frost. To postpone the ripening process, scientists incorporated the “antifreeze” gene of a flounder into a tomato. However, the sales were not profitable so the first genetically engineered tomato was removed from the market. Today, the U.S. Food and Drug Administration (FDA) approves the sale of genetically modified canola, corn, flax, cotton, soybeans, squash, and sugar beet, just to name a few.
Likewise, irrigation systems have been put in place all over the world to make use of arid lands. In hot and humid climates and in the absence of rain, this practice created an accumulation of salts on the soil surface due to the high evaporation rate of water from the soil. The only way to remove excess salts on the surface is to irrigate more. The increase in the salinity of the irrigation water, often recycled through many irrigation cycles, led to a decrease in the productivity of crops, especially beans, carrots, and onions [3, p. 236].
Meeting the food demand of the 21st century is an increasingly difficult challenge, since these new technologies have already been exploited to their maximum potentials, especially in developed countries. Food shortages are expected due to grain productivity decline and growth in the world demand for food.
The modern pharmaceutical industry was born in the 20th century with the mass production of new medicines. The fast growing field of biotechnology and biocatalysis provided the ability to explore new technological applications through a vital drug discovery process. Among the highlights of the pharmaceutical sector in the 20th century were the discovery and development of insulin, new antibiotics to fight a greater range of diseases, and the development of new drugs for cancer treatment.
The discovery of insulin, a hormone that regulates blood sugar, changed the lives of diabetic patients whose malfunctioning pancreas leads to an inability to produce the required hormone. In 1921, Canadian physician Frederick Banting first isolated the hormone. In the laboratories of Eli Lilly, now the 10th largest pharmaceutical company in the world, the process was developed to extract, purify, and mass produce insulin. Insulin was introduced commercially in 1923.
The second famous discovery happened in 1928 when Dr. Alexander Fleming, a bacteriologist at London’s Saint Mary’s Hospital, found that a “magic mold” resisted the action of bacteria. He named the mold penicillin. It was not until 1940 that penicillin was developed into a therapeutic agent by Oxford University scientists Howard Florey and Ernest Chain. Unfortunately, an insufficient supply of penicillin existed until the beginning of World War II, when several U.S.-based companies purified and mass produced penicillin to treat the wounds of U.S. soldiers on the battlefield. A long series of new antibiotics followed in the 1950s, known as the “decade of antibiotics.”
Substantial progress in the fight against cancer also occurred during the 20th century. Named karkinos by Hippocrates, a Greek physician and the father of medicine, cancer found its origin as early as 1500 BCE. Although typically grouped together, there are a wide variety of cancerous diseases. When cells in our organs continue to multiply without any need for them, a mass or growth called tumor appears. These masses of cells can either be benign (noncancerous, not life threatening, and easily removed) or malignant (cancerous, spread to tissue and organs). Malignant cells can be identified by magnetic resonance imaging (MRI) used in radiology to distinguish pathologic tissue such as a brain tumor from normal tissue. The fight against cancer was pursued with assiduity in the 20th century when chemotherapy and radiation therapy were discovered. The first chemotherapy agent for cancer was actually mustard gas used in World War I. However, the gas killed both healthy and cancerous cells. Since then, many antimetabolites (“any substance that interferes with growth of an organism by competing with or substituting for an essential nutrient in an enzymatic process” [4]) have been developed and deaths from all cancers combined declined.
John E. Niederhuber, M.D., the 13th director of the National Cancer Institute, opined on the growth of biotechnology and its impact on human health. “The continued decline in overall cancer rates documents the success we have had with our aggressive efforts to reduce risk in large populations, to provide for early detection, and to develop new therapies that have been successfully applied in this past decade. … Yet we cannot be content with this steady reduction in incidence and mortality. We must, in fact, accelerate our efforts to get individualized diagnoses and treatments to all Americans and our belief is that our research efforts and our vision are moving us rapidly in that direction” [5].
The contribution of the pharmaceutical sector to health and welfare, the importance of this sector to the economy, and the springboard it provided for research in the medical field have been unprecedented. The challenge of the pharmaceutical industry in the 21st century is to ensure the safety and efficacy of drugs on the market. Unexpected side effects lead to greater numbers of recalls, even after being approved by the FDA. In 2004, a nonsteroidal anti-inflammatory drug named Vioxx, marketed by Merck and prescribed for osteoarthritis, menstruation, and adult pain, was recalled from the U.S. market after it was discovered that the drug caused an increased risk of heart attacks and strokes. The challenge is to maximize the therapeutic benefits of the drug while eliminating or reducing the toxic side effects.
Industrialization and materialization came with a price, sometimes easily recognized but often more obscure. Over time, we have come to realize that the development and use of new chemicals is not without risk, and the associated risk of chemicals in the environment must be managed carefully. In 1962, Rachel Carson, in her well-known book Silent Spring, pointed out that “chemicals are the sinister and little-recognized partners of radiation in changing the very nature of the world––the very nature of life.” Our planet has been despoiled, and the environment in which we live today is one of a fear of chemicals, and a lack of recognition of their importance in our lives.
There are numerous examples of instances in which advances in chemistry and the introduction of new chemicals did not fully take into account their impacts on our lives. At times the negative side effects were covered over for many years after they were known, but more frequently this was simply a lack of knowledge.
Lead is a toxic metal found mostly in paint, dust, drinking water, and soil. According to the U.S. Environmental Protection Agency the walls of houses built before 1978 are likely to have lead-based paint. Lead from paint chips and lead dust from old painted toys and furniture are particularly dangerous to children, since children are more likely to put hands covered with lead dust in their mouths or eat paint chips containing lead. The growing body of a child absorbs lead rapidly, making a child more sensitive to lead’s destructive effects. Lead causes damage to the brain and nervous system, slowed growth, hearing problems, and behavior and learning problems. In late 1991, the Secretary of the Department of Health and Human Services, Louis W. Sullivan, called lead the “number one environmental threat to the health of children in the United States.” In 1996 requirements for sales and leases of older housing became effective under the “Residential Lead-Based Paint Disclosure Program Section 1018 of Title X.” In 2001 hazard standards for paint, dust, and soil were established by the EPA for most pre-1978 housing and child-occupied facilities.
In the late 1940s and into the following decade, biologists and chemists determined that thalidomide could be used by pregnant women to combat morning sickness and help them sleep. This was a remarkable advance in human health care, as it alleviated a major discomfort. However, all of the biological impacts of the drug within the body were not understood, especially as concerned the relationship with the growing fetus in the womb. From 1956 to 1962, approximately 10,000 children were born with malformations. Scientists had not understood that the use of the chemical could cause birth defects in children, outweighing all of the parental benefits from the use of the drug. This undesirable outcome caused outrage in the general public about the unintended effect of drugs and led to implementation of new governmental regulations for testing new drugs. In 1962, the use of thalidomide during pregnancy was discontinued (Highlight 1.1).
Limited understanding of the role of pharmaceuticals in contact with humans was paralleled by a limited understanding of the impact of chemicals in the environment. Examples of poor management of chemical waste abound.
On June 22, 1969, the Cuyahoga River in Cleveland, Ohio, caught on fire, when oil-soaked debris was ignited by the spark from a passing train car. Although only a brief river fire, this incident brought national attention to the poor state of the nation’s urban rivers.
The Love Canal in Niagara Falls, New York, was used as a waste disposal site by Hooker Chemical and the City of Niagara Falls from the 1930s to 1950s (Highlight 1.2). The site was later sold to the city for construction of a school, with Hooker disclosing that the site had been used as a waste repository. The school was built nearby in 1955.
In Times Beach, Missouri, the roads were sprayed with waste oil to reduce dust formation. Unfortunately, the contractor combined waste oil with other hazardous chemicals, including dioxin, one of the main components of Agent Orange. As a result of the contractor’s actions, the entire town of Times Beach was determined to be contaminated with dioxin, the town was quarantined, and the inhabitants were relocated by the government.
General Electric (GE) Corporation produced polychlorinated biphenyls (PCBs) at its plants in Fort Edward and Hudson Falls, New York, for use as dielectrics and coolant fluids in transformers, capacitors, and electric motors. From 1947 through 1977, they discharged the runoff from this process into the Hudson River. In 1983, the U.S. Environmental Protection Agency declared 200 miles of the Hudson River a superfund site, and sought to develop a cleanup and remediation plan to remove the PCBs that contaminated the sediment at the bottom of the river. Phase 1 cleanup was completed in 2009, at a cost to GE of $460,000,000. A projected Phase 2 effort will be even larger and more expensive (Highlight 1.3).
The industrial disaster of 1984 in Bhopal, India, was caused by the release of 40 tons of methyl isocyanate gas by a Union Carbide pesticide plant, resulting from a series of worker errors and safety issues that had not been properly addressed. The official government report documents 3787 deaths as a result of this leakage, although reports of as many as 20,000 deaths are widely accepted. Today, more than 100,000 people still suffer from painful symptoms, most of which doctors are not sure how to treat. Furthermore, most of the waste left behind is in evaporation ponds outside the factory walls and this poses a danger for the health of nearby residents who get their drinking water from hand pumps and wells. The plant is still not dismantled (Highlight 1.4), and legal wrangling over responsibility for cleanup of the site continues today.
With the growing environmental awareness throughout the 1960s and into the early 1970s, the United States initiated a series of legislative initiatives that controlled the release of toxic materials into the environment, and set standards for clean air and clean water. A brief and noncomprehensive timeline includes the following breakthrough actions:
The Pollution Prevention Act (also called P2 Act) of 1990 designated the EPA to embark on a mission of source reduction, rather than monitoring and cleanup (Highlight 1.5). “Congress declared it to be the national policy of the United States that pollution should be prevented or reduced at the source whenever feasible; pollution that cannot be prevented should be recycled in an environmentally safe manner, whenever feasible; pollution that cannot be prevented or recycled should be treated in an environmentally safe manner whenever feasible; and disposal or other release into the environment should be employed only as a last resort and should be conducted in an environmentally safe manner” [10].
At the same time the EPA was working on the Clean Air Act and the Clean Water Act, the first United Nations Conference on the Human Environment (UNCHE) was held in Stockholm, Sweden, in 1972. This conference acknowledged the need to reduce the impact of human activities on the environment, the specificity of the environmental issues to developing countries versus developed countries, as well as the need for international collaboration to work on these global problems. The United Nations Environmental Program (UNEP) whose mission is “to provide leadership and encourage partnership in caring for the environment by inspiring, informing, and enabling nations and peoples to improve their quality of life without compromising that of future generations” was launched as a result of this conference. A step forward defining sustainability was accomplished.
Recognizing that pollution does not respect the boundaries between countries, it became clear that international agreements would be required to control the more onerous of environmental issues. One of the most successful examples of such international cooperation has been the Montreal Protocol, signed by 100 countries in September 1987, and made effective in 1989. This agreement led to a ban on ozone-depleting chemicals, such as chlorofluorocarbons (CFCs).
Throughout their history, chemists have discovered some revolutionary molecules and synthetic pathways that bring new products and technologies to society. Production techniques have often neglected the impact of these materials and processes on the environment. Today, with the increased environmental awareness, it is crucial to discover new ways of producing the same or similar molecules with desirable properties but with zero waste and zero pollution. New materials that are inherently nontoxic and have functionality that replaces hazardous chemicals, and processes to make these materials without the use of toxic intermediates or release to the environment, need to be developed.
As mentioned in the fact sheet published in December 2005 by the State of Ohio Environmental Protection Agency [12]: “Each day we are exposed to risks. Some risks are the result of our own behavior: choices we make such as diet, smoking, speeding on the freeway or playing a contact sport. Other risks come from factors we don’t directly control: hazardous weather conditions, environmental pollutants, even our own genetic history.”
One accepts risk on a daily basis. When you drive in your car, there is a real potential that you will have a wreck, and that wreck might even lead to death. The insurance company can determine how likely you are to have an accident and, from that calculation, will assign you to a specific risk category, from which they determine the amount of the premium. The insurance company assumes a portion of your risk, and you pay a price for that.
We can also consider risk from a chemical standpoint. Consider two relatively similar chemicals. Benzene is a known carcinogen, is a likely mutagen, and is a known nervous system toxin. Based on its impact on humans, benzene is a particularly nasty chemical. Toluene, however, is an irritant without any known cancer-causing activity and is listed as possibly causing damage to the central nervous system. In short, benzene is known to be a particularly harmful chemical, whereas toluene is simply a chemical deserving of concern but without any special toxicity issues. You wouldn’t want to risk coming into contact with benzene without special protective equipment, but you might be willing to work with toluene.
Risk assessment is the process by which we evaluate the potential adverse health effects associated with people coming in contact with an environmental hazard. It consists of two specific activities:
Once these two components are determined, then the total risk may be determined through a combination of these two elements:
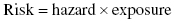
Traditional engineering approaches focus on risk management; understand the hazard and take steps to minimize the likelihood of exposure. This has resulted in the use of personal protective equipment, such as the safety goggles you wear in the laboratory. Safety protocols are in place throughout the manufacturing sector, and existed in Bhopal. Because these risk management initiatives are imperfect, hazardous chemicals continue to find their way into the environment.
Green chemistry, on the other hand, is more attuned to reducing the hazard through inherently safer design (ISD). If the chemical of concern is replaced by a less hazardous material, then that chemical cannot possibly be harmful, since it is removed from the process. An inherently safe process is one in which nothing bad can happen, even if something does go wrong. Inherently safer design reduces cost, since less expense is incurred in ensuring that workers and the public are not exposed to an unsafe material. As described by Berkeley (Buzz) Cue, Founder and President of BWC Pharma Consulting, LLC, inherently safer design is “just a question of changing your mind set.”
ISD requires the chemist and engineer to go back to the drawing board and think about alternative feedstocks, solvents, and synthetic pathways when developing a new process. It is about redesigning products. As an example, paint used to be made from oil-based materials, and its application created volatile organic compounds in the ambient air. Today, all paints are water-based (latex). They work the same as previous paints, but they are inherently less hazardous. Latex paint is a green chemistry product.
Green chemistry is not a new type of chemistry, but a new philosophy of chemistry, one focused on the reduction of risks and inherent safety. It is about new benign by design alternatives. It is not more complex than traditional chemistry but it is a distinctive approach based on the evaluation of toxicity of materials and their by-products when designing a safer, cleaner, and cost-efficient process.
In Stockholm, Sweden, in 1972, the First International Conference on the Human Environment focused the attention of the world on the transnational issues associated with pollution. Twenty years later, the United Nations Conference on Environment and Development (UNCED), also known as the Earth Summit, occurred in Rio de Janeiro, Brazil, in June 1992. This summit featured 178 countries discussing global problems such as poverty, war, and sustainable development. For the first time, the protection of the welfare of the planet was seen as the key driver to promote long-term economic and social progress. This was the Earth Summit’s most important achievement. Some notable results of the UNCED included:
Ten years after Rio’s Earth Summit, the United Nations World Summit on Sustainable Development (WWSD), also known as Earth Summit II or Rio +10, took place in Johannesburg, South Africa, in 2002. Even if some have argued that the agenda of this summit may have been too ambitious, it acknowledged that sustainable development addresses not just environmentally related issues but also economic and sociopolitical matters. Key points of discussion included water and sanitation, energy, human health, agricultural productivity, biodiversity, and ecosystem management. The main outcome of the summit was the Johannesburg Declaration, which reinforced agreements made at the Rio de Janeiro and Stockholm summits. Specific goals outlined in the declaration pointed at:
Heads of the richest industrialized countries (France, Germany, Italy, Japan, the United Kingdom, the United States, Canada, Russia), the President of the European Commission, and representatives from five developing countries, including Brazil, China, India, Mexico, and South Africa, referred to as the Outreach Five, attend annual G8 summits. At the 2008 G8 summit in Japan, world priorities such as the current global food crisis, African development, climate change, intellectual property rights, and some political issues were discussed. The eight richest nations in the world pledged to cut global greenhouse emissions by 50% by 2050. However, a baseline year was lacking for those cuts. Concerns and ideas were shared, challenges and long-term impacts were described, but the implementation of ideas did not happen.
Four years later, at the G8 summit in Camp David, Frederick County, Maryland, on May 18–19, 2012, the leaders of Britain, Canada, France, Germany, Italy, Japan, Russia, and the United States met to address major global economic, political, and security challenges. Most importantly, it was once again recognized that development and access to environmentally safe and sustainable sources of energy is essential to global economic growth. The issue of food security was addressed, and a goal of providing a new alliance with African leaders to allow 50 million people to live above the poverty level by 2022.
Energy, food, and water––resources fundamentally linked to one another––are becoming scarce in the 21st century. Called the “perfect storm” of food, energy, and water shortages by the 2009 U.K. government chief scientist Professor John Beddington, scarcity for these resources will happen at an international level. Professor Beddington predicts that as the world population will get to 8.3 billion by 2030, “demand for food and energy will increase by 50% and for fresh water by 30%” [13].
If these issues are not addressed now, widespread shortages will be followed by increased prices, that could create international conflicts. Security of these resources is now a high priority on the political agenda of the heads of many countries as the prices of oil and goods continue to rise.
One way to tackle the problem, according to Professor Beddington, is to improve agricultural productivity since “30 to 40% of all crops are lost due to pest and disease before they are harvested.” More disease- and pest-resistant crops as well as plants resistant to drought and salinity coupled with more efficient irrigation and harvesting practices are needed in the 21st century.
Many concerns have already emerged in the developed countries, but the developing countries face similar sustainability challenges. In his book, Natural Capitalism: Creating the Next Industrial Revolution with co-authors Amory Lovins and L. Hunter Lovins, Paul Hawken laid out some potential disasters coming up, such as decline in grain production and in fish per capita, decline in jobs (1 billion people looking for work), loss of rain forests, disappearing wetlands, nuclear waste cleanup, greenhouse gases, global warming, stratospheric ozone depletion, and rising global temperatures. Global warming and climate change are widely recognized among scientists, although controversy still rages among the political community. By the end of the 21st century temperatures may rise between 1.8 and 4 degrees Celsius (3.2 to 7.2 degrees Fahrenheit). Greater impact is observed in the polar regions, and Greenland has warmed by 4 °C since 1991. Impacts are seen on every continent and every ocean [14, pp. 26–27].
The abundance of coal and its low cost make it the world’s choice to provide the growing electricity demand. However, “worldwide coal-plants are responsible for 20% of human-caused greenhouse gas emissions” [14, p. 10]. The combustion of coal, natural gas, and biomass (according to the American Heritage Dictionary of the English language biomass is defined as “the total mass of living matter within a given unit of environmental area or as plant material, vegetation, or agricultural waste used as a fuel or energy source”), the rapidly growing population, and increasing development in Asia and Africa are adding to the challenge. Extreme dry weather has contributed to famine and the spread of disease, especially in African countries such as Ethiopia, which are heavily dependent on rain-fed agriculture and cannot afford adequate watering technology.
One way to measure the impact of an individual on the environment is through the carbon footprint, which varies substantially from region to region. As published in the magazine National Geographic, Special Report on “Changing Climate,” early in 2008, families from Botswana, the United States of America, and India have very different carbon footprints. In Botswana, the traditional way of cooking uses wood and biomass for fuel, which leads to annual CO2 emissions of 2.2 metric tons per person, twice the annual emissions per person in India. Since the population of Botswana is only 1.8 million, the global impact is small. In the United States, the total CO2 emissions in 2005 reached 5957 million metric tons, or approximately 18.5 metric tons per person. While most people in the United States are using energy-efficient appliances and taking other actions to reduce their personal carbon footprint, the average emissions include their contributions through the transportation, manufacturing, and residential sectors.
The United States is making strides in reducing its carbon footprint, with per capita emissions in 2011 falling to the lowest levels since 1992. The State of California is, in many respects, leading the way. In California, energy efficiency and conservation, sustainability, green building, and green purchasing are put into practice through Executive Order S-20-04 known as the “Green Building Initiative” and the Green Building Action Plan. Governor Arnold Schwarzenegger created the Green Action Team, leading the call for public buildings to be 20% more energy efficient by 2015. California, often referred to as “the Green State,” is devoted to creating healthier environments for its citizens in which to work, live, and learn (Highlight 1.6).
Development and population growth add to the global carbon footprint. In India, the growing population, strong economic development, and even a small increase in the living standard are having a tremendous impact on the average carbon footprint. Economists foresee that by 2050 more than 600 million automobiles will be on India’s roads, an increase that has never been seen in any other country in the world. The footprint of China, which represents a geographical area similar to that of the 48 contiguous states and has a population five times that of the United States, has grown to 5.3 metric tons per person and continues to increase. Growth in the developing countries is a central question, as voiced by Maurice Strong, the cofounder and chairman emeritus of the Earth Council: “The fate of the Earth will be decided in the developing world.” One asks, “Should the developing countries follow the model provided by the developed countries?” [16]. The Agenda 21/Rio summit states clearly that the developing world must have the right to strive for the same rights, chances, and justice as the developed world, but at what cost? Is there a path to prosperity for these regions that does not result in a similar level of emissions?
The current image of chemical industries is associated with pollution makers and environment destructors, and the image of chemicals is associated with hazards and toxicity. But the chemical industry has also been responsible for great advances in medicine and science, and the development of new materials such as plastics. Chemistry is a part of society, whether we want/like it or not. Chemistry is essential to maintain and improve the quality of life, but chemistry must work with other disciplines to develop the new technologies needed for the advancement of society. It goes hand-in-hand with disciplines such as ecology, biology, toxicology, and chemical engineering. Together with these additional disciplines, the chemical industry will be transformed into one that creates the materials and products demanded by society without the environmental consequences that society is no longer willing to accept.
Getting from “here to there” takes patience, collaboration across disciplines, and a sense of achievement and influence. This book will help to show that there are environmentally benign alternatives to current syntheses and chemicals. Understanding these inherently safer design alternatives will enable us to reduce our local and global impacts on the environment, health, society, and economy. As mentioned by L. Hunter Lovins: “We need to invent whole new institutions, new ways of doing business, and new ways of governing.” Where do we want to be 50 years from now? What do we want our planet to look like? How do we get out of our comfort zone and change our way of thinking? If you are interested in having the answers to these questions and if you are ready to pursue science in a creative, innovative, and responsible manner, then this book is for you.
Green Chemistry and Engineering: A Pathway to Sustainability is divided into 11 chapters. The first three chapters provide background on the practices and principles of green chemistry and engineering, the impacts of chemistry in natural systems, and some basic tenets of life cycle analysis and pollution prevention. The following four chapters of the book enable the readers to learn about matter, the heart of chemistry, to understand chemical reactions and processes commonly used and their greener alternatives, concepts about kinetics, catalysis, and reaction engineering from an environmentally benign point of view, and the role of thermodynamics and equilibrium. The last four chapters focus on applications of renewable materials and current and future states of energy sources, as well as the relationship between green chemistry and economics and green chemistry and toxicology.
Green chemistry and green engineering are not new scientific disciplines. There is even a belief that the word green will disappear because “green” will be the only way to do chemistry. This new way of thinking, doing, teaching, and learning is part of the solution to our global problems. It is a new philosophy of life beneficial to our own health, environment, business, and sense of community. Our own drive may be different from our neighbor’s but the end product will be the same. Let’s begin by looking at how it all started.
1. http://en.wikipedia.org/wiki/Chemistry#cite_note-0.
2. http://www.icistrainingsite.com.
3. Girard, J. Principles of Environmental Chemistry, 2nd ed., Jones and Bartlett Publishers, Sudbury, MA, 2010.
4. http://dictionary.reference.com/browse/anti+metabolites?s=t.
5. http://www.cancer.gov/newscenter/pressreleases/ReportNation2009Release.
6. http://www.epa.gov/history/topics/lovecanal/01.htm.
7. http://www.tropmed.org/rreh/vol1_10.htm.
8. http://www.epa.gov/superfund/.
9. http://www.epa.gov/oem/content/epcra/index.htm.
10. http://www.epa.gov/p2/pubs/basic.htm.
11. http://www.eoearth.org/article/Pollution_Prevention_ Act_of_1990,_United_States.
12. http://www.sbcapcd.org/airtoxics/OhioEPA-UnderstandingRiskAssessment.pdf.
13. http://www.guardian.co.uk/science/2009/mar/18/perfect-storm-john-beddington-energy-food-climate.
14. National Geographic Special Report June 2008.
15. http://www.green.ca.gov/default.htm.
16. Anderson, R. C. Mid-course Correction: Towards a Sustainable Enterprise: The Interface Model, Chelsea Green Publishing Company, White River Junction, VT, 1998.