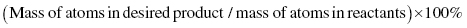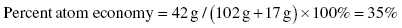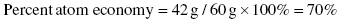2
PRINCIPLES OF GREEN CHEMISTRY AND GREEN ENGINEERING
2.1 INTRODUCTION
Green is the color of chlorophyll; it is also the color of money. For some countries it is associated with a political party, for others it may be everything that is beneficial to the environment. Green chemistry is not a new branch of chemistry or about the chemistry of molds, leaves, or plants. It is apolitical and is rather the new color of science. It is cleaner, smarter, and cheaper chemistry.
Green chemistry strives to minimize waste production, to promote the use of renewable and recycled resources, and to achieve the highest possible energy efficiency. It is based on the design or redesign of chemicals at the basic molecular level. When we asked ourselves in the first chapter: “Will future generations be able to live adequately with sufficient food supplies, energy resources, and means of transportation?” we realized that for the answer to be yes, our way of thinking had to change. “We’ve got to get over the idea that all of this is here for us,” said Janine Benyus, founder of the Biomimicry Guild and author of Biomimicry: Innovation Inspired by Nature [1]. “What we have to do is learn from what’s out there.” Nature should be our mentor. Effectively, “What use is a house if you haven’t got a tolerable planet to put it on?” asked Henry David Thoreau, American author and naturalist (1817–1862).
Green chemistry addresses the issue of “doing well by doing good” [2]. We all know that it is more efficient to prevent cancer than to try to cure it after being diagnosed. The same concept applies to pollution. Prevention of pollution rather than treating pollution and disposing of chemicals is a sustainable solution. We all have heard “An ounce of prevention is worth a pound of cure” by Benjamin Franklin, one of the Founding Fathers of the United States of America and renowned inventor (1706–1790). Green chemistry is not applying bandage technology anymore. As Paul Anastas, former Director of the Center for Green Chemistry and Green Engineering and Professor in the Practice of Green Chemistry in the School of Forestry and Environmental Studies at Yale University mentioned, “Green chemistry is powerful because it starts at the molecular level and ultimately delivers more environmentally benign products and processes” [3].
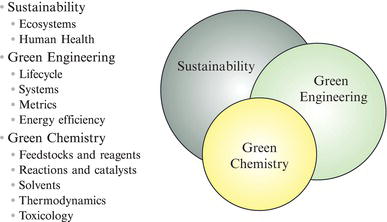
Green chemistry is distinct from green engineering, which is itself distinct from sustainability. Although the terms are often used interchangeably, each of these concepts embodies slightly different ideas and encompasses different scopes of activity. They are related terms with some overlap, but as indicated in Figure 2.1, they have unique foci that create distinct flavors in the application and use of these concepts.
Within the remainder of the chapter, we provide greater details on the definition of each of these terms. For now, it is worthwhile to consider the concepts in their unique flavor. Green chemistry focuses on the design of chemical products to minimize their inherent hazard, using fundamental principles of chemistry. Green engineering is more oriented toward the process and, as such, to identify ways in which manufactured products (including those that are not inherently chemical in nature) are produced and used. On the other hand, sustainability is focused on the system, not only the nature of the material and how it’s produced, but also on why it is needed in the first place. Because of its breadth and scope, issues of sustainability require more than just scientists and engineers; sustainability science requires contributions from social scientists, economists, health professionals, and more as it seeks to transform the fundamental value system of the human population.
2.2 GREEN CHEMISTRY
2.2.1 Definition
So what exactly is green chemistry? Green chemistry applies fundamental chemical principles to produce chemical products that are inherently less toxic, either to humans or to the ecosystem, than currently existing chemical products. Green chemistry may be applied to any of the various elements of the chemical product life cycle, from manufacture, to use, and ultimately to disposal. Thus, green chemistry may be applied to the production of a particular chemical to minimize the hazard associated with its use, or it may focus on the manufacture of the chemical to minimize the environmental consequences of the by-products or the synthesis, or it may equally well look into the development of more environmentally friendly alternatives to a specific chemical. Regardless, green chemistry seeks to reduce the hazard associated with chemical species.
Green chemistry is distinct from environmental chemistry. The latter focuses on understanding the natural environment, the interactions of chemicals in the ecosystem, and, ultimately, the nature and fate of pollutants within the environment. Environmental chemistry leads to an understanding of the interactions of chemical species in the environment; green chemistry emphasizes minimizing the addition of undesired species into the ecosystem.
Green chemistry was first defined by Paul Anastas and John Warner. In their revolutionary book Green Chemistry: Theory and Practice published in 1998, green chemistry is defined as “the utilization of a set of principles that reduces or eliminates the use or generation of hazardous substances in the design, manufacture and application of chemical products” [4, p. 11]. One of the distinct features of green chemistry is the idea of “benign by design,” that is, the deliberate action to make a chemical product or process inherently less hazardous. This chemical philosophy was described in the previous chapter as inherently safer design and is also referred to as “environmentally benign chemical synthesis” or “alternative synthetic pathways for pollution prevention.”
Green chemistry is an outgrowth of the 1990 Pollution Prevention Act (PPA) and draws from all chemical disciplines including organic, inorganic, analytical, biochemistry, and even physical chemistry. Prior to 1990, the U.S. government focused on command and control strategies that prescribed mechanisms to limit the impact of the chemical enterprise on the environment. Pollution was considered acceptable, as long as it was kept below some acceptable value. If a manufacturer exceeded the permitted emission limit, then a penalty would be imposed. Thus, the business could make an economic decision based on the cost of installing recovery or treatment equipment relative to the cost of violating a permissible emission limit. The PPA changed the economic equation, promoting an opportunity for the manufacturer to seek new technologies or chemical products that would be inherently benign, thereby making the regulation irrelevant. This is the inherent value of green chemistry; no longer would a company be forced to install certain types of treatment technology, but within the green chemistry paradigm the company is able to meet its environmental targets through innovation. Green chemistry is “an opportunity rather than a limitation” [4, p. 12].
Green chemistry is not just a way to protect the environment by preventing pollution before its creation; it is also a way to increase efficiency and reduce costs of production, an opportunity for businesses to lighten their environmental burdens and make money. To demonstrate how green chemistry can be applied, Anastas and Warner have provided some directions detailed in the 12 principles of green chemistry [4, pp. 29–55].
2.2.2 Principles of Green Chemistry and Examples
The 12 principles are summarized below and examples of each principle are provided.
1. Prevention: It is better to prevent waste than to treat or clean up waste after it has been created. Prevention starts by avoiding the use or generation of hazardous substances. If hazardous materials are not produced, then treatment and disposal are not required. Moreover, extraordinary safety measures needed for the manufacture of hazardous materials are not required, making the green product less costly to produce and easier to use. Why waste time, money, and effort to deal with these avoidable consequences? Remember the quote from Benjamin Franklin: “An ounce of prevention is worth a pound of cure.”
2. Atom economy: Synthetic methods should be designed to maximize the incorporation of all materials used in the process into the final product. The concept of atom economy was invented by Professor Barry Trost from Stanford University [5]. Atom economy is a measure of how much of the reactants are actually incorporated into the products. This concept differs completely from the concept of yield, which is still taught in traditional general chemistry courses. With yield, one is only focused on the molar quantity of product formed relative to the molar amount of reactant. Atom economy looks more closely at what happens to the side or waste products. One can easily imagine the case where a product is formed in 100% yield through an elimination reaction, but because the moiety being eliminated is large, the reaction may only have 50% or 75% atom economy. Atom economy is an essential tool in measuring how much waste is being produced and thus provides a valuable metric for reducing the use of nonrenewable resources, minimizing the amount of waste, and reducing the number of steps used to synthesize chemicals (Highlight 2.1). The ultimate measure of “reaction efficiency” is calculated as being the product of chemical yield by experimental atom economy.
3. Less hazardous chemical syntheses: Wherever practicable, synthetic methods should be designed to use and generate substances that possess little or no toxicity to human health and the environment. The use of hazardous chemicals is linked to risk of harm. Risk can be written as the product of exposure and hazard. A quick segue into nuclear power illustrates this concept perfectly. The hazard associated with a nuclear reactor meltdown is catastrophic. Because of the severity of the hazard, we demand that huge costs be expended to minimize the exposure. Ultimately, the combination of these two features makes nuclear power acceptable to a large segment of the population, despite the huge environmental risk. Put mathematically, if we multiply infinity (the hazard) by zero (the exposure), we get none. Since we can never reduce the exposure to zero, there is always a finite risk with any potentially hazardous material. On the other hand, chemists and scientists have the knowledge and skills to develop safer reactions and chemicals. Hazard is an intrinsic characteristic: it will not change. We can minimize the risk by reducing the hazard, and thus reduce the costs associated with minimizing the exposure, creating a new dynamic for determining an acceptable risk (Highlight 2.2).
4. Designing safer chemicals: Chemical products should be designed to achieve their desired function while minimizing their toxicity. Many chemicals are multifunctional, with a specific portion of the molecule providing the desired function while another portion of the molecule might impart some undesirable toxicity characteristics. Careful analysis of a selected chemical can elicit which elements of the molecule provide the desired and undesired functions. If the mechanism of action of a chemical is known, then it is possible to modify its structure and reduce its toxicity. Even if the mechanism of action is unknown, chemical structure analysis of the functional groups can be used to distinguish between the functional elements and the potentially hazardous moieties. By redesigning the molecule, the functionality related to the toxic effect can then be avoided, minimized, or totally suppressed, while the functionality providing the desired activity is retained (Highlight 2.3).
5. Safer solvents and auxiliaries: The use of auxiliary substances (e.g., solvents and separation agents) should be made unnecessary wherever possible and innocuous when used. Anecdotal information suggests that many reactions are performed in a selected solvent for expediency, and not for chemical or environmental reasons. In other words, a solvent that was once identified as “good” is simply chosen for all subsequent versions of the chemistry, without regard to whether it is “best.” But these solvents may be released into the environment, and if they don’t impart specific chemical benefit, then their value must be questioned. The impacts of solvents on the environment, health, and ecosystem are well known. Whenever possible, reactions should be developed with the environmental impacts of the solvent in mind, and benign solvents such as supercritical CO2 or water, immobilized solvents, or solventless systems should be considered. An example of a conversion to a solventless process is detailed in Highlight 2.4.
6. Design for energy efficiency: Energy requirements of chemical processes should be recognized for their environmental and economic impacts and should be minimized. Synthetic methods that are conducted at ambient temperature and atmospheric pressure consume less energy than those that are done at high temperature and pressure. Recovery of energy resources and reuse within the process can minimize the need for external energy resources. Analysis of the heating requirements of a reaction for maximum heat integration minimizes the overall need for fossil resources. An example of heat integration analysis is presented in Highlight 2.5.
7. Use of renewable feedstocks: A raw material or feedstock should be renewable rather than depleting whenever technically and economically practicable. Depleting or nonrenewable fossil resources are subject to the criterion of time and cannot be replenished nearly as rapidly as they are consumed. Renewable feedstocks are biological and plant-based starting materials that can be replaced through their natural processes. Because there are limited supplies of depleting resources, we must take care to use these judiciously and look for opportunities in which renewable materials may be substituted economically. Opportunities for renewable feedstocks as the basis for chemical production require novel chemical techniques and new processing methods, but should be considered as an important method to reduce the reliance on nonrenewable fossil resources. The reuse and recycle of metals and other nonrenewable natural resources must also be considered as an opportunity to minimize the consumption of these depleting materials.
8. Reduce derivatives: Unnecessary derivatization (use of blocking groups, protection/deprotection, temporary modification of physical/chemical processes) should be minimized or avoided if possible, because such steps require additional reagents and can generate waste. During the manufacture of pharmaceuticals, pesticides, and dyes, it is sometimes necessary to derivatize certain functional groups or parts of the molecule to protect them from reaction with reagents that facilitate a necessary transformation. Conceptually, protection/deprotection requires two extra steps to accomplish a simple addition or a reaction step to a particular functional group. Extra reaction steps mean extra processing steps and extra waste, all of which reduces the atom economy of the overall process. The unnecessary derivatization generates additional waste upon deprotection.
9. Catalysis: Catalytic reagents (as selective as possible) are superior to stoichiometric reagents. Catalysts increase the rates of chemical reactions but are not consumed in the reactions. As such, a catalyst may decrease the temperature at which a reaction can be performed economically, thus saving energy costs. Because a catalyst will accelerate the rates of certain reactions more than others, a catalyst can also be used to effect the selectivity in a sequence of reactions. In some reactions, stoichiometric reagents are used to promote a particular pathway or to influence a specific reaction step. Substitution of a catalytic material, particularly one that can easily be recovered, enhances the performance of the reaction in terms of speed and decreases the consumption of materials. Chapter 6 covers some of the basic catalytic tools used in typical chemical reactions and in industry.
10. Design for degradation: Chemical products should be designed so that at the end of their function they break down into innocuous degradation products and do not persist in the environment. Some chemicals such as plastics made from petroleum intermediates or pesticides persist in the environment. For example, there are billions of plastic bags manufactured every year. The usable average life of a plastic bag is only 20 minutes but it can take up to 1000 years for the bag to degrade under ambient conditions. New technologies are being developed to insert photoactive materials into plastic bags that would cause them to degrade in the presence of sunlight, or for them to be more easily digested by microorganisms in the environment. Today, only about 10% of plastic bags are biodegradable (Highlight 2.6).
11. Real-time analysis for pollution prevention: Analytical methodologies need to be further developed to allow for real-time, in-process monitoring and control prior to the formation of hazardous substances. The monitoring of a chemical process is beneficial for several reasons: the formation of toxic by-products can be detected early on and parameters can be adjusted to reduce or eliminate formation of these substances. Real-time monitoring ensures that the reaction is running smoothly, and reduces the amount of off-specification product formed. Control systems can be designed to autocorrect as process conditions change, ensuring optimal performance of the system.
12. Inherently safer chemistry for accident prevention: Substances and the form of a substance used in a chemical process should be chosen to minimize the potential for chemical accidents, including releases, explosions, and fires. Toxicity, explosivity, and flammability must be part of the design of chemical products and processes. Pollution prevention relies on accident prevention to minimize the likelihood of chemicals leaking into the environment. However, if one uses inherently safe materials, then even in the event of a leak, there is minimal hazard. For example, when water is used as a reaction solvent, the loss of solvent is of no concern, because the water is nonhazardous. On the other hand, elaborate precautions must be taken with the use of organic solvents, since volatile organic compounds in the atmosphere are hazardous to the environment and human health. Other techniques to minimize the presence of hazardous materials include their generation as part of the reaction scheme, followed by rapid consumption of these materials. By controlling the process conditions, the amount of the hazardous substance is kept to a minimum at all times, which minimizes the impact of that substance in the event of an accident or spill.
Highlight 2.1 Definition and Calculations Dealing with the Application of Atom Economy
Chemists are interested in assessing the reaction efficiency and, most of the time, use the percent yield of a reaction calculated as the ratio of the actual yield of a specific product divided by the theoretical yield (based on the limiting reactant). This tool is used to quantify the efficiency of a chemical reaction and to compare the expected product quantity to the actual one.
However, just looking at a percent yield does not tell the complete story. A percent yield calculation does not measure how efficiently the reactants have been used in generating the desired product and therefore does not take into account how much waste (toxic or benign) is created.
To express how efficiently reactant atoms are used in the products, Barry Trost of Stanford University developed the concept of atom economy, for which he received the Presidential Green Chemistry Challenge Award in 1998. The percent atom economy is calculated as:
This tool does not take into account the amount of reactants often used in excess as well as the yield of the reaction. However, it helps in making the final decision about the efficiency of a synthetic pathway versus another.
Let’s work out an example. Propene (C3H6) can be synthesized via two routes:
Route 1: Propene is generated from the reaction of trimethylpropylamine ion [C6H16N]+ with a strong base [OH−] under heat conditions. The by-products of this reaction are trimethylamine [C3H9N] and water.
Route 2: Propene can also be generated from the decomposition of 1-propanol [C3H8O] under heat and acidic conditions. In this case the only by-product is water.
When calculating the molecular mass of each reactant and product we need to add the atomic weight of all elements present in the molecule (see Chapter 5). The mass of propene is 42 g/mol (3 × 12 g/mol + 6 × 1 g/mol = 42 g/mol); the mass of trimethylpropylamine and OH− combined is 119 g/mol whereas the mass of 1-propanol is 60 g/mol.
Route 1:
Route 2:
We conclude that route 2 offers the highest atom economy (twice the one obtained for route 1) but the only by-product in route 2 is water; so by comparing these two synthetic pathways we can determine that route 2 is the most environmentally friendly way to produce propene.
We need to exercise caution when looking at atom economy. A synthetic pathway with the highest percent atom economy is not always the less toxic one. Assessing the toxicity and hazards associated with the by-products (in route 2 the only by-product was a benign compound, water) is essential to make the final call.
Highlight 2.2 Risk, Hazard, and Exposure?
How can we assess Risk = f (hazard, exposure)?
Risk is often expressed as the probability of adverse effects associated with a particular activity. For example, the fatality risk associated with smoking 10 cigarettes per day is 1 in 1000 compared to the risk associated with being hit by automobiles which is 4 in 100,000. Some risks are voluntary risks (such as smoking) while others are not (e.g., natural disasters).
The risk assessment process always starts with identification of hazards (e.g., what chemicals are potentially harmful based on toxicity score). The toxicity score is a function of the maximum concentration of a chemical and its acceptable daily intake. For example, when comparing toxicity score values for chlorobenzene (previously used in the manufacture of pesticides such as DDT) and BEHP (bis-2-ethylhexyl phthalate used as solvent in glowsticks and in the manufacture of PVC), the toxicity score for BEHP is 11,500 compared to 320 for chlorobenzene. The evaluation of other properties of the contaminant such as bioaccumulation and persistence in the environment as well as treatability is also important to assess. Most importantly, the second step in assessing risk is to look into the dose–response relationship. In other words, quantifying the dose in relation to its adverse effects will enable a safe dose to be established. There is normally a proportional relationship between an increase in the dose administered or received and the incidence of an adverse health effect and its severity for an exposed population. A dose is often quantified as mg chemical/kg of body weight. The third step is to assess means of exposure (occupational exposure, i.e., workplace and community exposure) and routes of exposure (dermal exposure through the skin, lung exposure through inhalation, and ingestion). Intake rate calculations can help identify chronic daily inhalation intake as a function of the concentration of contaminant, contact rate, frequency, exposure duration, and body weight. The final step in the risk assessment process is the integration of hazard identification, dose response, and exposure assessment. This ultimately leads to the characterization of a contaminant as carcinogenic or noncarcinogenic. Since data is often missing, educated guesses and assumptions are often necessary, which leads to uncertainty. Computational analysis is a very helpful tool to make more informed decisions regarding risk management (see Chapter 11 for general information on toxicity, and Section 11.5 for more on computational methods).
Highlight 2.3 Designing Molecules While Retaining Functionality and Suppressing Toxicity
In 2011 the Sherwin-Williams company reinvented oil-based paints by developing water-based acrylic alkyd paints with low levels of volatile organic compounds (VOCs). These new paints are made from recycled soda bottle plastic (polyethylene terephthalate or PET), acrylics, and soybean oil.
Oil-based “alkyd” paints generate high levels of VOCs as the paint dries. Acrylic-based paints were developed in the past with production of low VOCs but their performance was not satisfactory. Instead, Sherwin-Williams designed a new technology taking advantage of the combination of a PET-based polymer dispersion (for rigidity, hardness, and hydrolytic resistance) with acrylic (for improved dry times and durability) and soya functionality (for gloss formation and flexibility). This new technology combines the benefits of alkyd and acrylic paints with lower VOCs, without surfactants and with minimum odor. By redesigning the molecular content of this paint product, Sherwin-Williams was able to eliminate 800,000 pounds of VOC solvents and petroleum-based solvents since 2010. Sherwin-Williams won the 2011 Presidential Green Chemistry Challenge award in the designing greener chemicals category.
Highlight 2.4 Solvents: Are They Necessary?
The cosmetics and personal care manufacturers are heavy consumers of esters, usually synthesized using strong acids and hazardous organic solvents at high temperatures. In 2009, Eastman Chemical Company managed to replace strong acids and organic solvents and eliminated the production of undesirable by-products using immobilized enzymes (such as lipase involved in the formation or breaking of lipids) in the esters synthesis. These enzymes serve as biocatalysts in the production of esters and can easily be removed by filtration. They also allow the synthesis to be performed under mild processing conditions and therefore save energy and increase yield. This enzymatic route saves over 10 liters of organic solvent per kilogram of product. Overall, the quality of the product is superior to the one obtained via conventional esterification, while the cost and environmental footprint are improved. Eastman Chemical Company received the 2009 Presidential Green Chemistry Challenge Award in the greener synthetic pathways award category.
Highlight 2.5 Heat Integration for Sustainable Energy
Heat integration analysis (or process integration) is a way to save energy by examining the “potential of exchanging heat between heat sources and heat sinks via the use of heat exchangers and reducing the amount of external heating and cooling required”[6].
Several case studies have been published regarding hospitals as important thermal energy users [7, 8]. Herrera and co-workers [6] looked into a hospital complex including an institute, a general hospital, a regional laundry center, a sports center, and other public buildings. Input of high-priced diesel fuel in boilers to produce steam usually met the heat demand. However, several sinks of heat were identified such as the hot streams coming from the laundry and condensed steam not recovered in the condensation network. A heat integration analysis suggested the addition of four heat exchangers to use energy that would otherwise be wasted (two in the laundry, one in the machinery room to help heat the boiler feed water, and the last one in the condensation tank area that heats the sanitary water) [7]. Kemp furthered this analysis by suggesting various hospital operating procedures to further reduce energy consumption, such as changing the periods of high utility demands since energy consumption seemed to vary considerably between day and night, or recovering exhaust gases to supply a space heating system, or to invest in a standby diesel generator to supply emergency power rather than expand overall generation capacity [8]. New designs based on heat integration led to energy savings of over 30% in major producing companies. Heat integration can be applied equally well to small or large buildings and offices and is a way to promote ecobuilding technologies.
Highlight 2.6 Plastic Bags and Microbes?
Most of the traditional methods used to decompose plastic require high temperatures and harsh chemicals. In 2009, a 16-year-old high school student at Waterloo Collegiate Institute in Ottawa, Canada, won a science fair for finding an answer to the famous question: “Are there microorganisms able to decompose plastic bags?” Daniel Burd managed to find a way to breed microorganisms able to degrade plastic in a very short amount of time [9]. He immersed ground plastic in a yeast solution and was able to isolate the effective strains and maintain their growth so that 43% of the plastic degraded in 6 weeks. Unfortunately, the experiments were run under highly controlled environments that would need to be in place for the biodegradation to happen. Several bacteria-based solutions have been found since 2009 and microbial degradation is one of the most effective and environmentally friendly ways to incorporate green chemistry in a new design pathway.
2.2.3 Presidential Green Chemistry Challenge Awards
The Presidential Green Chemistry Challenge Program was created in 1996 to promote innovative research in and uses of green chemistry for pollution prevention. This partnership program is led by the EPA’s Office of Chemical Safety and Pollution Prevention in cooperation with the American Chemical Society, other EPA offices, other federal agencies, and others. Although the program has had a varying scope over the years, including a research component, the program is currently limited to the administration of the Presidential Green Chemistry Challenge Awards.
Every year, the Presidential Green Chemistry Challenge Awards Program seeks nominations from individuals, groups, and both nonprofit and for profit organizations, including academia, government, and industry to compete for awards in recognition of innovative technology incorporating the principles of green chemistry. Nominations are judged by an independent panel of experts. Three areas of focus describe these awards.
2.2.3.1 Use of Greener Synthetic Pathways
The use of greener synthetic pathways involves either implementing a novel, green pathway for a new chemical product or redesigning the synthesis of an existing chemical product. According to the EPA, “examples include synthetic pathways that:
- Use greener feedstocks that are innocuous or renewable (e.g., biomass, natural oils).
- Use novel reagents or catalysts, including biocatalysts and microorganisms (e.g., protein enzymes).
- Are natural processes, such as fermentation or biomimetic synthesis (replication of reactions occurring in nature using in vitro system).
- Are atom-economical (this is the topic of principle 2 of green chemistry).
- Are convergent syntheses. Convergent syntheses aim at improving the yield of multistep chemical reactions by reordering the reaction steps, which serves to increase the final yield of the desired product. Convergent synthesis can be highly effective when applied to the production of complex molecules such as proteins made up of 300 amino acids.”
A recent use of greener synthetic pathways was exemplified in the production of 1,4-butanediol using a microbial synthetic pathway and genetic engineering, for which the company Genomatica won the 2011 Presidential Green Chemistry Challenge Award. Genomatica is involved in the production of basic and intermediate chemicals made from renewable feedstocks such as sugars and biomass. Genomatica created a successful bio-based fermentation process of sugars for production of 1,4-butanediol [HO(CH2)4OH] as feedstock for polymers and commodity chemicals that are used in products such as spandex, automative plastics, and running shoes. Several outcomes were observed: reduction of energy by 60% compared to the acetylene-based process; reduction of carbon dioxide emissions by 70%; no organic solvent was used in the fermentation process and the water used was recycled; and a safer working environment. This bio-based process is expected to be economically competitive with traditional processes based on current oil and natural gas prices.
2.2.3.2 Use of Greener Reaction Conditions
The use of greener reaction conditions includes:
- Replacing hazardous solvents with solvents that reduce the impact on human health and the environment.
- Operating under solventless reaction conditions and performing solid-state reactions.
- Novel processing methods.
- Eliminating energy- or material-intensive separation and purification steps.
- Improving energy efficiency, including running reactions closer to ambient conditions.
As an example, biocatalysis has found its way into greener manufacturing of drugs. This is the case for the second generation production of sitagliptin, the active ingredient in JanuviaTM, used in the treatment of type-2 diabetes. Prominent companies in this field are Merck, a leading health-care and pharmaceutical proider, and Codexis, a leading provider of biocatalysts. Merck and Codexis collaborated to design a greener manufacture of sitagliptin using biocatalysts while reducing waste, improving yield and safety, and removing the need for a metal-based catalyst. This new route relies on the use of evolved transaminases (important enzymes in the production of amino acids). The traditional pathway involved a high-pressure catalytic hydrogenation step with a rhodium catalyst, ultimately leading to the production of undesired enantiomers. Major benefits include a 56% improvement in productivity, a 10–13% increase in yield, and a 19% reduction in waste generation mostly due to the elimination of the purification steps. Both Merck and Codexis won awards individually in 2006 and co-received the 2010 Presidential Green Challenge Award in the greener reaction conditions category.
2.2.3.3 Design of Greener Chemicals
Designing greener chemicals involves the development of new materials that are less hazardous than chemicals used in traditional technologies. Greener chemicals would have the following characteristics:
- Less toxic than currently available chemical products.
- Inherently safer with regard to accident potential.
- More easily recycled or biodegradable after use.
- Reduction in atmospheric impacts (e.g., nonozone depleting or smog forming).
In this electronic world, the dependence on paper products has decreased, but the paper and packaging industry still plays an important role in the economy of the United States, employing about 400,000 people and posting sales up to $115 billion per year. The traditional pathway to increase strength and quality of paper from cellulose in wood relies on either significant mechanical treatments and therefore intense energy requirements, or the use of nonrenewable chemicals. Buckman International, Inc. based in Memphis, Tennessee, developed Maximize® enzymes to increase the number of fibrils in cellulose, thereby improving the strength and quality of paper without additional mechanical or chemical treatment. Maximize enzymes are a combination of cellulose enzymes and enzymes from natural sources produced from fermentation. While these enzymes use less energy and electricity for refining, the overall treatment is also less toxic, safer to handle than the current treatments, and also completely recyclable. The commercialization of this biotechnology took place in 2010. One company using Maximize technology saved over $1 million per year and another one claimed that the machine speed increased by 20 feet per minute for a 2% increase in production. It is expected that this new technology will be applied in over 50 paper mills in the United States and beyond.
Five awards are typically given to an academic investigator for a green chemistry technology in any of the three focus areas mentioned above, to a small business* for a green chemistry technology in any of the three focus areas, to an industry sponsor for the use of greener synthetic pathways, to a second industry sponsor for the use of greener reaction conditions, and to a third industry sponsor for the design of greener chemicals.
A complete list of the award winners can be accessed on the EPA website: http://www.epa.gov/greenchemistry/pubs/pgcc/past.html.
2.3 GREEN ENGINEERING
2.3.1 Definition
The Environmental Protection Agency (EPA) defines green engineering as “the design, commercialization, and use of processes and products that are feasible and economical while minimizing (1) generation of pollution at the source and (2) risk to human health and the environment” [9]. The goal of green engineering is to minimize the impact of chemical processes on human health and the environment. The impact of environmentally conscious strategies is to reduce risks to ecosystems, workers, consumers, and the general population while manufacturers and suppliers are cutting their cost of production.
Engineers apply risk assessment to pollution prevention in their strategies. Risk is expressed as a mathematical function of hazards and exposures. Risk assessment methods help quantify the degree of environmental impact for individual chemicals. Engineers apply technologies to control the risks as an element of the design processes and products, taking into account the likelihood that certain actions will occur. Thus, a fundamental difference between green engineering and green chemistry is the assumption of risk as an acceptable element to be controlled, rather than attempting to design the risk out of the particular chemical species.
As mentioned by the EPA: “By applying risk assessment concepts to processes and products, the engineer can accomplish the following:
- Estimate the health and environmental impacts of specific chemicals on people and ecosystems.
- Prioritize those chemicals that should be minimized or eliminated.
- Optimize the process design to avoid or reduce health and environmental impacts.
- Assess feed and recycle streams based on risk, rather than volume, when evaluating the impact of a chemical process.
- Design greener products and processes.”
Green engineering also involves systems analysis, creating an opportunity to consider the life cycle impacts of a particular product or process. In a life cycle analysis, one considers the environmental impacts at various stages of the process. For example, one may consider that the electric vehicle is “zero” emissions. However, when one recognizes that the electricity that is required to maintain charge on the battery is produced from a power plant (often a coal-fired power plant), then it is appropriate to assign a portion of the environmental impacts of that power plant to the operation of the electric vehicle, and it is no longer truly “zero” emissions. Similar types of analyses allow one to properly assign all of the impacts of a product and to thus choose the product that has the least environmental impact over its entire life cycle. Failure to look at the full life cycle results in the selection of a process improvement that enhances the environmental performance in a particular aspect while creating greater environmental challenges in the overall consumption of the product.
2.3.2 Principles of Green Engineering
The 12 principles of green engineering were proposed by Paul Anastas and Julie Zimmerman and published in Environmental Science and Technology on March 1, 2003 [10]. According to Anastas and Zimmerman, there are two fundamental concepts that designers should integrate in their design: “life cycle considerations and the first principle of green engineering, inherency.”
1. Inherent rather than circumstantial: Designers need to strive to ensure that all material and energy inputs and outputs are as inherently nonhazardous as possible. Materials and energy sources that are inherently benign minimize the possibility of building hazardous substances into the design of the product or process. Thus, it is inherently preferred to use water as a solvent than an organic solvent, since water is inherently benign, whereas the vapors of the organic solvent need to be controlled and the solvent itself needs to be recovered and recycled. The use of inherently benign materials reduces the need for constant monitoring and control throughout the hazard’s lifetime.
2. Prevention instead of treatment: It is better to prevent waste than to treat or clean up waste after it is formed. Waste involves a resource that is not used. Any material that is not used, even if it is inherently innocuous, needs to be recovered and recycled. Recovery requires additional processing steps, the use of additional materials, and the consumption of increased energy resources. If the material cannot be recovered, it must be disposed of in some way, and thus it creates a burden on the environment. From a business standpoint, the company pays once for the material it does not need, and then a second time to dispose of the waste. Clearly, reducing the amount of waste enhances the process, both environmentally and economically.
3. Design for separation: Separation and purification operations should be designed to minimize energy consumption and materials use. Separation and purification steps consume significant amounts of energy and may also be materials intensive, using large amounts of hazardous solvents. The separation process is often one of the most energy-intensive steps in the manufacturing process. Thus, the incorporation of self-separated reaction products prevents the need to spend energy and materials to recover the output product.
4. Products, processes, and systems should be designed to maximize mass, energy, space, and time efficiency. The concept of efficiency focuses on proper consumption of a resource. Processes that occur in small spaces and over short times can contain the hazard, minimizing the need for elaborate safety and risk management methods. Mass efficiency again refers to the incorporation of the maximum mass into the process. The second law of thermodynamics, which states that the amount of energy embodied in a product can never be fully recovered, suggests that it is best to maximize the energy intensity in the process. That is, small periods of intense energy use are preferred to using low energy levels over extended periods.
5. Output-pulled versus input-pushed: Products, processes, and systems should be output-pulled rather than input-pushed through the use of energy and materials. This approach is based on Le Châtelier’s principle, which states that “when a stress is applied to a system at equilibrium, the system readjusts to relieve or offset the applied stress.” Stress is defined as temperature, pressure, or concentration gradient. It is possible to increase the productivity of a process by overwhelming the feed with a specific reactant. That leads to incredible excess of the reactant in the product stream, which requires separation, recovery, and recycle. On the other hand, if you can remove a product from the process stream as it is produced, then the reaction will drive to greater quantities of product, without any need for use of excess reactants.
6. Conserve complexity: Embedded entropy and complexity must be viewed as an investment when making design choices on recycle, reuse, or beneficial disposition. Highly complex materials can be broken down into simple molecules, which can then be used in the production of different complex materials. However, this is often more energy and material intensive than using the natural complexity of the existing material. In a similar way, complex end products that can be reused are easier to recycle than those that need to be broken down into individual subcomponents.
7. Durability rather than immortality: Targeted durability, not immortality, should be a design goal. Immortality of a product is associated with environmental issues such as persistence and bioaccumulation, and ultimately the need for secure waste disposal. Products and processes should be designed to accomplish their task through their anticipated operating conditions and lifetimes, but then be recyclable and reusable afterwards. The “greener” design should take into account effective and efficient maintenance and repair without the addition of extra material and energy. By designing a durable and biodegradable product, long-term environmental impacts are significantly reduced.
8. Meet need, minimize excess: Design for unnecessary capacity or capability (e.g., “one size fits all” solutions) should be considered a design flaw. Unnecessary resources are often spent to “overdesign” a product or process with the intention of designing for worst-case scenarios regardless of local time, space, and operating conditions. This tendency often results in a lot of excess materials and energy wasted throughout the process. Greener designs need to incorporate the specific needs and functions of materials without going overboard.
9. Minimize material diversity: Material diversity in multicomponent products should be minimized to promote disassembly and value retention. Earlier principles discuss the desire for materials to be recovered and recycled. Minimizing the number of unique substances included within any product simplifies the product and makes recovery easier. A single material such as a polymer with tailoring backbones specifically engineered to accomplish desired properties is easier to recycle and reuse than multicomponent materials.
10. Integrate local material and energy flows: Design of products, processes, and systems must include integration and interconnectivity with available energy and materials flows. Utilizing existing energy and material flows will increase efficiency. Reusing wasted heat or existing materials from adjacent processes reduces the consumption of raw materials and improves the life cycle efficiency of the process and the sustainability of the product. The waste product from one facility may be a raw material for another facility. Energy can be recovered from the wastewater stream of an industrial plant. Integrating processes provides the maximum opportunity for recovery of energy and material resources.
11. Design for commercial “afterlife”: Products, processes, and systems should be designed for performance after their intended function has been completed. Afterlife should be part of the design strategy of a product. Incorporating components whose function and value can be recovered for reuse and reconfiguration after a premature end of life or a change in style is necessary. For example, pesticides will remain in the environment after the crop has been harvested. A pesticide that will decompose to innocuous materials in the natural environment in a reasonable time frame minimizes its long-term impact.
12. Renewable rather than depleting: Material and energy inputs should be from renewable resources to the maximum extent possible. Clearly, material and energy resources that are consumed at a rate that exceeds the ability of the natural environment to replenish that resource will eventually be fully depleted and not available for future use. Thus, processes and products should be designed to consume raw materials only at the rate at which they can be replenished. Because materials can be recycled, net consumption is the important parameter to be considered. In other words, aluminum that is obtained from recycled material does not deplete the raw material in the ground and thus may be considered renewable if there is sufficient recycled aluminum to fully meet the process needs.
2.4 SUSTAINABILITY
The idea of sustainability goes beyond the engineering and science associated with green chemistry and engineering, to incorporate social/health and economic factors into the discussion of the most appropriate technologies to implement. At the beginning of the chapter, we indicated that sustainability includes the concepts of ecosystems and human health. Incorporating these issues into engineering design creates new challenges in terms of valuation; one must consider the value of the rain forest relative to the value of clean drinking water or sufficient food supply. Clearly, sustainability involves analysis of the system, and thinking on a global scale.
According to the Brundtland Commission (formerly known as the World Commission on Environment and Development), sustainable development is generally defined as “providing for human needs without compromising the ability of future generations to meet their needs” [11].
The U.S. Environmental Protection Agency describes sustainability from two perspectives [12]: A public policy perspective would define sustainability as the satisfaction of basic economic, social, and security needs now and in the future without undermining the natural resource base and environmental quality on which life depends. From a business perspective, the goal of sustainability is to increase long-term shareholder and social value, while decreasing industry’s use of materials and reducing negative impacts on the environment.
An alternative business perspective can also be used to describe the concepts of sustainability in terms of the triple bottom line [13]:
Economic viability: the business aspects of a project.
Social concerns: human health and social welfare.
Natural or ecological issues: environmental stewardship.
Regardless of the perspective one takes, sustainability involves the intersection of environment, economics, and social welfare and requires analysis of the trade-offs that weigh the value of each of these goals. In January 2004, a group of 65 engineers, scientists, and others met on the shores of San Destin, Florida, to develop a set of principles for sustainable engineering. These principles, with further analysis, follow [14].
Sustainable engineering transforms existing engineering disciplines and practices to those that promote sustainability. Sustainable engineering incorporates development and implementation of technologically and economically viable products, processes, and systems that promote human welfare while protecting human health and elevating the protection of the biosphere as a criterion in engineering solutions.
To fully implement sustainable engineering solutions, engineers use the following principles:
- Engineer processes and products holistically, use systems analysis, and integrate environmental impact assessment tools.
One must consider all of the impacts and all of the opportunities available to deliver a desirable service for society. The choice of how this outcome can be delivered results from a combination of discrete decisions that lead to the development of a system for the delivery of that service. Transportation provides a good opportunity to consider systems analysis. The use of the internal combustion engine as the power source for the automobile requires the presence of fueling stations throughout the country. In Europe, where the diesel engine is more commonly used, fueling stations provide diesel fuel. Trade-offs are made through the choice of power source: diesel engines produce greater particulates than Otto-cycle engines, but they are also more efficient. Which is preferred? Thinking beyond the choice of the fuel source, the same activity could be achieved without the use of a personal vehicle, if a robust mass transit system was in place. Again, decisions are made that impact delivery of the service. In Europe, a significant rail presence exists and many people travel by train on a regular basis. In the United States, the car is king, and trains are rarely a viable alternative.
- Conserve and improve natural ecosystems while protecting human health and well-being.
What is an ecosystem? There are several different definitions, but all focus on the interactions of plants, animals, and microorganisms within their environment. Natural ecosystems are those that exist without input from humans. The preservation of natural ecosystems relates to the concept of using renewable resources, but goes further to reveal that even the use of renewable materials will disturb the natural cycles in nature. Maintaining these cycles reduces the impact of humanity on the planet and allows historical interactions to continue unabated.
- Use life cycle thinking in all engineering activities.
Life cycle is a key concept of green engineering and simply requires that one consider all aspects of the development of a product or process. While the use of a particular product may not lead to great hazard, the manufacture of that product may be particularly problematic and thus should be developed with great care. For example, a solar panel has very limited environmental impact while in use, but because of its need for hazardous and rare metals, it could have significant impacts during manufacturing or in disposal. Decisions must be made to weigh the impact of the product throughout its life cycle, to determine the true costs of the product against a sustainable outcome.
- Ensure that all material and energy inputs and outputs are as inherently safe and benign as possible.
This concept returns to familiar topics from the principles of green chemistry and green engineering. The use of benign materials is preferred, since these materials do not require elaborate engineering schemes to be used safely. And, if a material is truly benign, then disposal can usually be achieved without risk to the natural environment, since it will be assimilated into the environment through natural processes.
- Minimize depletion of natural resources.
Whereas the second principle discusses preservation of ecosystems, this principle focuses on conservation of natural resources. Recognizing that natural resources can be depleted, their use should be minimized. Attempts to recycle and reuse resources can minimize their depletion and leave them available for future generations.
Although seemingly straightforward, this concept is deceptively complex. A waste is a resource that is not available for another purpose. But when one brings systems together, it is possible that the waste stream from one process can become the feed for a second process. The wastewater of a chemical plant can be fed into a digester to produce methane gas that can become the fuel to operate a fuel cell, providing electricity to power the lights of the neighboring village.
- Develop and apply engineering solutions, while being cognizant of local geography, aspirations, and cultures.
The best solution for one area in the world may not be a viable solution for another. In the developed world, electricity is produced in large power plants and transported by power lines to locations in which it is needed. But in a world in which electricity is not reliable, local sources of power generation become critical, and the use of small-scale solar arrays that produce only sufficient energy for one family is more practical.
- Create engineering solutions beyond current or dominant technologies; improve, innovate and invent (technologies) to achieve sustainability.
One must recognize that the technologies that have been used to create the world in which we currently live will not be sufficient to provide a sustainable world for our children. It is critical to look beyond what is currently known, to seek out bold new ideas that deliver services in a way which is not yet conceived.
- Actively engage communities and stakeholders in development of engineering solutions.
Because sustainability is fundamentally a systems analysis, in which social aspects are equally important to engineering solutions, it is critical that the stakeholder community become involved in the selection of choices to deliver a desired service. If one needs a transportation solution in a community without cars, delivering a fueling station will provide no value. Delivering workforce training via the Internet to an inner city neighborhood in which people do not have access to a computer cannot be effective. One must understand the resources of the stakeholders, and their cultural limitations, in order to provide a solution of value.
- There is a duty to inform society of the practice of sustainable engineering.
The participants in the workshop were not satisfied simply stating a series of principles, and hoping that society would accept these concepts as self-evident. Because sustainability is built on the aggregation of billions of individual choices, it is vitally important that all of society understands the trade-offs associated with these choices. When individuals seek out the best choice for society as a whole, rather than the easy choice for themselves, the future of the world’s ecosystems will be sustained.
REFERENCES
1. Benyus, J. M. In: Biomimicry: Innovation Inspired by Nature, First Quill Edition, 1998.
2. Anderson, R. C. In: Mid-course Correction: Toward a Sustainable Enterprise: The Interface Model, Chelsea Green Publishing Company, White River Junction, VT, 1998, p. 63.
3. American Chemical Society website—Green Chemistry Educational Resources: Bleaching With Green Oxidation Chemistry, Chemistry and Compost Chemistry and Compost, Cleaning Up With Atom Economy, Fuel Cells: Energy From Gases Instead of Gasoline, Green Chemistry: A Greener Clean, Phytochemistry Activity, and Simple Green Cleans with Green Chemistry. Available at http://portal.acs.org/portal/acs/corg/content?_nfpb=true&_pageLabel=PP_SUPERARTICLE&node_id=1444&use_sec=false&sec_url_var=region1.
4. Anastas, P. T.; Warner, J. C. Green Chemistry: Theory and Practice, Oxford University Press, New York, 1998.
5. http://www.epa.gov/greenchemistry/pubs/pgcc/winners/aa98a.html.
6. Herrerra, A.; Islas, J.; Arriola, A. Applied Thermal Engineering, 2003, 23, 127–139.
7. Kemp, I. Pinch Analysis and Process Integration. A User Guide on Process Integration for Efficient Use of Energy, Butterworth-Heinemann Elsevier, New York, 2007.
8. http://www.mnn.com/green-tech/research-innovations/blogs/boy-discovers-microbe-that-eats-plastic.
9. http://www.epa.gov/opptintr/greenengineering/.
10. Anastas, P. T.; Zimmerman, J. B. Environ. Sci. Technol., 2003, 37(5), 94A–101A.
11. http://www.un-documents.net/wced-ocf.htm.
12. http://www.epa.gov/sustainability/basicinfo.htm.
13. Barrera-Roldan, A.; Saldivar-Valdes, A. Ecol. Indic., 2002, 2, 251–256.
14. Abraham, M. A., Sustainability Science and Engineering, Volume 1: Defining Principles, Elsevier Science, New York, 2006.