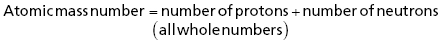
Matter surrounds us. It is in everything we taste, touch, smell, and see. It constitutes most of the observable universe. From the time we use a tablespoon to stir sugar or cream in our coffee or tea in the morning, to the time we travel to work, and finally when we sleep, chemistry is occurring and chemical transformations are taking place. Chemistry is the study of matter and its transformations from one form to another. Understanding the properties of matter allows for the design of more environmentally benign materials. In this chapter we discuss matter and its properties, the three states of matter, their applications in green chemistry and green engineering, and how understanding the intrinsic nature of materials can lead to an improved design and a reduction in the environmental impact of the product.
Matter is commonly defined as anything that occupies space and has mass. All matter is made up of tiny particles called atoms. An atom is the smallest particle of an element.
One of the milestones in the study of matter was the theory published by English chemist John Dalton (1766–1844) in 1803, now commonly known as the Dalton’s atomic theory. Dalton postulated that all matter could be described according to the following rules:
In 1869, Dmitri Mendeleev (1834–1907) noted the recurring trends in the properties of the elements and used this to classify the elements, leading to the modern periodic table arrangement. Mendeleev arranged the elements in order of increasing atomic weights, putting similar elements in the same column. At that time there were still gaps in the knowledge of the elements, so Mendeleev left space and made predictions for where other elements would place in his chart. The modern periodic table is now based on Moseley’s (1887–1915) consideration to arrange the elements in order of increasing atomic numbers. In the periodic table a common representation of an element is shown as below:
Atomic number (whole number)
Symbol
Atomic weight (decimal number; unit in g/mol)
Elements with similar properties such as comparable atomic radius are placed in the same columns called groups or families (1–8 or 1–18). Because of their similar properties, four main regions in the periodic table can be distinguished:
TABLE 4.1. Some Good, Bad, and Ugly Common Elements
| Good Elements | Bad Elements | Ugly Elements |
| Nitrogen and explosives Hydrogen and energy (hydrogen fuel cells) Fluorine and fluorocarbons Calcium and bones Iron and anemia (iron-poor blood) Lithium and batteries Oxygen and respiration Sodium and food Magnesium and enzymes Aluminum and household utensils Phosphorus and DNA Strontium and fireworks Antimony and leishmaniasis Gold and jewelry | Lead and paint Mercury and aquatic life Chlorine and environment Polonium and radioactivity | Arsenic and poisoning |
The transition metals and metalloids, as well as lead, are commonly referred to as heavy metals. The term “heavy metals” is misleading and is not based on any scientific definition. Heavy metals are often used in catalysis and other chemical processes. Many of these metals are also hazardous to the environment and/or human health, leading them to be sometimes known as toxic metals.
The current periodic table contains 118 elements all associated with a unique symbol as of June 2012. All elements from atomic numbers 1 (hydrogen) to 118 (ununoctium) have been isolated and, of these, all up to and including californium exist naturally. Two recently accepted elements were named “flerovium” (114) and “livermorium” (116). The most recent discovery in 2010 of an element was the result of an international collaboration between Oak Ridge National Laboratory (U.S. Department of Energy), the Joint Institute of Nuclear Research (Dubna, Russia), the Research Institute for Advanced Reactors (Dimitrovgrad), Lawrence Livermore National Laboratory, Vanderbilt University, and the University of Nevada Las Vegas. This team claimed to have synthesized six atoms of the temporarily named element of ununseptium (element 117) [1].
Elements in the periodic table can be classified as good, bad, and ugly ones. Categories with listed common elements are presented in Table 4.1. A few of these elements are discussed in more detail here.
A common element aluminum (with the symbol Al) is present in cooking utensils and is one of the safest metals. Although it was postulated that it was involved in Alzheimer’s disease, the correlation has not been confirmed. Aluminum can be recovered from its ore, bauxite, or it can be recycled from recovered aluminum product. Recycling aluminum is more energy efficient than extraction from ore, and aluminum can be recycled many times without a decrease in its quality.
Like aluminum, mercury (Hg) is also an element naturally found in the environment. But it is among the most toxic elements, through inhalation over long periods of time, through contact with the skin, or if swallowed. It is hard to believe then that mercury has been used for dental fillings for quite a while (Highlight 4.1).
Severe mercury poisoning can lead to Minamata disease, which is a neurological disease exhibited by numbness in the hands and feet, general muscle weakness, and damage to sight, hearing, and speech, eventually leading to death in extreme cases. This disease, first discovered in Minamata City in Japan in 1956, was caused by the release of a derivative of mercury, called methyl mercury, in the wastewater from a local chemical factory. The bioaccumulation of this highly toxic chemical in fish and shellfish in Minamata Bay resulted in mercury poisoning for the local population who ate the fish. It was not until 2001 that the chemical manufacturer, Chisso, initially producing fertilizers, and the local Japanese government finally recognized the extent of the pollution, which caused almost 2000 deaths.
In the same category of elements, lead (Pb) has been the topic of attention since 3800 BCE when the Romans were drinking acidic beverages from lead containers and even adding lead salts to acidic wine to sweeten the taste. As a consequence, lead was present in a higher level in the bones of Romans than those of modern humans. As mentioned in Chapter 1, lead is extremely toxic, especially to children because it accumulates in their growing bodies very fast. In the early 1970s, lead additives were banned from petroleum in the United States. In the United States, lead is banned from paints, but the presence of lead in red and yellow paints used for manufactured wooden toys from China has resulted in numerous product recalls (Highlight 4.2). Lead is naturally present in petroleum and has been one of the major causes of lead pollution.
The reactivity and chemical properties of the elements are highly dependent on the atomic structure. The atom is comprised of two major components:
Figure 4.1 shows a representation of atomic structure, illustrating the relationship of the nucleus (containing protons and neutrons) and the space around it that is occupied by electrons.
Table 4.2 summarizes the characteristics of each particle.
The atomic weight of the atom can be calculated by adding together the weights of all of the subparticles that make up the atom. Very often, the atomic weight is given in terms of atomic mass units (amu), equivalent to the mass of one proton. Thus, one also can define the mass of the atom in terms of atomic mass number, which is simply equal to the sum of the number of protons and number of neutrons.
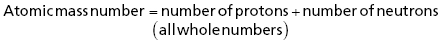
Figure 4.1. Representation of atomic structure [4].
TABLE 4.2. Characteristics of Protons, Electrons, and Neutrons in Terms of Charge, Charge Number, and Mass

Because we must have whole numbers of subatomic particles, one would expect that the atomic weight of the elements would all be whole numbers. But a quick look at the periodic table indicates that is not the case. How can that be? It turns out that many of the elements may have multiple varieties, or isotopes, that vary only in the number of neutrons the element contains. In total, 80 elements in the periodic table have stable isotopes. In order to calculate the atomic weight (in g/mol) of the element, an average of atomic weight of all isotopes of the same element is calculated, based on the normal abundance of the element as found in nature. As an example, chlorine has two isotopes, one having an atomic mass of 35 and found in 75.8% of the chlorine atoms (this is written as 35Cl), and the other isotope having an atomic mass of 37 (37Cl) and found in 24.2% of the chlorine atoms. When a weighted average of these two isotopes is calculated, the average atomic mass is calculated to be 35.5 (g/mol or amu), which is the number reported in the periodic table.
Matter can be classified according to its physical state. The three principal states of matter include the solid, liquid, and gaseous phases, in decreasing levels of structure and atomic order.
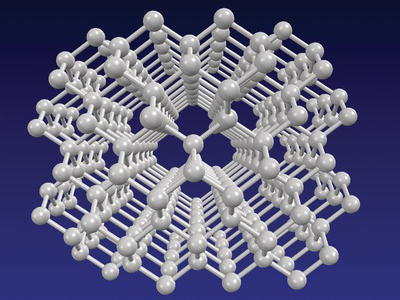
Solids have a fixed volume and rigid shape. They cannot be compressed. In a solid, the atoms of an element are highly ordered and are tightly packed. A diamond is an example of a solid containing all atoms of carbon (Figure 4.2). It is the hardest naturally occurring mineral.
Liquids have a definite volume but will take the shape of the container into which they are placed. There is a more random arrangement of particles in liquids. You can pour milk in a glass, in a cup, or in a bowl. Milk is a liquid and it takes the shape of its container. Water, such as that found in a lake or a river, or the water that we drink, is also a liquid.
Gases are the least structured of the phases. A gas will take the shape and volume of the container in which it is placed. Particles in a gas are highly disordered and are relatively far apart. In gases, atoms are moving around randomly and do not occupy any specific position in space.
Figure 4.2. Crystalline structure of diamond (all represented atoms are those of carbon).
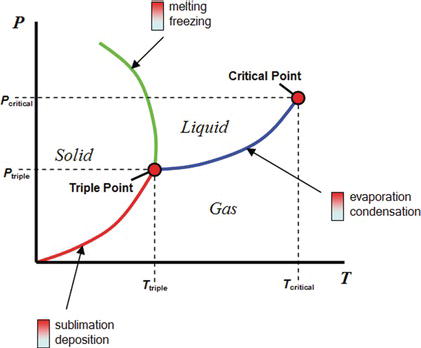
These phases define the physical condition of the material, and not the chemical structure. The same material may interchange between the various phases. The same species may exist as either vapor, liquid, or solid, or may coexist as multiple phases, depending on the specific conditions of temperature and pressure. The phase behavior of the species is illustrated in a phase diagram, which illustrates the conditions at which a phase change can occur. The curves in the phase diagram describe the phase boundaries and the point of transition between phases (Figure 4.3). There are two unique points on the phase diagram that bear notice. The first is the triple point, which is the single condition at which a pure species can exist as a solid, liquid, and gas at the same time. The other is the critical point, which marks the end of the liquid–gas phase boundary. Above the critical point, it is no longer possible to induce a phase change and the fluid moves continuously from liquid-like to gas-like properties.
The physical properties of the material depend on the phase of the material. When undergoing a phase change, the substance remains chemically identical, but its appearance (the way it appears to the naked eye) is different. The physical state, size, or shape may have changed but there is no creation of a new substance in a physical change. The identity of the substance is preserved. Important physical properties in engineering and chemistry include density, thermal conductivity (the ability of a substance to transfer heat energy), and specific heat (the ability of the substance to retain energy).
Species (atoms, molecules, and ions) that are not chemically bonded to each other may interact with one another through intermolecular forces. The strength of intermolecular forces dictates the inherent properties of solids, liquids, and gases. Compounds with very strong intermolecular forces are normally solids at room temperature, whereas compounds with intermediate intermolecular forces are liquids, and those with extremely weak intermolecular forces are gases. The strength of these intermolecular forces explains why a solid such as sucrose melts at 185 °C, whereas ice water melts at 0 °C and molecular nitrogen boils at −196 °C.
Figure 4.3. The phase diagram of water [5].
The strongest of intermolecular forces within pure substances is hydrogen bonding. Hydrogen bonds are formed between the hydrogen of the H2O molecule and a highly electronegative atom such as F, N, or O. Hydrogen bonding occurs between molecules of water but also between carboxylic acid molecules, which can often form dimers through hydrogen bonding in the vapor phase.
All metals in the periodic table are solids, except mercury (Hg), which is a liquid. At room temperature, nonmetals can be solids such as phosphorus (P), sulfur (S), and iodine (I2); liquids such as bromine (Br2); or gases such as hydrogen (H2), nitrogen (N2), and oxygen (O2). Note that for some elements, we normally write their structure with the subscript 2 attached. That is because these elements (such as iodine, bromine, hydrogen, nitrogen, and oxygen) do not exist in nature as individual atoms, but rather they exist as a diatomic molecule. A diatomic molecule is the simplest molecular form and occurs when two atoms of the same type are connected to one another.
When two or more atoms are connected or combine together, they form compounds. The simplest unit of a compound is called a molecule. A molecule can contain identical elements such as in the homonuclear (same nucleus) diatomic molecule of oxygen (O2) or different elements (heteronuclear) such as in water (H2O).
Chemical compounds can either consist of individual molecules (formation of molecular compounds) or of positively and negatively charged atoms or groups of atoms called ions (formation of ionic compounds). Ionic bonding is stronger than hydrogen bonding, but it can only occur in heteronuclear molecules; the strength of ionic bonds is the reason that salts (such as sodium chloride, NaCl, ordinary table salt) are normally solids at room temperature.
Recall that the atom is made up of protons and neutrons in the nucleus, and electrons orbiting in an external shell. Because of the way the electrons distribute, there are multiple layers in which the electrons can orbit. The most important electrons are in the outer layer, furthest away from the nucleus, and are denoted as the valence electrons. It is the interaction of these valence electrons that leads to the formation of molecules.
Each grouping of atoms wants to exist in its lowest energy state, which is achieved when it has a complete outer shell of electrons. In order to achieve this completion of the shell, the atom may share electrons with a neighboring atom. When two elements each share one of their valence electrons, they share a pair of electrons and a covalent bond is formed. In some cases, two elements can complete their outer shell if one of the elements donates an electron to a neighboring element. In this case, an ionic bond is formed. Ionic compounds will be described in Section 4.4.2.
The periodic table provides more information about the atomic structure than just the mass number, atomic number, and some of the chemical behaviors of elements. Elements in a column have the same number of valence electrons. The valence electrons are the electrons involved in the formation of covalent bonds. In fact, for the main group elements (group A), the number of valence electrons is equal to the group number (with the exception of helium).
For the first three rows of the periodic table, when molecules are formed (with just a few exceptions), the complete outer shell of each atom involved in the formation of a covalent bond contains eight electrons. For example, the diatomic molecule of fluorine (F2) is made from two atoms of fluorine (F) (each with seven valence electrons). When these two atoms combine, they each share a pair of electrons to form a covalent bond and six electrons remain on each fluorine atom. The outer shell of each fluorine atom contains eight electrons, the six original electrons remaining with each atom and the two that are now shared, thus forming a stable molecule.
One way to represent the sharing of electrons is through the Lewis dot representation. Here, the nucleus of the atom is represented by the letter representation from the periodic table, then the electrons in the outer shell are indicated as dots surrounding the nucleus. The two atoms that share an electron are shown with the two electrons between the nuclei, providing a visual clarification that a stable material is produced. When more than two types of atoms are involved in a molecule, it is often necessary to define a central atom, with the other atoms in the molecule being strategically placed to illustrate how the electron sharing occurs (Figure 4.4).
Figure 4.4. Examples of Lewis structures of CO2, SO2, and CH4.
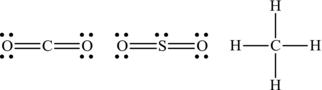
Figure 4.5. Example of Lewis structure for which the central atom has more than eight electrons.
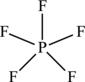
Figure 4.6. Lewis structure for the borane molecule.
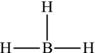
There are numerous exceptions to the octet rule, particularly as you move down the periodic table to the higher molecular weight elements. Elements in period three and beyond have more than eight valence electrons. In this case, they form molecules or ions with an expanded octet. One such example is the element phosphorus. Phosphorus is in group 5A and therefore has a total of five valence electrons. It can form five covalent bonds such as in the molecule of phosphorus pentafluoride (PF5), a highly reactive colorless gas at room temperature and pressure. Fluorine, which is in group 7, has seven valence electrons. So phosphorus may share one of its valence electrons with each of the F atoms, creating a shell with eight electrons for each fluorine atom, and thus a stable molecular species. The phosphorus atom is an exception to the octet rule in this case, because it has 10 shared electrons, as seen in the Lewis structure of Figure 4.5.
On the other hand some central atoms have fewer than eight valence electrons and still form stable molecules. Boranes, whose parent member is BH3, are extremely reactive and are involved in the formation of cage-like materials used in catalysis. Boron is in group 3A and only has three valence electrons to share. When B forms three bonds with three hydrogen atoms, it has a total of six electrons around it, as shown in Figure 4.6. Therefore, boron cannot achieve the octet rule since it will never have a total of eight valence electrons around it. Similar elements to B are found in group 3A, such as aluminum (Al) and gallium (Ga).
For transition elements also known as transition metals (periods 4 through 7), the outer valence shell may contain as many as 18 electrons, distributed into suborbitals. Transition metals are involved in the formation of coordination complexes, in which the transition metal is the central atom and the ions or molecules surrounding the central atom are called ligands. The type of bond connecting the central atom to the ligands is known as a coordinate covalent bond, where donation of electrons occurs from the ligands to an empty metal orbital. Coordination complexes are used extensively as catalysts in a variety of organic reactions. Examples of coordination complexes include the anticancer drug cisplatin [Pt(NH3)2Cl2], where Pt2+ is the central metal cation surrounded by NH3 and Cl− ligands. This complex is neutral since the two negative charges from Cl− balance the two positive charges from Pt2+. Another well-known example of a ligand is EDTA4−, drawn as shown in Figure 4.7.
Figure 4.7. The EDTA ligand.
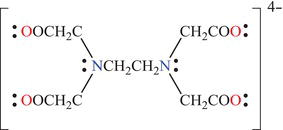
Figure 4.8. Binding of EDTA ligand to copper.
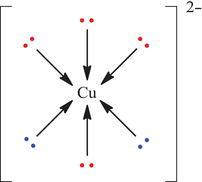
Because EDTA4− binds strongly with many metal species, it is often added to commercial salad dressing to remove traces of metal ions from solution (the presence of metal ions will accelerate the oxidation of oils in salad dressings). The other useful need for EDTA4− is in the treatment of lead and mercury poisoning since EDTA4− anions can form coordination complexes with the metal cations of Cu2+, Pb2+ and Hg2+ (Figure 4.8). When bound to a metal atom, the six available lone pairs of electrons can wrap around the central metal ion, creating a stable coordinate covalent molecule, as shown here for Cu(EDTA).
Inorganic compounds typically do not have any carbon covalently bonded to oxygen, hydrogen, or nitrogen. Now that we know a little about how inorganic compounds are formed, it is also important to know how to name them. For binary inorganic compounds some rules apply.
The most useful Greek prefixes are the following:
Typical inorganic compounds include titanium tetrachloride (TiCl4), dinitrogen pentoxide (N2O5), phosphorus pentachloride (PCl5), and aluminum trichloride (AlCl3).
Molecules that contain only the elements C, H, O, N, S, and P may be organic compounds. Not all molecules containing these elements are organic, but those derived from living species and based on the structure of carbon as a building block are generally included. Nearly all materials derived from fossil fuels are organic compounds. Through derivatization (replacement of a hydrogen with another element), halogens (Cl, Br, etc.) may also be incorporated into an organic compound.
Organic compounds can be classified based on the types of bonds and atoms that they contain. Hydrocarbons are a large class of organic compounds that contain only the elements hydrogen and carbon, and may be subclassified into groups of alkanes such as propane (C3H8), a gas used in household heating, and alkenes such as ethene or ethylene, the basic building block of polyethylene, a major group of plastics. Alcohols contain an oxygen atom bound to a hydrogen as an end group, such as ethanol (C2H5OH) commonly found in alcoholic beverages such as wine and beer (as a side note, ethanol was also added to gasoline for many years). Carboxylic acids contain an oxygen double-bonded to carbon plus an OH group; an example is ethanoic or acetic acid (CH3COOH) commonly found in salad dressings. There are many other classes of organic compounds.
There are three different ways to represent a molecular compound, as described for propane, an alkane containing three carbon atoms linked together with hydrogen atoms forming the remaining bonds.
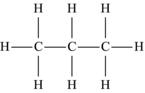
The simplest hydrocarbon structure is that of the alkane, formed from simple bonds of carbon and hydrogen atoms. Alkanes are made up of a chain of carbon atoms bound to each other, with only hydrogen atoms surrounding the carbon atoms. When two carbon atoms bond together in a single manner, and each of the carbon atoms is then bound to three additional hydrogen atoms, this is the two-carbon alkane known as ethane. Similarly, three carbon atoms in a chain make up propane, and similarly named substances exist for every possible alkane. The name, molecular formula, and structural formula of the first ten alkanes are summarized in Table 4.3; note that the structure of an alkane always has a formula that can be represented as CnH2n+2.
It is also possible to make an alkane that is nonlinear, otherwise known as a branched alkane. Such species have a central carbon atom that is bound to three carbon atoms, and thus three branches are created. The simplest of the branched alkanes is methylpropane, also called isobutane (Figure 4.9).
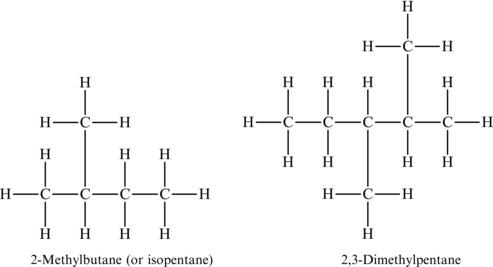
When naming a branched alkane, you start with the longest single chain to identify the root structure, and then add the side chain with the proper term as if you were naming the sidechain as a separate species. Some examples of branched alkanes are shown, with the proper name for the species also listed (Figure 4.10).
TABLE 4.3. The First Ten Alkanes
| Name of Alkane | Molecular Formula | Structural Formula |
| Methane | CH4 | 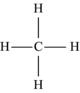 |
| Ethane | C2H6 | 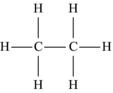 |
| Propane | C3H8 | 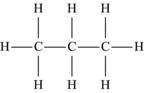 |
| Butane | C4H10 | 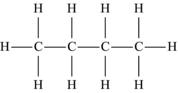 |
| Pentane | C5H12 | 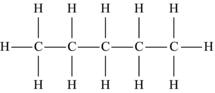 |
| Hexane | C6H14 | 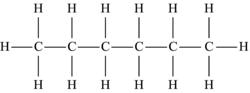 |
| Heptane | C7H16 | 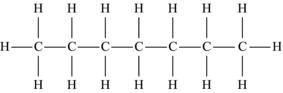 |
| Octane | C8H18 | 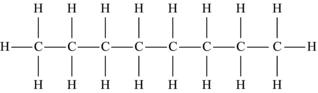 |
| Nonane | C9H20 | 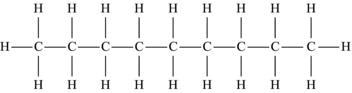 |
| Decane | C10H22 | 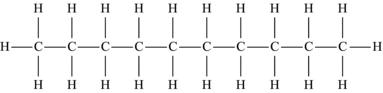 |
Figure 4.9. Structural formula of methylpropane.
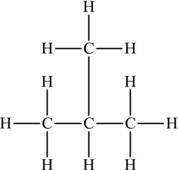
Figure 4.10. Examples of branched alkanes and structural formulas.
When you look at the carbon element present in group 14 or 4A, the number of valence electrons is four. In other words, carbon can form a maximum of four bonds. As you probably noticed all C–C bonds in alkanes are single bonds. But it is also possible for carbon atoms to share multiple electrons, and form double or triple bonds. When two carbon atoms share two electrons, they form a double bond to produce a molecule known as an alkene. The simplest of the alkenes is ethene, or ethylene, which contains two C atoms and four H atoms. All alkenes can be represented by the general structure CnH2n, and the molecules are named in a manner analogous to alkanes. Thus, a three-carbon molecule containing a double bond would be propene (or propylene, an older form of the name).
A common colorless and flammable gas at room temperature and pressure, propene is generally produced from coal or petroleum and is a nonrenewable resource. However, it can be used in combination with hydrogen peroxide (H2O2) and employed as an inexpensive rocket fuel propellant. Alkenes can also form branched compounds, just as the alkanes (Figure 4.11). However, in the case of the alkene, the main part of the name is always the portion containing the double bond. And, it is important to identify the carbon atom from which the branch comes. Some examples of the names of alkenes are provided here. Note the convention on naming, but also know that the naming conventions become very complicated as the molecules become larger and more complex.
Figure 4.11. Examples of branched alkenes.
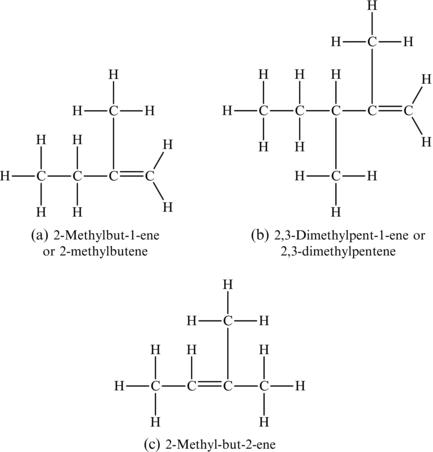
Note that in the examples in Figure 4.11a,b, since all double bonds start on the first carbon (counting from the right), it is not essential to include the 1 in the name of the alkene. However, since the double bond starts on carbon number two (counting from all possible starting carbons) in the example in Figure 4.11c, it is necessary to mention the position of the double bond in the name.
Since carbon can only form a total of four bonds, it is possible for a carbon atom to contain one triple bond and one single bond; hydrocarbons containing a triple bond are termed alkynes. Alkynes are named in parallel to the alkanes and alkenes, as described previously, and can also be branched and linear. Propyne, also known as methylacetylene, has the condensed formula CH3C ≡ CH and is a component of MAPP gas, a tradename of the Petromont, previously of the Dow Chemical Company. MAPP stands for methylacetylene-propadiene propane. Propyne is usually used in combination with oxygen for welding and is about two to four times more expensive than propane.
That takes care of the molecules made up exclusively of carbon and hydrogen, but there are also many important organic compounds that contain oxygen (and other atoms, but we’ll focus on the oxygenated molecules for now). In order to distinguish one compound from others and to be able to name it, we look at their specific functional groups. Useful functional groups covered in this chapter are summarized in Table 4.4.
TABLE 4.4. Useful Functional Groups in Organic Chemistry
| Class of Organic Compound | Functional Group |
| Alcohols | R–OH |
| Aldehydes | 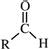 |
| Carboxylic acids | 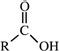 |
| Ketones | 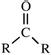 |
| Ethers | R–O–R′ |
| Esters | 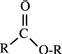 |
| Amines | NR1R2R3 |
R and R′ are the hydrocarbon frameworks with one hydrogen removed for each functional group added. R1, R2, and R3 in amines are alkyl groups, typically –CH3 (methyl) or –CH2CH3 (ethyl).
The general structures and names are important to know, since chemists use the names to properly describe molecules of importance. The names of alcohols, aldehydes, and ketones are derived directly from the names of their respective alkanes. Alcohols end in “ol,” aldehydes end in “al,” and ketones end in “one.” In naming alcohols, the longest chain of carbon atoms is numbered so that the C atom attached to the –OH functional group has the lowest number.
For example, the simplest alcohol, methanol, which is a flammable and poisonous liquid, but which is also used in the production of biodiesel, is derived from methane and has the structural formula shown in Figure 4.12.
Butanol or butan-1-ol, which is based on butane (having four C atoms in its hydrocarbon chain), would be represented as in Figure 4.13.
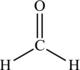
Methanal, the methane-derived aldehyde also called formaldehyde, would be drawn as in Figure 4.14.
For ketones, a number is used to designate the position of the −C=O (also called carbonyl) group, keeping in mind that the numbering of the longest chain of carbons should give the carbonyl group the smallest number.
Figure 4.12. Structural formula of methanol.
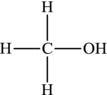
Figure 4.13. Structural formula of butanol.
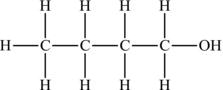
Figure 4.14. Structural formula of methanal.
Butan-2-one would have the structural formula shown in Figure 4.15.
Carboxylic acids are named after dropping the final “e” of the corresponding alkane and adding “oic acid.” Butanoic acid would be represented as in Figure 4.16.
Ethers are named by identifying the two alkyl sections on either side of the oxygen and then adding the name ether. Thus, methylethylether (also called methoxyethane) is written as CH3OCH2CH3 and has the structural formula shown in Figure 4.17.
An ester is a compound that is similar to a carboxylic acid, except the hydrogen atom bound to the oxygen is replaced with another alkyl group. The naming of an ester derives from the name of the two alkyl groups in the molecule, with the second group identified through the suffix “oate” at the end (Figure 4.18). It is important to keep in mind that for aldehydes and carboxylic acids, the carbon atom part of the functional group is always numbered one.
Functional groups provide the relationship between the chemical structure and the compound activity. Often, the physical properties of similar types of molecules may be related, and thus it is possible to substitute an undesired molecule for a less hazardous one. These structure–activity relationships give the green chemist the opportunity to “tune” the molecule to achieve a more environmentally responsible process. For example, the use of a solvent from the same functional group but of a higher molecular weight may prove advantageous if the volatility of the solvent is a concern. Specifically, diethanolamine is seen to be a better solvent for CO2 capture than is monoethanolamine because of its reduced volatility.
Classes of raw materials and reactions can help to eliminate exposure to toxic functional groups such as isocyanate (whose functional group is R–N=C=O), thereby preventing harm to humans or the environment. Alternative elements and structures are required that provide the functionality of the desired group but not the toxicity. Researchers at NanoTech Industries were able to produce hybrid non-isocyanate polyurethane (HNIPU) through reaction of cyclocarbonate with an amine, avoiding the formation of the undesirable isocyanate species. HNIPU is currently marketed in the United States and Europe as an alternative to polyurethane foam used in paints, coatings, sealers, and adhesives as well as in insulation and packaging.
Figure 4.15. Structural formula of butan-2-one.
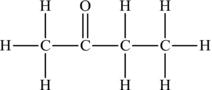
Figure 4.16. Structural formula of butanoic acid.
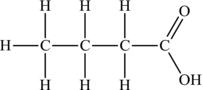
Figure 4.17. Structural formula of methylethylether.
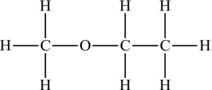
Figure 4.18. Examples of esters (methylpropanoate and ethylethanoate).
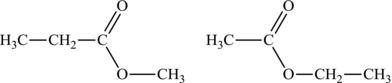
Organic molecules can be much more complicated than the linear species already considered, forming ring-like structures and multiple rings. When carbon atoms in a molecule are connected in a ring, the term cyclo- is added to the front of the structure. So cyclohexane, containing six carbon atoms all connected in a ring (with two hydrogen atoms attached to each carbon), is drawn as in Figure 4.19.
Cyclic molecules can also contain double bonds or may be functionalized. If two of the carbon atoms in a six-carbon ring are connected with a double bond, then we would have cyclohexene. And we could also have functionality in a cyclic ring, creating cyclohexanol or cyclohexanone, as shown in Figure 4.20.
Figure 4.19. Structural formula of cyclohexane.
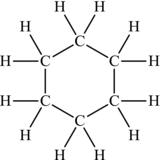
Figure 4.20. Structural formula of (a) cyclohexanol and (b) cyclohexanone.
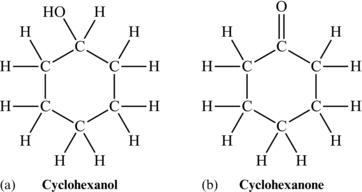
Figure 4.21. Structural formula of benzene.
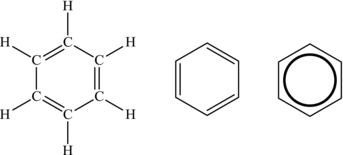
As we begin to form multiple double bonds in a ring structure, the nature of the double bond changes, forming a stable ring with shared single and double bonds. Benzene is the most common of these materials, with each carbon atom containing one single bond and one double bond (plus one bond to a hydrogen atom). Because these molecules share the double bonds, its properties are unlike those of other cyclic compounds they look like. It is these types of complicated molecules that make up the structure of oil and coal, as well as biomass. The molecule of benzene is drawn in Figure 4.21. Note that all these different representations are equivalent.
Benzene is a colorless, highly flammable liquid and is a known carcinogen. It is a natural constituent of crude oil and also an important industrial solvent and precursor in the production of drugs, plastics, synthetic rubber, and dyes. In March 2006, the official Food Standards Agency in Britain found that four brands of soft drinks among 150 studied contained benzene levels above World Health Organization limits. One way that pure benzene can cause harm is when it oxidizes in the body to produce benzene oxide, which is not excreted readily and can cause DNA strand breaking as well as chromosomal damage.
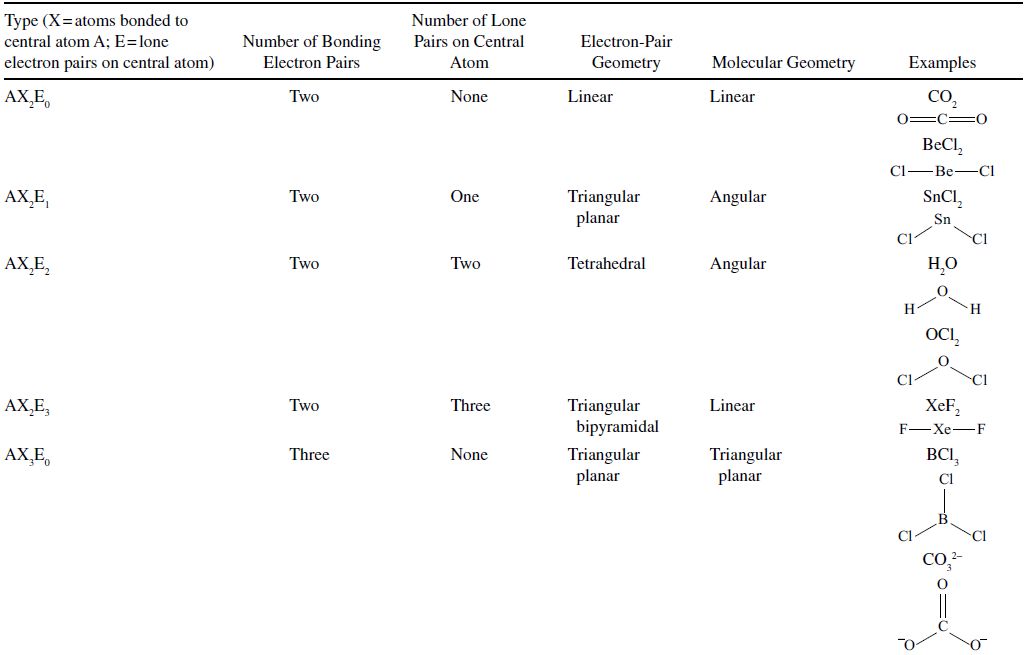
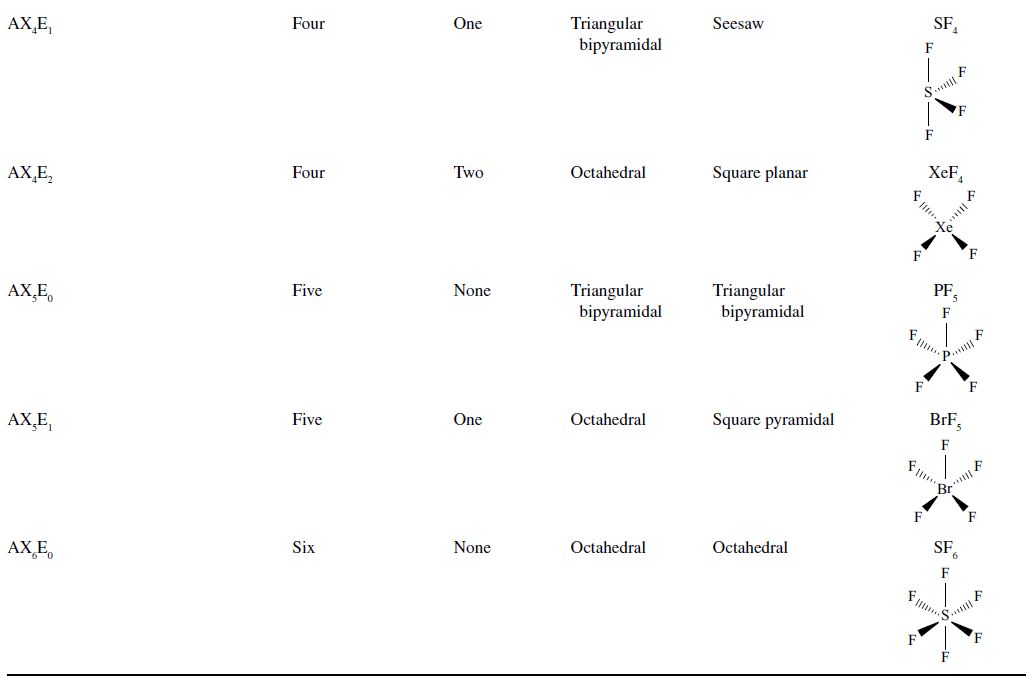
The Lewis representation of a molecule, and all other methods of describing the molecules mentioned previously, does not give any clue about the three-dimensional nature of the molecule. The atoms in the molecule form complex shapes based on the repulsive and attractive forces that exist between pairs of bonding and lone pairs of electrons. The specific positions of each of the atoms in the molecule are critical determinants of the properties and behavior of molecules in living organisms, in the environment, and in drugs. Because of the importance of the shape of molecules, a model is necessary to predict the shapes of molecules. This model is called VSEPR for valence-shell electron-pair repulsion. The geometries predicted according to the VSPER model are summarized in Table 4.5.
Before leaving the subject of covalent bonding structure, we should look at one more type of molecule. In some cases, a semistable molecule may be formed, which has an overall odd number of valence electrons. These molecules, containing an unpaired electron, are called free radicals. These species appear during chemical reactions, including reactions used by nature. For example, nitric oxide or nitrogen monoxide (NO) is an important intermediate molecule involved in physiological and pathological processes in mammalian bodies including humans. Nitrogen oxide NO has a total of 11 valence electrons. Nitrogen is in group 5A and therefore has five valence electrons. N uses two of its five valence electrons to make a double bond with the O and three valence electrons remain on the N. Free radicals are extremely reactive and can react with the oxygen in the air and lead to the formation of tropospheric ozone, O3, an air pollutant well-known to affect the respiratory system (Chapter 3).
In a molecular compound, covalent bonding happens when two nonmetals share electrons. Covalent bonds are defined not only by the number of electrons shared but also by their length and strength. Covalent bonds have a specific bond length and bond energy. In a homonuclear diatomic molecule where both atoms are identical, the pair or pairs of electrons is/are shared equally between the two atoms. However, in a heteronuclear diatomic molecule, one shared pair(s) will be more attracted to one atom than the other.
The tendency of an atom to attract shared electrons is called electronegativity. The more electronegative, the higher the electron density, and the higher the probability of finding the bonding electrons near this atom. Again, the periodic table arrangement describes this trend with the more electronegative atoms on the right side (except for the noble gases at the far right). Electronegativity decreases along a group and increases across periods. Fluorine is the most electronegative element whereas francium (Fr) is the least electronegative.
TABLE 4.5. Geometries Predicted by the VSEPR Model

When the difference in electronegativity between two elements is less than about 0.5, the bond is considered nonpolar. When the difference is between 0.5 and 2, the bond is defined as polar. In each case, the type of bond is still a covalent bond; in a homonuclear molecule, the covalent bond is nonpolar; in a heteronuclear molecule, the covalent bond is polar if the electronegativity difference is sufficiently large. In a polar molecule, one region of the molecule has a partial positive charge, whereas the opposite region has an equivalent partial negative charge, and thus the molecule has a net zero charge.
When the difference in electronegativity is greater than 2.0, the electrons are so greatly associated with one of the atoms that a transfer of electrons to the more electronegative species is now involved. Under these conditions, the molecule is no longer considered polar, but rather an ionic compound is formed. One of the most common ionic species is NaCl, common table salt. In this species, the sodium atom (Na) “donates” an electron to the chlorine, leaving it with an empty outer shell and the chlorine with an outer shell containing eight electrons. Because of the extra electron, the chlorine atom contains a strong negative charge, whereas the sodium, which is missing an electron, has an equally strong positive charge.
Metals that donate electrons to an ionic pair form cations with a positive charge (fewer electrons than protons) whereas nonmetals tend to gain electrons to form anions with a negative charge (more electrons than protons). In NaCl, the metal sodium is the cation Na+ and the chlorine atom becomes the chloride anion, Cl−. For metals, the (positive) charge is equal to the group number. For nonmetals, the (negative) charge is equal to: 8 − group number. Thus, in the example above, the sodium atom has a +1 charge and is written as Na+1 whereas the chlorine has a −1 charge and is written as Cl−1; together these ions form the stable species NaCl. Ionic compounds consist of arrays of cations and anions.
There are two types of ions: monoatomic (single atom) and polyatomic (two or more atoms possessing an overall charge). The naming protocols for these species are precise, but difficult to learn. The following rules should help you.
For monoatomic cations:
For monoatomic anions: replace ending from element name with “-ide” suffix.
Example: F (fluorine) becomes F− or fluoride
For our example of table salt (NaCl), the proper chemical name is sodium chloride.
For polyatomic anions, some guidelines are useful.
Oxoanions: polyatomic ions that contain oxygen.
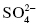 , sulfate;
, sulfate; 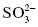 , sulfite
, sulfite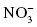 , nitrate;
, nitrate; 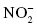 , nitrite
, nitrite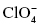 , perchlorate
, perchlorate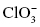 , chlorate
, chlorate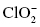 , chlorite
, chlorite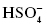 , hydrogen sulfate ion
, hydrogen sulfate ion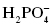 , dihydrogen phosphate
, dihydrogen phosphate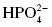 , monohydrogen phosphate
, monohydrogen phosphateTo name ionic compounds, always name the cation first, followed by the proper form of the anion.
Some alternative solvents in green chemistry are based on complex ionic species. Ionic liquids are ionic salts whose melting point is relatively low (typically below 100 °C) and thus form a liquid near room temperature. The cation is usually a large nitrogen-containing organic-type species, whereas the anion is a smaller inorganic ion. A typical cation would be 1-butyl-3-methyl imidazolium (BMIM) whereas traditional anions would include a halogen such as in [AlCl4]− and [PF6]− (Figure 4.22).
Figure 4.22. Example of ionic liquid containing BMIM cation and [PF6]− anion.
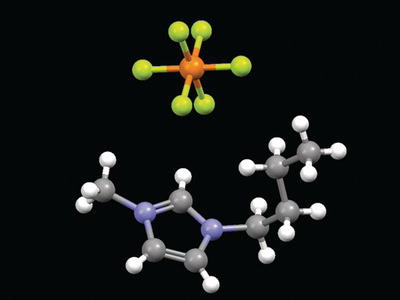
Because of the mismatch in size, structure, and shape, these species are less rigid than a simple ionic species, leading to their low melting points. Ionic liquids are important green solvents, because they do not vaporize upon heating (and thus produce no toxic air emissions, in contrast to organic solvents). However, not all ionic liquids are environmentally friendly, as expected from the anion structures indicated previously. New anions are now being developed based on organic species which do not contain any halide and therefore prevent the halide waste formation.
Matter is not inert. It undergoes chemical and/or physical changes. Chemical changes called reactions involve transformations of one or more substances (the reactants) into one or more different substances (the products). It is through these chemical reactions that all chemicals are derived from their raw materials, or one chemical is converted into another.
A chemical reaction is represented as
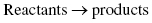
with the arrow meaning “forming” or “transforming into” or “leads to.”
One fairly common reaction is the oxidation reaction, in which an oxygen atom is added to the chemical structure of the molecule. The most common of the oxidation reactions is combustion, in which a molecule is heated to a high temperature in the presence of air and decomposed to produce CO2 and water. This reaction forms the basis by which energy is generated from fossil resources, with the production of CO2 as a by-product. Combustion of fossil resources has led to an increase in the atmospheric content of CO2. However, under conditions of restricted oxygen, the oxidation of hydrocarbons CxHy to carbon dioxide is not complete, and the toxic gas carbon monoxide may also be formed (1 atom of C and 1 atom of O forms CO) (Chapter 3).
Renewable raw materials such as wheat straw, corn, and sugar are highly complex materials containing structures similar to those present in the simpler molecules that have been described above. Biomass may be burned through combustion to produce energy. Biomass combustion also produces CO2 as a by-product, but a life cycle analysis reveals that the carbon was originally derived from atmospheric CO2 and thus the net CO2 generation is negligible. Because additional energy is required for the production of the biomass, and fertilizer is consumed to grow the crop, these activities do add to the overall atmospheric CO2 content, but this is certainly a reduced quantity compared with fossil resources.
In order for renewable materials to serve as a feedstock for chemical processes, it is important to break them apart. Thermal routes to chemical products involve the partial combustion of the renewable material into CO and H2, a reaction that is partially controlled by the amount of oxygen present in the reactor. This mixture of gases is commonly referred to as synthesis gas (or syngas) and can be used as the raw feed to a number of other chemical processes that produce compounds that would traditionally have been produced from nonrenewable fossil resources. It may also be possible to break down the biomass directly into functional molecules using enzymatic methods, simplifying and greening the production of desired molecules.
Oxidation of individual compounds can also be completed under controlled conditions to produce molecules of commercial interest. You may have noticed that in alcohols there is a single C–O bond, in aldehydes there is a double C=O bond, and in carboxylic acids there are three C–O bonds (two from the C=O double bond and one from the C attached to the OH group). We can conclude that the oxidation of primary alcohols (where the –OH group is at the end of the hydrocarbon chain producing a straight chain and not a branch) gives aldehydes, which are then oxidized into carboxylic acids.
Primary alcohol → aldehyde → carboxylic acid in the presence of oxidizing agents such as potassium permanganate (KMnO4) (Figure 4.23).
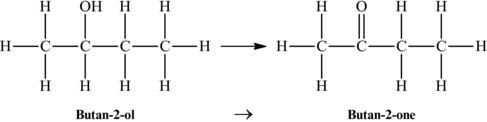
In the case of a secondary alcohol such as 2-butanol or butan-2-ol, the product of oxidation would be a ketone (Figure 4.24). In this case, the oxidation of 2-butanol produces 2-butanone or butan-2-one.
Chemists have the power to use and control chemical reactions. As described previously in the 12 principles of green chemistry, chemists can modify chemical reactions to take advantage of renewable resources, use more environmentally benign solvents in which to carry out reactions, and employ catalysts to promote the formation of desired products.
In Chapter 2, we discussed one example in which chemists and engineers are looking to use renewable feedstocks to produce chemicals of interest. Other examples include the production of polymers commonly used in consumer products like automobiles and food packaging from renewable feedstocks such as soy and corn. Biodegradable polymers can be made from the microbial fermentation of glucose, a common compound with the formula C6H12O6.
Figure 4.23. Conversion of butanol to butanal to butanoic acid.
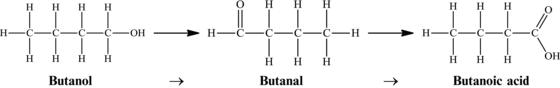
Figure 4.24. Conversion of the secondary alcohol of butan-2-ol to the ketone butan-2-one.
A lot of research is being devoted to the replacement of volatile organic compounds (VOCs) or hazardous air pollutants (HAPs), which are flammable, toxic, or carcinogenic and frequently used as solvents for chemical reactions. For example, carbon dioxide in its supercritical state (i.e., at a temperature above its critical temperature of 31.1 °C and pressure above its critical pressure of 74 bar) has several advantages as a benign solvent. It is nontoxic, nonflammable, and inexpensive. Supercritical CO2 has properties midway between a gas and a liquid and therefore has found many applications in chemical extractions such as the decaffeination of coffee and in the dry cleaning industry where it replaced perchloroethylene as a solvent.
A catalyst can sometimes be used to provide an alternative reaction step that reduces the energy barrier to reaction, or directs the reaction to produce more of the desired product without the formation of undesired by-products. Enzymes are natural catalysts that are often highly selective and are thus excellent green chemistry tools. Catalysis will be covered in Chapter 6. Types of chemical reactions and processes will be discussed in detail in Chapter 5.
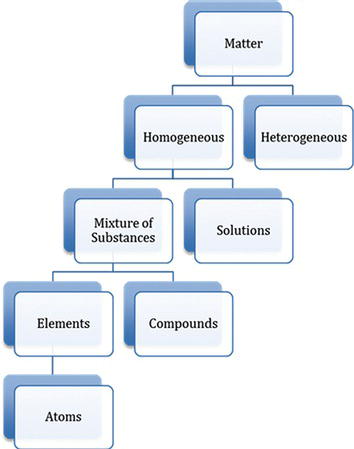
Matter can exist in the form of either a pure substance or a mixture. Pure substances can be either elements that contain only one type of atoms or compounds that are comprised of at least two elements. Compounds in a mixture are not chemically bonded to one another. Although chemicals are often identified as pure, pure substances are actually quite rare. More often, even a pure substance contains small amounts of impurities. Most of the substances surrounding us are mixtures rather than pure substances.
Mixtures can be classified as homogeneous or heterogeneous. In a homogeneous mixture, the composition is uniform throughout the entire sample; in a heterogeneous mixture, the composition is nonuniform and each individual component can be distinguished from others. Depending on particle sizes, homogeneous mixtures can be further classified as solutions, colloids, and suspensions (Figure 4.25).
Drinking water is a common substance that is a homogeneous mixture. Analysis of drinking water may reveal the presence of dissolved calcium cations, Ca2+, or chloride anions, Cl−, added for disinfection in water treatment plants. An analysis of any volume of the water would reveal an identical composition. Because these components are evenly distributed, they cannot be separated through mechanical means.
On the other hand, a heterogeneous mixture is one in which different compounds and/or substances are mechanically blended together and create a nonuniform distribution of substances. This is the case for concrete. Concrete is usually made by mixing cement, limestone or granite, and water, just to name a few concrete components. Limestone is made up of calcium carbonate (CaCO3), which is decomposed to calcium oxide, releasing CO2 in the process. Recently, the use of recycled materials to make concrete has gained popularity. Fly-ash, a by-product of coal-fired electric generating power plants, is now used to supplement the commonly used Portland cement. The impact of cement-replacement technology such as this will play an important role in cutting further the carbon dioxide emissions as cement production generates massive amounts of carbon dioxide.
Figure 4.25. Classification of matter.
Solutions are generally defined as clear mixtures, meaning the components are dissolved. Our naked eye cannot observe a particle settling out over time. Components in a solution are either the solute (minor component) or the solvent (major component). For example, when sugar is put in a hot cup of water, the sugar dissolves in the hot water. The hot water is termed the solvent, the sugar is the solute, and the overall mixture is an aqueous solution (aqua for water).
Colloids have larger particles and often appear cloudy. Our naked eye cannot distinguish suspended particles, which are too small to be filtered. A typical example of a colloid is paint, which is an emulsion that encompasses tiny droplets containing the pigment randomly dispersed in a liquid solvent. The use of water-based paints has led to substantial decreases in the emission of hazardous air pollutants.
Suspensions contain particles with different densities. When particles are larger than about 1000 nm in diameter, they can separate from solution upon standing.
Because a mixture is made from multiple species, there are other intermolecular forces present, depending on the types of molecules present in the mixture. For example, polar molecules may interact through hydrogen bonding, whereas ionic species may dissociate in a highly polar solvent such as water.
A general rule of thumb is “like dissolves like.” An organic solvent is generally needed for an organic molecule, whereas ionic species will dissolve in a highly polar solvent such as water. On the other hand, nonpolar solutes usually do not dissolve in polar solvents such as water. Water and oil do not form a solution, but with proper additional ingredients and sufficient mixing, you can get them to form a homogeneous mixture. The example of sugar dissolving in a cup of coffee leads us to assume that sugar is a polar solute. As a matter of fact, sugar has several hydroxyl groups, –OH, and is therefore a polar molecule. The molecules of sugar form a solution with the molecules of water through hydrogen bonding. The challenge of dissolving organic molecules into nonhazardous solvents is one of the central concerns of green chemistry.
Acids and bases are an important class of compounds dissolved in aqueous solution and often found in common household products as well as in many drugs. When you eat a grapefruit or a lemon, it tastes sour due to the presence of acids. Coffee and tonic water, on the other hand, taste bitter, due to the presence of bases. The definition of an acid is based on the Brønsted–Lowry theory, which specifies that acids produce protons (H+) in aqueous solution, that is, they are proton donors.
The dissociation of acids in water can be represented according to the following chemical equation:
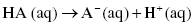
or also written as
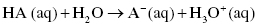
On the other end, Brønsted–Lowry bases produce hydroxide ions (OH−), that is, they are proton acceptors.
The dissociation of bases in water can be represented as follows:
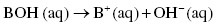
Acids and bases can be classified as strong and/or weak acids/bases depending on their tendency to ionize. Strong acids completely ionize into H+, whereas weak acids only partially ionize or dissociate in water.
The acid present in vinegar is acetic acid, which is a weak acid. Hydrochloric acid, found in our stomach, is a strong acid, meaning that it completely dissociates in water.
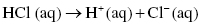
Complete dissociation is written as a chemical reaction involving a single arrow. When the amounts of products and reactants remain constant (which means the system has reached equilibrium) no HCl will be left.
Acetic acid or ethanoic acid only partially dissociates in water, the partial dissociation being symbolized by the double arrow. At equilibrium, there would be molecules of acetic acid as well as acetate CH3COO− and hydronium cations H3O+. Acetic acid is a weak acid.
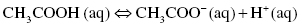
or also written as
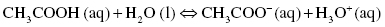
Strong bases completely ionize into OH−, whereas weak bases only partially ionize in water. Ammonia, found mostly in window cleaners, is a weak base whereas sodium hydroxide, found in oven cleaners, is a strong base.
The most common strong base is sodium hydroxide NaOH. Sodium hydroxide is used mainly in the manufacture of pulp and paper, soaps, and detergents, but also in the production of biodiesel, where sodium hydroxide is used as a catalyst.
Sodium hydroxide is a very strong base and completely decomposes into sodium cations and hydroxide anions. At equilibrium no NaOH remains.
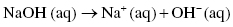
Ammonia is a weak base and only partially dissociates into NH4+ and OH−. At equilibrium molecules of NH3, NH4+, and OH− will coexist.
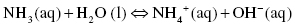
Aspirin and ibuprofen are acids; caffeine and nicotine are bases. More examples of acid–base reactions will be seen in Chapter 5.
In order to quantitatively measure the strength of acids and bases, a scale called the pH scale was developed. pH values are numbers between 0.0 and 14.0. An acidic solution is defined when pH < 7.0, a basic solution when pH > 7.0, and a neutral solution when pH = 7.0.
The pH value is directly related to the concentration of protons, H+, as follows:
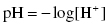
the concentration or molarity of any substance being expressed in moles of solute/liters of solution symbolized as M.
Therefore, to express the concentration of protons in base 10: [H+] = 10−pH.
For aqueous solutions containing acids and bases, a general expression links the concentrations of protons and hydroxides as follows:
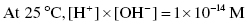
Common chemicals have a specific pH as shown in Table 4.6.
TABLE 4.6. Common Chemicals and Their Respective pH [6]
| Name | pH |
| Hydrochloric acid (HCl) | 0 |
| Battery acid (H2SO4 sulfuric acid) | 1.0 |
| Lemon juice | 2.0 |
| Vinegar | 2.2 |
| Apples | 3.0 |
| Wine and beer | 4.0 |
| Tomatoes | 4.5 |
| Milk | 6.6 |
| Pure water | 7.0 |
| Human blood | 7.4 |
| Baking soda (NaHCO3 sodium bicarbonate) | 8.3 |
| Milk of magnesia | 10.5 |
| Ammonia | 11.0 |
| Lime (Ca(OH)2 calcium hydroxide) | 12.4 |
| Lye | 13.0 |
| Caustic soda (NaOH sodium hydroxide) | 14.0 |
Brønsted–Lowry acids and bases are well-known and are generally naturally occurring substances. In 1923, Gilbert Lewis extended the definition of acids and bases when he published the following definition of an acid: “An acid substance is one which can employ an electron lone pair from another molecule in completing the stable group of one of its own atoms” [7]. Similarly, a Lewis base is one which can donate a pair of electrons when reacting with a Lewis acid and therefore forming a Lewis complex or adduct.
Lewis acids are used as homogeneous catalysts in fine and specialty manufacturing processes. Aluminum chloride (AlCl3), where the central atom of aluminum is covalently bonded to three chlorine atoms, is the most widely used Lewis acid. It is very stable, reactive, soluble in many organic solvents, and inexpensive. However, because of its strength, it often promotes unwanted side reactions and the release of hazardous substances in the environment. In addition, it is difficult to separate aluminum chloride from the reaction products and large volumes of hazardous waste can be generated during product recovery. The performance of Lewis acids can be improved by immobilizing them on supports such as silica (SiO2) or zirconia (ZrO2), which results in the formation of reusable heterogeneous catalysts discussed in detail in Chapter 7. The outcomes of using this type of catalysts are consistent with the principles of green chemistry: the selectivity of reactions is improved, the volume of hazardous waste is significantly reduced, and moderate reaction conditions can be used.
1. Oganessian, Y. T. et al. Synthesis of a new element with atomic number Z = 117, Phys. Rev. Lett., 2010, 104, 142502.
2. http://en.wikipedia.org/wiki/Dental_amalgam_controversy.
4. http://en.wikipedia.org/wiki/File:Atom_diagram.png.
5. http://www.marspedia.org/index.php?title=File:Phase_diagram_water.png.
6. http://chemistry.about.com/od/acidsbases/a/phtable.htm.
7. Lewis, G. N. Valence and the Structure of Atoms and Molecules, The Chemical Catalog Company, 1923, p. 142.