
In the previous chapters, the reader has been introduced to a brief history of chemistry and the transition currently occurring in the approaches and thinking that are needed to address the 21st century environmental and sustainability challenges that society globally is now facing. The reader has been introduced and versed in the 12 principles of green chemistry [1] and the 12 principles of green engineering [2] and the contributing roles they play in society and the science and engineering disciplines. Novel concepts have also been introduced to the current and next generation of research and researchers that includes the interaction of chemistry with the environment, the development and application of sustainability indicators and metrics and life cycle assessment (LCA) methodologies for assessing the current and potential states of sustainability for a system (e.g., a process, reaction, or supply chain), and the use of renewable materials as feedstocks for sustainable materials management. Additionally, for readers who are chemists, they have been introduced to chemical engineering concepts such as reactor design and kinetics, reactor and process engineering, thermodynamics, separations, and energy and heat transfer. These are concepts chemists now need to consider and integrate into their research during the chemical synthesis design phase. What is being developed, up to this point, is the demonstrated need for research to no longer be focused on one stage of the process or be constrained to a closed system, but the need for research and researchers to think holistically about the challenge they are solving, or the new technology they are developing. This chapter introduces and demonstrates the economic and, in turn, the correlated societal and environmental benefits that are gained when the principles of green chemistry and green engineering are introduced into a technology.
To place this chapter, and in fact the entire book, into perspective, the reader must understand the roles that green chemistry and green engineering play in the concept of sustainability. While there are many definitions for sustainability and these can also be modified to meet one’s needs or desires, the definition the authors employ is a combination of the Bruntland Commission definition from 1992 [3] integrated with the mission of the U.S. Environmental Protection Agency (EPA) [4]. This combination arrives at a definition of “protecting human health and safeguarding the environment to meet the needs of current and future generations.” The authors feel this definition captures the three pillars of sustainability—environmental, societal, and economics—as well as emphasizing the role of environmental protection and its contribution to increasing the sustainability of a system.
While the contributions of green chemistry and green engineering over the past 20 years have been significant, it must be recognized they are tools, which contribute to achieving an increased level of sustainability for a system as a whole. And as tools, they comprise a larger methodology, and that methodology is sustainable chemistry. In this context, sustainable chemistry applies a life cycle perspective to the social, economic, and environmental impact of a good, service, chemical, or product across its entire life cycle.
As evidenced in literature and practice, the goal of sustainability is now being applied to the chemical sector to reduce the negative effects on the environment and its health. To achieve sustainability, for the lifecycle of a chemical, researchers must have the ability not only to minimize or eliminate this risk across the lifecycle but also to be able to access and quantify any remaining risk and ensure the research direction taken is in a more sustainable direction. As the lifecycle of a chemical is mapped out, it is evident there are many opportunities that exist for improvement to current technologies as well as research areas for development of novel and innovative processes. Sustainability of a chemical synthesis not only occurs at the synthesis stage but also can be manipulated at any stage of the process lifecycle with direct and indirect benefits and consequences.
The chemical industry has made drastic improvements in the quality of life for society, but at the same time has not fully considered the effect on the health of the environment. For many decades, dilution was the solution to pollution. As a result, we are now seeing the effect of this approach of years of hazardous materials entering the environment from a number of human, animal, and environmental causes. Natural resources once seen as abundant and meeting every need of the human population have reached a point where it is obvious that our consumption exceeds the current supply. With concerns rising for pollution generation and natural resource consumption, sustainability has moved beyond the status of a buzzword to the forefront of industrial management. The concept of sustainability necessitates a shift to renewable resources, nonhazardous materials, and a decrease in the amount of waste being produced and released into the environment. Identifying the best means to achieve these goals is still a difficult task for research in any discipline. Even if we develop new and innovative processes or products with their own increased sustainability, sustainability does not have an endpoint and there is always more that can be done to achieve global sustainability. It is routinely acknowledged and demonstrated that the current methods for many industrial chemical production routes are inefficient. After scale up, many of these processes must be reexamined at a smaller scale to increase efficiency and production volume, decrease waste production and cost, and minimize energy consumption. This results in wasted time, personnel-hours, and increased costs to redesign the existing process to be more sustainable. While this process is transpiring, the continued large production of the inefficient process is still consuming copious amounts of materials, energy, and capital.
The premise of employing green chemistry and green engineering approaches is to contribute to the development of sustainable manufacturing processes for chemicals and products that simplify the reaction strategy and minimize resource and energy consumption, process time, and environmental impacts throughout the product or chemicals life cycle. In the absence of green chemistry and green engineering advancements, a major factor of any technology is the economic impact that can be expected. Oftentimes, the perceived bottom line is the final determinant companies contemplate when investigating a new process or synthesis option. Therefore, the economics of green chemistry are vital to its introduction into the marketplace and its eventual success. This chapter introduces the concepts, economic benefits, and needed thinking in order to increase the viability and introduction of technologies that employ green chemistry and green engineering into practice and the marketplace.
To understand the economics of green chemistry and green engineering, it is first necessary to understand basic economic theory as it applies to chemical manufacturing. Traditionally, chemists at the bench scale develop viable product pathways to meet scientific and corporate demands while engineers design optimized processes to implement these pathways using production costs as the primary design criterion. This approach to product design is limited because it treats chemical manufacturing as an isolated action and neglects the multiple levels of economic influence that can impact a chemical product. In actuality, economic theory can be applied with increasing complexity to three levels of scale: (1) microscale or plant scale, (2) corporate scale, and (3) macroscale or economy scale. Each level is now briefly defined only as a means to provide a context for understanding the economic influence of green principles.
Economic theory at the plant scale is an integral part of product design and development. The profit derived from a given product is largely impacted by the cost to produce it. Although typical chemists and engineers might be aware of key cost factors, there is a much larger set of factors that govern production costs, many of which might not be considered during product design. The complete set of factors can be divided into two types––capital expenditures and operational expenditures.
Capital expenditures, or CAPEX, include all necessary costs to build an operational process or plant and are comprised of land, equipment, construction, and miscellaneous administrative costs [5]. Companies must first purchase land for development, with prices determined by local real estate prices. Equipment costs account for the purchase of reaction vessels, separators, furnaces, boilers, heat exchangers, pumps, piping, control systems, heat tracing, insulation, spare parts, and such. These costs will depend on manufacturers’ prices for the specified equipment quantities and sizes. Typical construction costs involve land development, infrastructure (buildings, roads, sewer systems, etc.), labor (assembly, welding, steel and concrete fabrication, etc.), equipment (cranes, mixers, etc.), contractors’ fees and incentives, utilities, auxiliaries, insurance, engineering services, and project management. Once a plant or process has been constructed, there are additional administrative costs that must be incurred prior to operation. These include training, testing, inspection and permitting, and start-up. Companies will often put up only a portion of the capital costs with cash on hand (equity) and finance the rest through low-interest loans (debt). A process or product will only have net profitability once the total CAPEX has been recovered. The timetable to achieve this profitability is set by the period of time to attain a return on investment or ROI.
The operational expenditures, or OPEX, account for all fixed and variable costs associated with product manufacturing [5]. Fixed costs cover all yearly costs that are not dependent on the production levels (running time). Examples of fixed costs are capital expenses (CAPEX including equipment depreciation), maintenance, basic payroll and benefits, insurance premiums, safety, property taxes, and utilities for workspace facilities. Conversely, variable costs are costs directly related to production time. Examples of variable costs are raw materials, process utilities, payroll overtime, waste disposal, and product storage. Once a company makes the decision to move forward with implementing a new process or product, it will specify a desired rate of return on investment and set product prices accordingly.
At the corporate level, the economics associated with chemical manufacturing are less related to actual manufacturing and instead focus on product management including research and development, logistics, and product image. Successful companies must first be willing to invest in research and development (R&D) to identify novel and/or improved products. This investment can be quite costly in terms of personnel and resources. However, a thorough R&D effort can help ensure the scale-up and transition from concept to production is more easily achieved in a shorter amount of time. Without R&D or at least a strong R&D effort, a company’s market position can grow stagnant and become detrimental to the well-being of the company as consumers’ needs evolve. Highly profitable companies have the ability to quickly reinvest profits in R&D to maintain continued growth and strengthen their market position. So how do companies operating with negligible profits compete? For smaller companies, it may become necessary to solicit funds from venture capitalists, government grants, and other private investors to help raise the necessary capital for R&D investment. Larger companies can raise this capital by selling a stake in the company as stock on public stock exchanges or issuing corporate bonds to purchasers guaranteeing future repayment of debt. Regardless of the approach, the use of external capital will reduce the potential profit of a product because these investments must be paid back and are always made with the expectation of a profitable return to the lender. Lenders exposed to higher risk will demand a larger ROI.
A second key aspect of product management is the logistics of product storage and distribution. At the plant level, the primary objective is to manufacture the product with minimal production costs. At the corporate scale, companies must develop networks to efficiently deliver the product to customers. For example, after gasoline is refined from crude oil, it is transported to regional storage terminals for further delivery to customers (regional distributors and/or gas stations). The proper location of these storage terminals within the distribution network relative to both the manufacturing plant and their customers is critical because the cost of transporting the gasoline can be significant given current fuel costs. For gasoline, product storage itself will also impact the associated cost and profit because it can be an indicator of supply relative to demand. When gasoline stocks become significant, product demand is considered low and the price customers are willing to pay will be lowered accordingly. To counteract this effect, oil companies will control the fraction of raw materials (crude oil) converted to gasoline to avoid excess production levels.
Product image is vital to the success of any product. The three main aspects a company can use to control its product image are through marketing, industry trade, and regulatory guidance [6, 7]. Marketing costs are a necessary part of business to help sway customer preference, as well as maintain customers. Traditional costs involve ad campaigns and distribution of product literature. The instant availability of information that has accompanied the technological revolution spurred by the growth of the Internet has forced marketing tactics to evolve and added to these marketing costs. Companies must pay to design and maintain Internet domains for product marketing either using contractors or hiring information technologists. In addition, companies must be proactive against the threat of negative product press presented online in customer and product reviews as these can shape customer product perceptions and affect sales. For many industries, companies with similar products find it advantageous to form trade associations to help develop product standards and distribute the costs associated with building a common product image. For example, the American Wood Protection Association develops and implements standards for wood treatment solutions that can be used to sell the benefits of the various products [8]. Trade associations can also help companies with shaping regulatory policy for product markets. Traditional economic theory predicts environmental regulation negatively impacts company profits [7]. Therefore, companies typically incur costs associated with engaging policy makers on regulatory issues. Individually, a company can hire lobbyists to represent its needs. However, a collective industrial voice will carry more weight in decisions. Trade associations can carry out product safety testing and lobby on behalf of their constituents to positively influence regulatory decisions for the industry. These services will only increase the resulting product costs for companies.
The chemical industry as a whole functions within the larger context of regional, national, and global economies, which are subject to societal and environmental stressors. At the regional level, product demand dictates price and can fluctuate in response to local stressors. For example, pesticide manufacturers will see a decline in profits when customers are experiencing drought conditions. If the pesticides are manufactured using bioprocesses, the company could experience a further decline in profits because raw material prices would escalate in response to drought-impacted crop production. At the national level, societal concerns and government policy will impact product markets and profitability. In some cases, the impact will be negative like the soaring cost of cigarettes in response to antismoking health campaigns and tobacco taxes. Recent government-mandated changes to product labeling are designed to further minimize profits for tobacco companies by eliminating consumer demand. At the same time, various national governments are providing incentives through tax credits and purchase rebates to increase demand (and profits) for alternative energy products in response to evolving societal preferences for sustainability. In some cases, national governments are passing legislation to prioritize sustainable development within industries. In 2005, China passed initiatives to promote a circular economy through the promotion of green chemistry and green engineering [9]. Globally, product economics are largely influenced by the politics of cultural differences and the distribution of raw materials. As societies have advanced technologically, so has the demand for raw materials. The raw materials needed to produce fuels and chemicals, mainly crude oil, are unevenly distributed throughout the world with a select number of oil-producing countries controlling the market, most of which are still developing socially. As wars and civil unrest destabilize these regions, oil-dependent countries are facing steep increases in raw material costs that permeate throughout regional and national economies. Likewise, electronic manufacturers are facing ever increasing costs for specialty materials such as rare earth elements because approximately 97% of the global supply is controlled by one country [10]. Another major influence on product economics at the global scale is the transition from local product markets to global supply chains. Business transactions at this level will be subject to trade policies and taxation that will influence pricing and profitability.
The combination of economic factors affecting chemical manufacturing when considering all levels of scale should convey the complex economic system companies must navigate when making decisions. These various factors make it possible to provide an understanding for how the principles of green chemistry and green engineering can be related to economic theory. The hope is this knowledge can then be applied to develop better “green solutions” with more attractive incentives to persuade companies to implement them.
Although the principles of green chemistry and green engineering have been established for well over a decade, only recently have companies begun large-scale implementation and use of these concepts for industrial applications, often with emphasis on developing renewable feedstocks for chemical processes. The slow growth in the application of the green principles can be attributed to their misguided worth within the business model. Green principles are most often associated with “environmentally friendly” products. Thus, the only perceived benefits for the company are the environmental outcomes of the manufacturing processes or products themselves. If consumers can be swayed to use these products based on the “ecofriendly” label, then a company will likely incorporate green principles into its strategy to increase market share. However, this business view of green principles is naive and excludes many of the fundamental economic benefits that are offered from their use. To better understand this point, Table 10.1 demonstrates how each of the green chemistry and green engineering principles are related to the economic factors discussed in Section 10.2 and the respective relevance from a lifecycle perspective.
At the microscale, each of the 12 green chemistry principles can be related to a number of the economic factors discussed in Section 10.1. In all, 10 of the 12 principles can impact safety and insurance costs, 9 principles can impact waste disposal costs, 7 principles can impact material costs, 6 principles can impact equipment costs, 4 principles can impact utility costs, and 4 principles can impact land use costs. At the corporate scale, all 12 principles will impact R&D costs, 11 of the 12 principles can impact product image costs, and 3 principles can impact logistics costs. At the macroscale, all 12 principles can impact global supply chain costs while 9 principles can impact government policy costs. More important than the numbers are how and why the principles will impact these costs.
For the sake of this discussion, first consider the simple one-step reaction sequence:

where A and B are reactants and C is the desired product, a high-demand ultrapure precursor for specialty chemicals. The stoichiometric coefficient for all chemicals in this reaction are one (1). This reaction is carried out at room temperature with a 100% conversion and 100% yield and involves no solvents or downstream processing for product recovery. Thus, it is an ideal reaction from a chemist and chemical engineer viewpoint. This ideal chemical process represents the minimum cost scenario because it (1) utilizes maximum reaction efficiency, atom economy, and yield; (2) uses a minimal quantity of raw materials (A and B) to produce a given quantity of product (C); (3) requires a minimal energy input; and (4) eliminates the need for waste disposal.
TABLE 10.1. Relating the 12 Principles of Green Chemistry and Green Engineering to Economic Factors and Relevance to a Life Cycle Perspective
Source: Adapted from Ruiz-Mercado et al. [11].

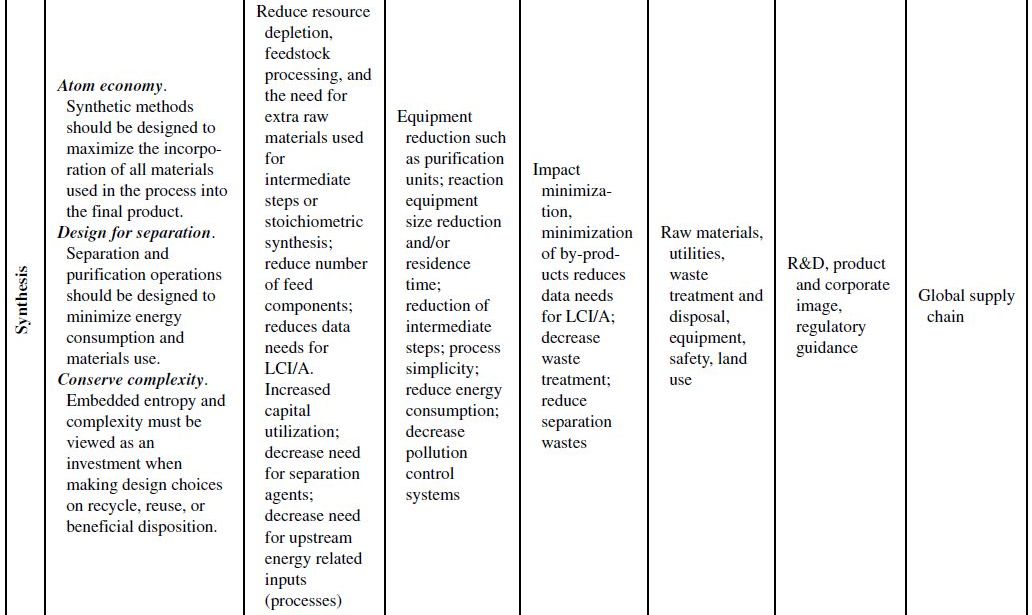
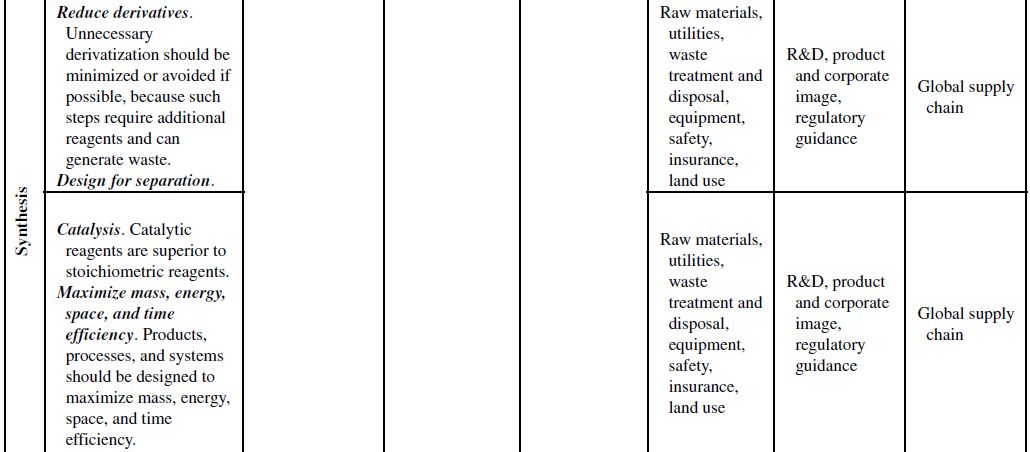
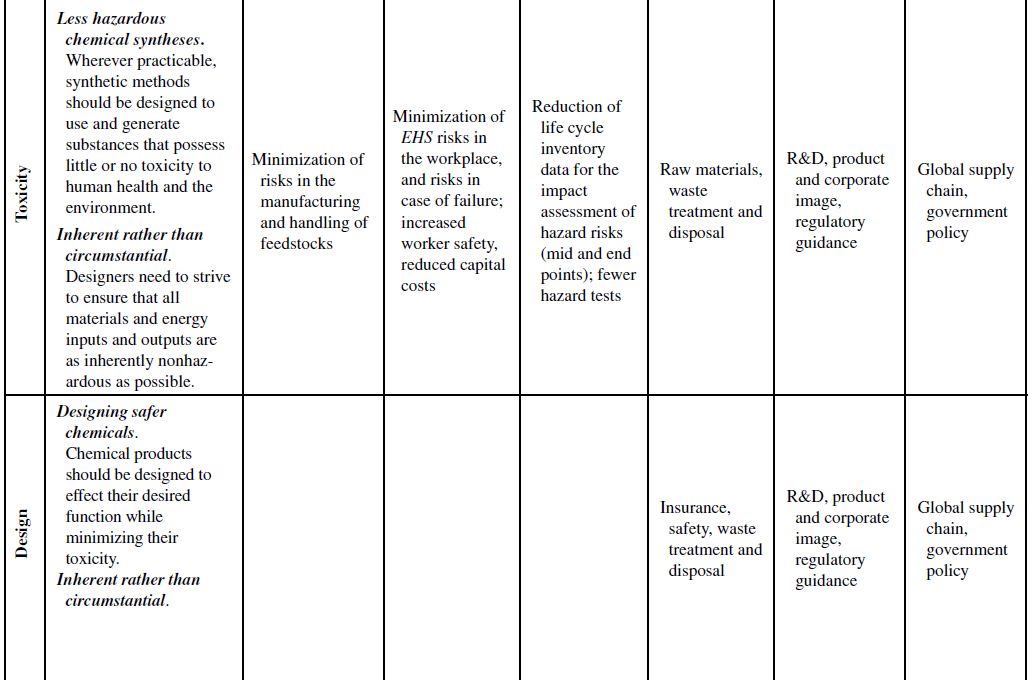
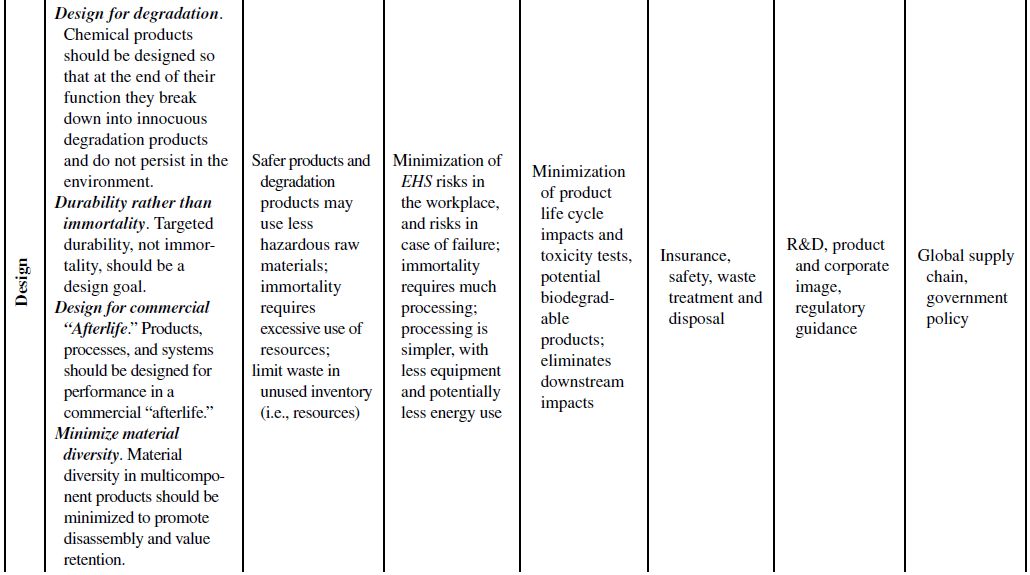
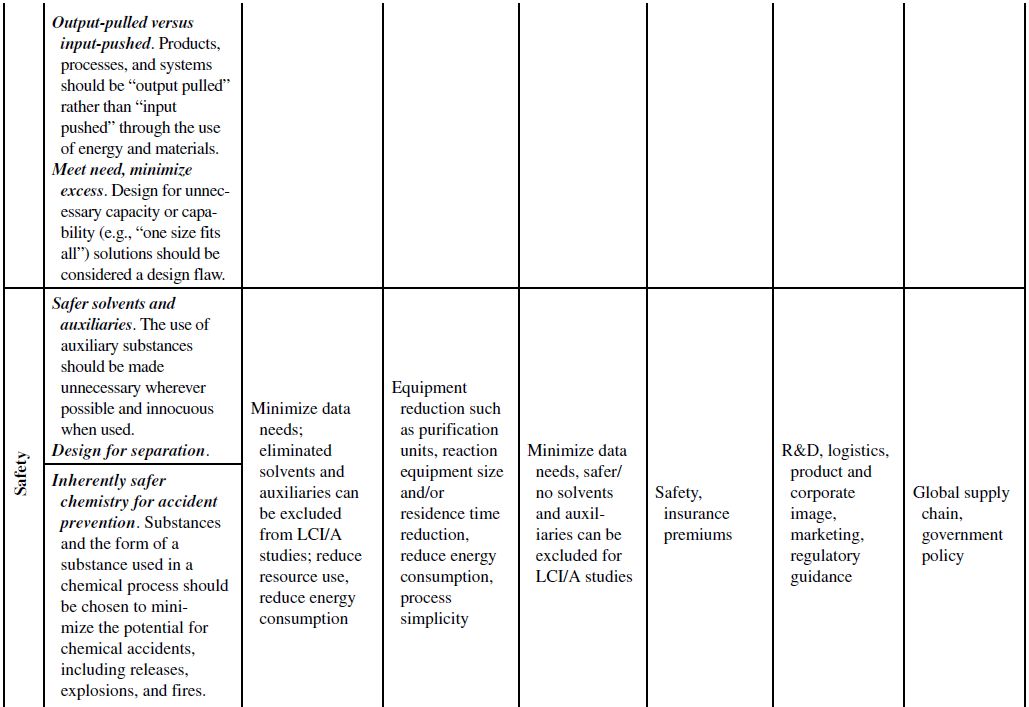
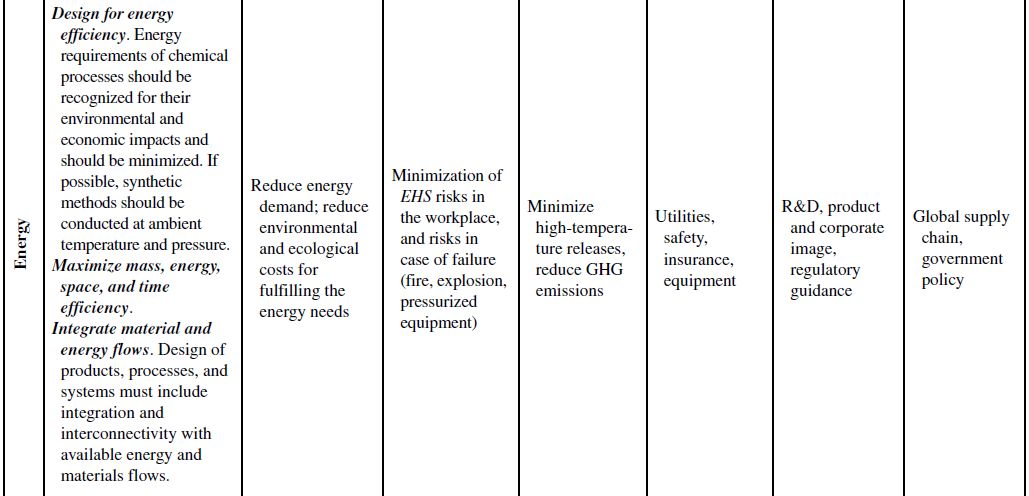
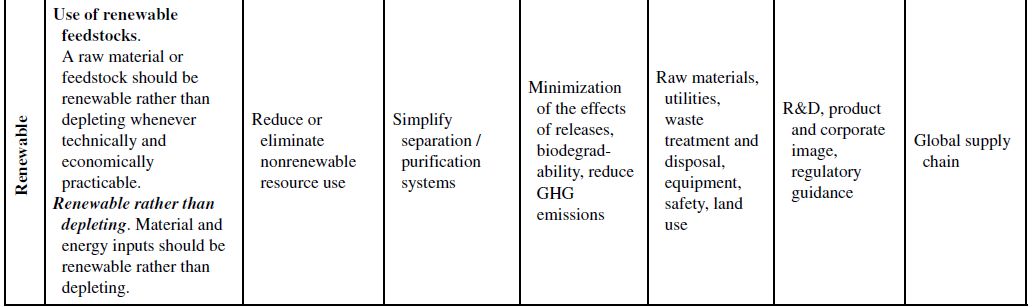
Figure 10.1. A theoretical traditional process for the production of a chemical product illustrating the complex and costly nature of processes designed without incorporating green chemistry.
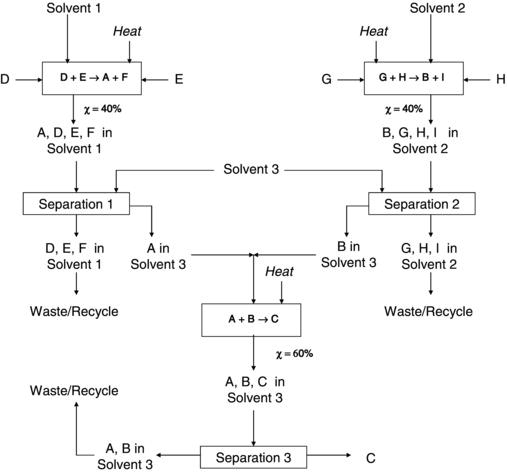
Now suppose we compare this ideal chemical process to a more complex process that is currently in operation by a company to obtain product C, as shown in Figure 10.1. Instead of the ideal case above, there are now four high-cost raw materials (D, E, G, H), two nonvaluable waste products (F, I), three organic solvents, three reaction steps, three separation steps, and a need for supplied heat to make the desired product C. The individual reaction steps themselves are inefficient with conversions much less than 100%. The microscale costs associated with such a process can be substantial. Based on the conversions listed, only 24% of the initial reactants actually end up as viable product. Thus, a large quantity of raw materials (reactants and solvents) would be required to produce a significant quantity of product, resulting in a larger cost of materials. If the addition of recycle feed loops was to be considered, further complexity and cost to this scenario would be the result. In addition to the cost of acquiring materials, a company must also be mindful of the cost of disposal for generated waste materials. Even if all of the solvents and reactants are recovered and recycled as in Figure 10.1, the company must dispose of the waste products F and I. Depending on the hazardous nature and volume of these materials, disposal cost can quickly drive up the operating cost of the process. Another consideration that will impact production cost is product monitoring. Because the product, C, must be ultrapure, the presence of so many other chemicals increases the chances of contamination and makes it necessary to continuously analyze process outputs, as well as adding to the cost of purification and decreasing process throughput.
Use of this process will also require energy to supply the required heat for reaction and/or separation, power the process equipment (pumps, blowers, etc.), and provide process control. The large number of process steps will require more personnel to run the equipment. This translates into the company being required to pay more for insurance premiums due to a number of factors including a larger number of potential victims and the use of hazardous chemicals. The additional costs associated with security, training, certification and permitting, and transportation cannot be overlooked. Likewise, the large number of processes will require a larger plant footprint in terms of land use. At the corporate scale, the complexity of the process will require a substantial R&D investment to obtain the needed product purity. The combination of low yield and high raw material costs will make it challenging to establish a cost-effective distribution network. Regional locations for product storage sites will be limited, which could possibly drive up land costs based on the greater number of constraints narrowing real estate choices. If the price for the product is on the high side of the industry average, the company will have to spend more on marketing strategies to convince downstream customers why their product is the best choice and worth the added cost. The added cost of manufacturing could then impede the ability of the company to participate in trade associations to aid with oversight and regulation of the product market. At the macroscale, governments are increasingly encouraging policy development with sustainability in mind. Given the typical long-scale return on investment for most new processes, it is possible that investing in such a wasteful process now could lead to unforeseen penalties and cost in the future if the process must be modified to remain within newly imposed regulatory guidelines. In the end, all of these factors will translate to a more expensive product and cost of operation with a higher risk for achieving the desired return on investment.
How will the principles of green chemistry impact this scenario? The answer to this question will illustrate the true economic value of green chemistry from a business perspective. For the example above, now assume that a second company, which also manufactures chemical product C, uses a newly designed modular process developed through the application of the principles of green chemistry. This novel process is detailed in Figure 10.2. When compared to the original process, this process requires less raw materials and energy, generates no wastes, utilizes fewer processing steps (one reaction and separation), and is overall more efficient. Most of these improvements can be attributed to the beneficial influence of encouraged catalysis use (principle 9) for more efficient reactions. In addition to material benefits, the catalytic pathways can require milder operating conditions (temperatures, pressures, and solvents), which drive process energy requirements. For this example, a common catalyst is used to perform a “one-pot” reaction where the two reactants (D, G) can be transformed into the necessary intermediates (A, B) without additional chemicals and then directly reacted to form the product (C). In addition, the reaction can be carried out in water at room temperature, eliminating the use of energy and organic solvents. The modular nature of the process allows production volumes to be continuously scaled to reflect product demands and maintain optimal profit margins. More detailed analysis of process intensification and modularization will be saved for subsequent discussions on green marketing and business strategies in the next section.
Figure 10.2. An alternative modular process for the production of a chemical product demonstrating the advantages of incorporating green chemistry into process design.
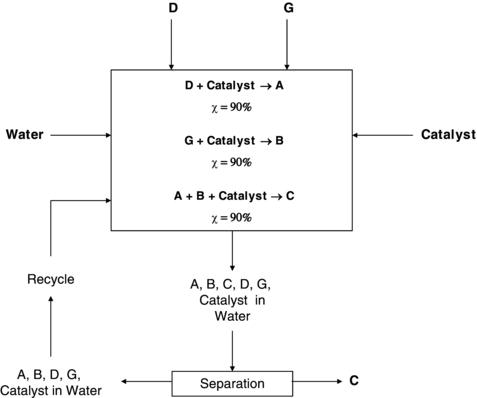
At the microscale, this process is more beneficial in terms of both capital and operating costs when compared to the original process. In general, the capital costs will be less for this process due to the application of green chemistry principles 1, 2, 5, 8, and 9 leading to fewer processing steps requiring less equipment to achieve desired production quantities. Additional savings in capital cost are possible given the assumption that smaller processes will require less land and construction time. The associated operating costs will also be smaller when compared to the original process. Although the use of catalyst will add cost to the process, it has eliminated the need for reactants E and H (principles 1 and 2) and now allows for water to be used in place of an organic solvent(s) (principle 5). The reaction efficiency at each step has been increased to 90%, which means 81% of the primary reactants (D, G) are converted to viable product C (principle 2), versus 24% in the traditional process. This increase translates into more efficient use of feedstocks and material conservation. The elimination of wastes and the use of a common solvent provides for easy recycle loops for the unreacted starting materials. When all of this is combined with the lower operating temperature (principle 6), the raw material and energy costs for this process will be much less than the costs for the traditional process. The fewer processing steps will also mean a reduced size operating staff, which should contribute to a reduction in payroll and benefits. The benign nature of the process could also reduce the associated insurance and permitting costs. In the end, all of these cost reductions can result in a lower price for product C in order to obtain the same desired return on investment, while offering higher profitability.
At the corporate scale, the development of this improved process may require more R&D investment to identify the necessary catalytic pathways. This increase should be offset by the reduced need for the development of separation processes after eliminating the use of organic solvents and nonvaluable waste products. The modular and scalable nature of the process when combined with a smaller plant footprint should remove a number of constraints from the design of the product distribution network and contribute to a reduced cost of logistics for the company. These reduced costs should make the company highly competitive in the product market based on price alone. This inherent competitive edge from pricing should require smaller marketing investments and further increase net revenue. This additional revenue can be applied toward active participation in trade groups and regulatory product development for the product market. The benign nature of the process will eliminate the burden (and therefore costs) of studying health effects during production to maintain regulatory compliance. The gains in profitability provide the company with capital to reinvest into the company and offer additional greener processes to other product lines. This should lead to a strengthening of market position and recognition as a leader in sustainability.
At the macroscale, the reduction in raw material and energy requirements will help protect the profitability of the process from externalities such as price fluctuations in the upstream global supply chain that have become a dominant effect of escalating sociopolitical tensions throughout the world. The greener nature of the process will increase the demand for the resulting product with downstream chemical manufacturers responding to shifting societal demands for sustainable development. The improved environmental performance will guarantee that the long-term returns on the process will be realized even as national environmental policies evolve with society. Ultimately, implementation of green chemistry and green engineering can help maximize long-term profitability while minimizing the perceived investment risks.
This example, although ideal, represents the benefits that should frame the mindset of implementing green chemistry within industry. Although the environmental benefits of green chemistry were not discussed in the example above, they should not be dismissed. However, these benefits alone will not be enough to compel industries to adopt the principles of green chemistry (see Section 10.4). The ability to alter the bottom-line will ultimately govern business decisions. Providing a clear understanding to decision makers of how green chemistry can influence not only the microscale economics of a process but also the larger scale economic factors for corporate profitability is essential to the successful incorporation of the principles of green chemistry into process/product development and design.
According to Pike Research, the current value of the global chemical industry is roughly US$3 trillion [12]. At the same time, the green chemical industry only accounts for 0.1% or US$3 billion with a projected value of US$100 billion by 2020. These numbers are significantly smaller than one would expect based on the potential economic benefits outlined in Section 10.2. From that discussion, companies have significantly resisted the implementation of green chemistry because of a misguided belief by investors and business managers that the development of green processes is motivated by environmental consciousness. The key challenge facing chemists and engineers who realize the broader benefits of green process design is identifying strategies to help grow support for deploying green concepts on a wider scale within the chemical industry. Effective implementation will require stakeholders throughout the entire life cycle of a chemical product (i.e., investors, manufacturers, consumers, governments) to be aware of the inherent benefits that can be realized when incorporating green chemistry (and engineering). This concept of sustainability marketing has been discussed by Iles and is based on the Porter hypothesis, which states that companies can improve commercial success through an efficient use of resources resulting from strict environmental regulations [13]. However, the Porter hypothesis itself poses a challenge to the acceptance of green chemistry because its reasoning is counterintuitive to traditional economic theory, which holds “environmental requirements are unavoidably increasing the private costs of the economy, resulting in the decreased competitiveness of the government and companies” [7]. Therefore, it is crucial to understand the challenges facing the application of green chemistry as a result of these competing economic views and how these challenges might be resolved. This knowledge, when combined with the numerous demonstrated and potential economic benefits of green chemistry, will provide a solid business strategy for the application of green chemistry.
Application of green chemistry begins with R&D. Not coincidentally, R&D investment poses one of the largest challenges to green chemistry because it is often viewed as an environmental investment [6, 7, 14]. As discussed in Section 10.2, companies at the corporate level require sufficient R&D investment to develop new processes and products, which are vital to maintaining a strong market share. Larger companies that typically have the necessary capital on hand or can easily obtain external funding are reluctant to invest in projects with unproven technologies, especially technologies viewed as environmental, because the resulting processes carry a much higher risk as a result of uncertainties surrounding scale-up and reliability [14]. Murovec and co-workers note that studies have shown successful companies experiencing growth are more likely to gamble on innovative, environmentally friendly technologies, provided the company possesses a more proactive attitude toward environmental stewardship and sees it as a means to gain market shares [7]. For smaller companies, profits are typically sufficiently reduced and limit the ability to internally fund R&D projects. These companies undergo much greater scrutiny when trying to secure external funding in response to their perceived economic weakness [7]. To complicate matters, external funding sources (banks, venture capitalist, etc.) may be even less inclined to back the development of “green processes” because the implied environmental gains would suggest a negative return on investment when placed in the context of traditional economic theory.
The negative perception regarding investment in green chemistry is indicative of a larger aversion to sustainable development and has been described by Pacheco and co-workers as a “prisoner’s dilemma problem” [6]. In traditional game theory, the prisoner’s dilemma is typically used to demonstrate how interrelated individuals who reject a group mentality and act in their own best interest will do worse than had they embraced the collective good. The idea is that the best outcome for an individual occurs when he/she alone rejects the group, while the worst outcome occurs when an individual chooses the group and everyone else rejects the group. In fact, it is actually better for an individual if everyone rejects the group versus everyone but him/her rejecting the group. All participants of the game are aware of these outcomes and fear they will be the only one to choose the group. This fear drives everyone to reject the group, knowing that they will at least perform better than the worst possible outcome. When applying this theory to green process development, companies and investors are the “players” in the game and industrial sectors are the “groups.” The underlying fear keeping each player in the “green prison” is that he/she will be the only one to incur the substantial R&D costs needed to pursue green technologies. This will result in a power shift within the group whereby competitors can sell cheaper products and gain a competitive edge. According to market estimates, a US$40 billion savings in process costs for the global chemical industry is possible through waste minimization alone [12]. This value could be much higher if the other benefits provided by green chemistry are included. As opposed to groups realizing the mutual benefits of green design, companies are willing to settle for the option of maintaining a level playing field and everyone rejecting the need to invest in green chemistry because this is at least a better outcome than the worst case scenario inspiring the fear.
Perhaps the underlying cause contributing to the reluctance to develop and implement green chemistry and green engineering is society’s lack of a clear voice when it comes to the question of environmental preservation and sustainability. In the United States, environmental protection based on national regulations has recently (as of 2013) been sensationalized in the media as a “job killer,” supporting classic economic theory about the impact on companies and economies by pursuing environmental benefits. This message is driven by the agendas of powerful industrial lobbies responding to what had been a growing trend in consumer preferences for sustainable products and services such as alternative energy and green buildings. With unemployment levels hovering at all time highs, the “job killing” message has created a societal “prisoner’s dilemma.” While individuals may understand the ultimate good of society is best served in the long run by pursuing sustainability, the fear of near-term financial suffering can potentially mute the willingness of consumers to absorb the extra costs associated with this endeavor. If companies perceive a negative shift in consumer preference, this too will serve as a deterrent when contemplating future investments in green chemistry and green engineering. A shift in public attitude can also impact the ability of governments to enact environmental regulatory policies. For example, recent efforts by the U.S. government to regulate greenhouse gas emissions and address climate change have been blocked by political forces responding to the efforts of lobbyists acting on behalf of industries and citizens fearing the economic ramifications of positive environmental actions. For government decision makers, this was both discouraging and confusing given the regulations were developed to meet the demands set forth during global environmental summits. Without enforceable environmental standards to incentivize against economic risk, companies will continually default to the prisoner’s mentality when considering emerging environmental technologies and pass on the opportunity to realize the full benefits of sustainability and green product development.
Although these challenges may seem daunting, they are not insurmountable. When studying entrepreneurship and investment in green technologies, Pacheco and co-workers propose a solution to escape from the “green prison” by changing the rules of the game [6]. This can be accomplished in one of three ways. The first way to change the rules is by instituting industry norms. Like society, norms in industry will instill a uniformity of behavior. If the companies in an industrial sector can agree upon a self-imposed code of conduct to implement green principles, then the group option of the prisoner’s dilemma will become the most attractive outcome for the game. The second way to change the rules is through the use of property rights. For example, industries that establish an emissions trading policy do so with the expectation that only members who are actively pursuing improvements to their own environmental performance will be allowed to trade. The apparent benefits of the trade system will force companies to comply or risk giving competitors an advantage.
The final way Pacheco and co-workers propose to change the rules of the game is through government legislation. The use of government legislation is the most effective way to address the prisoner’s dilemma because the promise of rewards (tax breaks, grants, etc.) or penalties (fines, legal charges, etc.) will reinforce the need to act for the best interest of the collective and discourage rejection of the group. A key example of the use of government policy and legislation to encourage implementation of green chemistry is the efforts of China to create a national circular economy [9, 15]. For many years during the latter parts of the 20th century, the primary goal of the Chinese government was to become a leading world economy. The demand for growth was pursued with little regard for the potential environmental impacts it might bring. As environmental problems mounted and societies worldwide became increasingly interested in sustainable development, China recognized its own need to better manage its environmental resources and began implementing policies to this effect. In 2001, the Institute for Process Engineering was established as a center for research on green chemical processes [9]. By 2003, China adopted a national regulatory program to manage a variety of chemicals. In 2005, the National Program for Experimental Units of Circular Economy was launched, the foundation of which has significant overlap with the 12 principles of green chemistry. The goal of this program is to create a closed loop of material flows (a circular economy) within the Chinese economy [15]. A recent study by Matus has examined the impact of these programs in response to policy changes and identified the current barriers companies are facing. While companies want to participate in the cultural shift, many are ill-equipped to handle the development of green technologies in-house. Instead, R&D is contracted out to academic centers, oftentimes using guidance from the Chinese government to help form the right industry–academia relationships. A drawback to this approach is the control it has given industry to define the goal and scope of green chemistry research to be more applied to the extent of eliminating basic science-oriented research. The advantage is the Chinese government is willing to help financially support these collaborations. This is extremely beneficial for smaller companies operating with a much tighter profit margin. Now that the demand for green technologies is escalating, other factors such as employee training must also be considered. Despite the remaining barriers, the actions of the Chinese government have helped the chemical industry escape the prisoner’s dilemma problem.
Governments aren’t the only entities with the ability to enact policies to promote green technologies. In 2009, Walmart, the largest retailer in the world, helped found The Sustainability Consortium (TSC), whose mission is to implement standards for communicating the sustainability of consumer products [16, 17]. The TSC is made up of 83 major retailers working with 9 NGOs and government agencies [17]. The product labels being developed should be considered norms because they offer no chance for rewards or threat of penalty, but instead will provide uniformity in retail. This can be a powerful tool to help break the prisoner’s dilemma because the desire of Walmart and other retailers to promote sustainable products will force manufacturers to decide either to go with the group and implement green technologies throughout the product supply chain (including upstream vendors) or to continue with their current manufacturing processes and face potential revenue losses as TSC retailers begin to focus their advertising campaigns on compliant products. The power of large-scale policy is necessary for encouraging the use of green chemistry and green engineering. As TSC demonstrates, this power can be evenly distributed between governments and industry.
The purpose of this discussion was to provide a business strategy to improve the ability to incorporate green chemistry and green engineering in chemical manufacturing. The key to a successful strategy is that it must be based on economic theory that can accept economic growth as a positive effect of environmental technology. The strategy should first address the stereotypical belief that the business value of environmental technologies is primarily the ability to market sustainability. This will shift the cost analysis from a society base to an industry base and allow for incorporation of a greater number of the economic factors discussed in Section 10.2. The inclusion of a broader set of factors will provide the potential for a better return on investment to offset the normal risks associated with new process development. Next, the strategy should encourage investment in R&D by providing investors with a means to escape the “green prison.” To this end, companies should make a stronger effort to work more collaboratively to develop industry norms that make green technologies more favorable. Such collaborations will help provide a strong industry voice to influence formal policy development at a larger level and help lock industry players into the “group” mentality. Finally, a company should work with partners upstream and downstream in the supply chain to develop more sustainable products in a manner that could more evenly distribute the associated costs.
The availability of literature explicitly detailing the economic benefits of green chemistry is limited [18]. Instead, the benefits of green chemistry in process design fall under the larger topic of sustainable process design. This is because the true value of green chemistry technically transcends economic savings and can encompass environmental and societal benefits too. The goal of sustainable design is to consider environmental and societal issues early in the design process as opposed to the end-of-pipe mentality that has dominated traditional process design. This type of thinking encourages chemists and engineers to work together from the bench-top to the final scaled-up process to develop processes and products that satisfy the criteria for sustainability by using the principles of green chemistry and green engineering. However, the broad system boundaries that must be considered for sustainable design pose a challenge to the design process because of the difficulty in estimating the true benefits and impacts of green chemistry across the supply chain, especially the economic benefits [18]. The development of tools to aid in the design of sustainable processes is a growing field. Over time, these tools have included decision-support methodologies and metrics, design algorithms, and computer software. These tools can be all-encompassing, addressing an entire process, or focus on specific aspects of the process such as solvent selection.
The starting point when developing design tools is establishing suitable metrics for evaluation of green and/or sustainable processes. A set of sustainability metrics typically includes environmental, social, and economic factors [18, 19, 20, 21]. In addition, green chemistry metrics have been developed to characterize reaction pathways. Chemical factors can include the effective mass yield, E-factor (environmental impact factor), atom economy, and mass intensity [22]. These factors are used to assess how efficient a chemical process is when converting starting materials to product. Differences among the four factors are related to how nonreactants and nonproducts (waste, catalyst, and solvents) are treated. Evaluation of the eco footprint associated with chemicals as they go from raw materials to final disposal is often made using the life cycle assessment (LCA) methodology as defined in ISO 14044 (ISO 2006). Typical impact categories include global warming/climate change, stratospheric ozone depletion, human toxicity, ecotoxicity, photo-oxidant formation, acidification, eutrophication, land use, and resource depletion [23, 24]. Economic or cost factors can include any of the following: capital cost (material, equipment, and labor), net present value (NPV), product revenue, waste treatment, training, insurance, and health and safety compliance. Societal metrics include provision of employment, the health and safety of workers and area residents, odor, noise, and public acceptability of products and/or processes [19].
When comparing potential processing or product alternatives, the various metrics can be calculated and weighted to determine which option is the best. However, this can prove to be a difficult task given the lack of standard weighting methods and has led to numerous publications offering the aforementioned tools for green and/or sustainable design. These methodologies will now be discussed, beginning with the simple environmental tools and following the incorporation of such tools into larger sustainability frameworks that encompass economic and societal concerns.
A major factor in the application of green chemistry is the choice of solvents for reaction media. An ideal solvent will provide maximum product yield and efficiency while minimizing the environmental, health, and safety (EHS) impacts of the process. Unfortunately, many solvents that produce favorable reaction conditions do so at the expense of EHS impacts. Therefore, it is necessary to identify solvents that optimize the trade-offs between the various performance criteria. Gani and co-workers have proposed a framework to guide the selection of a green solvent for an organic reaction [25]. The selection process involves two levels of evaluation. During initial evaluation, all possible solvents are listed and ranked based on basic user-defined reaction constraints using computer-aided molecular design and preexisting reaction data. The best candidates from this ranking are further evaluated at the second level using detailed calculations and experimentation that determine the specific performance and EHS impact of the solvent candidates. These results are then used to identify the optimal solvent. Folic and co-workers have extended the application of this methodology to multistage reaction systems [26].
Capello and co-workers have also proposed a comprehensive framework for the environmental assessment of solvents (both single and mixture) [27]. The tool combines EHS analysis of potential solvent hazards with LCA results for environmental impact in a simple three-step procedure. First, a solvent is scored using the EHS method for nine effect categories. These include the potential for release, acute toxicity, chronic toxicity, fire/explosion and reaction/decomposition, persistency, and air and water hazard. The second step involves application of LCA, as described above using the software tool Ecosolvent to calculate the impact scores for the solvent. Finally, the two assessment scores are combined and used to rate the solvents.
Andraos has devised an algorithm to evaluate processes using the green chemistry metrics reaction yield, atom economy, reaction mass efficiency, and E-factor [28]. In this algorithm, a process is first analyzed at the kernel (process step) level. These results are then combined at the global level to rate the performance of the entire process. This decomposition technique accounts for the potential use of by-products from one reaction in subsequent reactions within the process. When applied to existing processes, the kernel level scores can be used to identify potential areas of improvement within a process. For comparison of alternative processes, the global score can be used to select the “greenest” option.
Halim and Srinivasan have proposed an intelligent simulation-optimization framework for waste (E-factor) minimization of batch processes [29]. The procedure involves five steps: material flow representation, decomposition analysis for identifying design alternatives, detailed simulation of design alternatives, synthesis of a recycle network, and integrated simulation-optimization analysis for multi-objective solutions. This approach combines both qualitative and quantitative design tools through its use of heuristic design, process simulation, network synthesis, and optimization. This framework has resulted in the creation of a software tool, BATCH-ENVOPExpert, for batch process design.
Charpentier proposes a different view of green process design [30, 31]. Instead of stepwise frameworks, Charpentier discusses the need for multiscale and multidisciplinary design practices through the use of a triplet molecular processes–product–process engineering (3PE) approach. His premise is that emerging technologies such as nanotechnology coupled with growing trends in cost control and labor management will require a more integrated approach to process design and intensification to obtain more sustainable (or greener) manufacturing practices and products that are economically viable. Essentially, successful process design must utilize tools that enable multiscale modeling accounting for system performance at all length and time scales from the atomic to the macro level. These tools can encompass traditional engineering theory (reactor design, separations, thermodynamics, and material transport), molecular simulation, computational fluid dynamics, various forms of microscopy, environmental and health analysis (LCA, EHS, risk assessment), and waste minimization. The goal is to first design processes at the atomic scale using property-based simulations that can be used as a basis to predict scaled-up plant performance. At each stage of the design, the incorporation of environmental and health impact assessments can be used to guide the choices for material and processing alternatives to produce more sustainable systems
For all of the tools discussed, the basic goal of the design process is to minimize material usage and waste generation while alleviating the environmental impacts that often accompany chemical processing. Even when considering process options from this environmental perspective only, the task of identifying a “best alternative” can be challenging given the multi-objective nature of the problem. This process becomes even more daunting when economical and societal factors are also to be considered due to the large number of unknowns that must be evaluated and optimized. In addition, the selection, evaluation, and weighting of suitable metrics can be subjective in nature and produce varying results depending on the stakeholder and the decision criteria. This can be especially difficult when considering novel processing options because of the lack of knowledge and data concerning actual scaled-up performance. For these reasons, numerous decision-support tools for sustainable process design have been created. Some focus on how to incorporate sustainability analysis in general into the design process while others offer detailed accounts of how to obtain the optimized solution to the multi-objective design formulation.
GREENSCOPE (Gauging Reaction Effectiveness for Environmental Sustainability of Chemistries with a multi-Objective Process Evaluator) is a systematic methodology and software tool that can assist researchers from industry, academia, and government agencies in developing more sustainable processes [21, 32]. The sustainability of a process is measured in terms of environmental, efficiency, energy, and economic indicators (the four E’s), with each indicator being mathematically defined. The indicators (140) express diverse aspects of performance in a format that is easily understood, supporting realistic usage. The indicators enable and demonstrate the effectiveness of the application of green chemistry and green engineering principles in the sustainability context.
To evaluate the environmental aspects of alternative chemistries or technologies, GREENSCOPE employs the Waste Reduction (WAR) algorithm [33]. The WAR algorithm determines the potential environmental impacts of releases from a process in eight impact categories: human toxicity by ingestion, human toxicity by dermal/inhalation routes, aquatic toxicity, terrestrial toxicity, acidification, photochemical oxidation, global warming, and ozone depletion. While these potential impacts are defined as midpoint indicators (as opposed to endpoint indicators), the measures for the categories are well defined, which is a substantial improvement over arbitrary environmental or mass-based scores.
Efficiencies for chemical reactions are reflected in values such as conversion and selectivity, which track yields, product distributions, and recycle flows needed to make a desired amount of product. Another measure of how green a reaction is can be obtained from the atom economy (i.e., how many atoms from the feeds are in the product). These measures, which are well known in green chemistry, are related to environmental impacts since the product distribution defines what chemicals (and amounts) may leave a process. These efficiencies represent a bridge between the lab-scale experiments of a chemist and further engineering calculations.
Energy is a basic component of chemical processes. Its use depletes resources and creates potential environmental impacts. Connecting to yet another sustainability indicator, a less efficient process can be expected to use more energy.
Without a positive economic performance, no industrial process is sustainable. The economics of processes are measured according to their costs. For economists, this is an oversimplified view of markets, but for engineering calculations, the annualized costs are significant measures. The costs are tied into the process through efficiencies, energy, and environmental impacts.
A novel aspect of the GREENSCOPE methodology and tool is that each indicator is placed on a sustainability scale enclosed by scenarios representing the best target (100% of sustainability) and the worst case (0% of sustainability). This sustainability scale allows the transformation of any indicator score to a dimensionless form using the worst and best scenarios. A process that is better in environmental, efficiency, energy, and economic terms will most likely be sustainable, although one can expect that trade-offs will need to be made.
Azapagic and co-workers offer a “systems approach” method for sustainable design that is based on four basic stages: project initiation, preliminary design, detailed design, and final design [19]. As opposed to traditional process design with boundaries that isolate the desired process as a unique system, the system in sustainable design is expanded to include the product and process life cycles. Project initiation involves identification of stakeholders and high-priority design criteria. The various process alternatives are listed and evaluated using the selected metrics. Preliminary design begins with selection of the best sustainable process based on the initial criteria. A working flowsheet for the process is constructed including material and energy flows, equipment design, process control, and identification of potential safety considerations. This data can then be used to conduct preliminary cost analysis consisting of microeconomic (capital and operating costs and profitability) and macroeconomic (added-value, potential environmental liability) indicators. The list of sustainability metrics is expanded to include all desired constraints and an environmental analysis of the system is performed using LCA. These analyses can then be coupled with societal considerations (health and safety, public acceptance, odor, noise, visual impact) to evaluate the sustainability of the system. The detailed design stage involves an iterative optimization of the preliminary design calculations to maximize the sustainability of the process. The final design stage occurs once a process has been designed that satisfies the defined criteria and involves the preparation of drawings and plans for construction.
Simlarly, Diwekar uses a systems analysis perspective to generate a framework for sustainable process design [34]. Unlike Azapagic and co-workers, Diwekar’s methods couple this perspective with multi-objective problem solving to address the stochastic nature of sustainability. With multi-objective problem solving, a mathematical function is developed for each of the desired metrics based on quantifiable process variables and is assigned a weighting factor. The choice of metrics and weighting factors is subjective and left to the discretion of the stakeholder. Metrics for all three aspects of sustainability (environment, economics, society) can be incorporated. Nonlinear computational theory is then applied to the system to arrive at an optimized solution. While this may not sound difficult, the actual theory can involve highly sophisticated algorithms that use artificial intelligence to account for both the subjective weighting scheme and process variable uncertainty. The end result is a ranking of alternatives for decision makers based on trade-offs between the metrics. Diwekar provides several examples to aid in the formulation of metric functions.
The use of multi-objective optimization is a growing trend in sustainable design. Hugo and co-workers offer a process design methodology that focuses only on the minimization of environmental impacts while considering process cost and excluding societal impacts [35]. The Eco-Indicator 99 assessment model is incorporated into traditional economical-based process design to generate a set of objective functions. Unlike previous methodologies, the solution algorithm minimizes subjectivity in the calculations by analyzing all possible weighting schemes. The output is a set of Pareto optimal solutions, or best alternatives. The subjective decision making is then carried out based on these alternatives. Li and co-workers have adopted this same approach, but using their own environmental impact model in place of the Eco-Indicator 99 methodology [36, 37]. Khan and co-workers have developed the software GreenPro-I to aid with multicriteria decision making during sustainable design based on LCA [38, 39]. This is another computational tool that is capable of performing multi-objective optimization of sustainability criteria to identify the best compromise solution for a specified design objective.
A prevailing thought in sustainable design is that green thinking must be incorporated into the design process as early as possible. Kralisch and co-workers illustrate this approach when developing the ECO (ecological and economical optimization) method to identify the most sustainable solution to a design problem during research and development (R&D) [40]. The methodology uses a simplified version of LCA combined with rudimentary economic analysis similar to life cycle costing (LCC) to overcome the data limitations often encountered during R&D. LCC offers an added benefit over traditional cost analysis because it accounts for the costs associated with a chemical throughout its life cycle, including equipment and energy costs for manufacture and disposal. The multicriteria ECO problem formulation involves three objective functions describing energy consumption (EF), health and environmental risk (EHF), and cost (CF) that can be applied to each step in the proposed process. The Pareto optimal solution can be identified using decision theory, resulting in the ability to optimize each process step to arrive at the best process. Even if an optimal solution cannot be identified, the ranking of alternatives can be used to eliminate obvious adverse options and focus research efforts to reduce the cost of R&D.
Yu and co-workers recommend the use of an analytic hierarchy process (AHP) approach when considering the environmental and economic trade-offs during sustainable product design [41]. LCA and LCC are both used to assess product performance. AHP is a multicriteria decision making method that can be used to organize the complexity of a design problem into four manageable levels: goal, criteria, subcriteria, and alternatives. For sustainable design using this method, the goal is an optimized life cycle assessment of a product based on criteria of total life cycle cost and total environmental impact. The subcriteria are the individual impact categories (LCA and LCC categories) calculated for each of the alternative products to be considered. AHP is subjective in nature because it requires user input to establish a weighting matrix for ranking criteria during calculation of total cost and impact.
Yan and co-workers have devised a sustainable product conceptualization system (SPCS) as a framework for sustainable design [42]. The system first uses a design knowledge hierarchy to break a potential product down by category, then component, and finally part and part option. This hierarchy is then used in conjunction with the initial design criteria (e.g., materials, manufacturing feasibility, marketability, distribution) as specified by the product developers to generate a Hopefield network that can be solved to identify the best design options based on limited and preliminary data that may contain varying degrees of uncertainty. The best alternatives are then subjected to a more rigorous evaluation of sustainability using environmental and cost objectives specified by higher-level decision makers. The sustainability results are then used by decision makers to select the most feasible product design. This approach is interesting because it attempts to divide the decision making responsibilities among the various stakeholders and allow “experts” to make the necessary decisions at each level. So chemists and engineers can handle the selection of materials and processes while business managers can evaluate the acceptable trade-offs between product performance and environmental and economic impact.
Lapkin and co-workers address sustainable design through the use of a hierarchy ranking scheme to organize selected indicators [18]. The first indicator level is for products and processes and includes green chemistry metrics and energy usage as defined by process chemists and engineers. The next level describes company criteria set by business managers. It can include added process/product value based on gains in utility usage, environmental impact, and product safety. The third level examines infrastructure indicators based on environmental and energy requirements set by governments. The final society level indicators examine the total sustainability of a product or process based on the needs of end users and the general public. This approach allows the boundaries for analysis to be successively widened at each level and provides a way to integrate the technical, economical, and societal concerns of the various stakeholders in an efficient manner. The solution of the resulting multi-optimization problem can be accomplished using any of the techniques outlined above.
Not all process design will involve new processes. GREENSCOPE and Sustain-Pro by Carvalho and co-workers were created to evaluate process retrofits. Sustain-Pro, an Excel-based software, can be used to retrofit existing processes for a more sustainable operation [20]. The methodology consists of six steps: (1) steady-state data collection; (2) flowsheet decomposition to understand material and energy interactions among the various units within the system; (3) calculation of safety, environmental, and cost indicators; (4) sensitivity analysis to optimize indicators; (5) identification of design variables most affecting indicators; and (6) creation of alternative process steps that satisfy indicator targets. Much like GREENSCOPE, the benefit of this tool is its ability to identify the hot spots for the various indicators within a process, thereby focusing the redesign efforts. This approach captures the essence of green chemistry and green engineering principles by forcing the reevaluation of traditional process design practices to develop cheaper, more efficient processing strategies. They differ from other methodologies because the system boundaries are drawn around the process and its interaction with the environment, excluding the upstream and downstream implications of the process during its life cycle.
The various methodologies all attempt to provide the user a systematic approach to sustainable design. The difficulty of applying these tools will increase as the number of design parameters (metrics) increases. As with any decision making process, the ranking of criteria (weighting) will depend on the stakeholders and their decision criteria. The economic impact of green decisions can involve more than mere processing costs and will require a global assessment including potential upstream and downstream benefits to fully realize their influence.
Although many benefits can arise from the use of green chemistry and green engineering and design, the economic impact of such technologies is probably the most important from an industrial perspective. For this reason, a number of comprehensive technology assessments have been published which include detailed economic analyses. Some of these case studies were developed as examples of how to apply sustainable design methodologies described previously, while others have been performed using the traditional principles of process economics. Applications ranging from biofuels refining to traditional solvent processing have been addressed. Selected case studies will now be presented to offer the reader a better understanding of the potential economic impacts by applying green design.
The production of vinyl chloride monomer (VCM) from ethylene and chlorine is a common case study used when discussing sustainable design because of the large-scale use of VCM, mainly for the production of polyvinyl chloride (PVC).The key synthesis steps involve the formation of ethylene dichloride (EDC) followed by cracking to form VCM. Multiple undesirable by-products (carbon dioxide, trichloromethane, chloroethane, trichloroethane, and tetrachloroethane) are formed during the EDC synthesis process, while by-products hydrochloric acid and trichloroethane result from the hydrocracking process. Azapagic and co-workers apply a simplified case study to this example to demonstrate the application of their PDfS methodology [19]. This case study considers potential changes to traditional VCM production processes, including alternative feedstocks and changes to a key reaction step, and examines how to identify the most sustainable alternative early in the design process.In the end, the PDfS methodology found that economic sustainability is attainable using present technologies while environmental sustainability will require creation of new processing alternatives.
Carvalho and co-workers also attempted to redesign a typical VCM plant using their Sustain-Pro flowsheet to improve the sustainability [20]. The VCM process is first divided into the corresponding five sections and then analyzed using sustainability metrics to identify the areas of potential improvement. Once a hot spot is located, in this case the formation of EDC, alternatives are evaluated based on available data and used to find the most sustainable solution. These alternatives include inserting a recycle purge, improving existing separation units, inserting new separation processes, and improving reaction conversion. Of these, only the use of a new separation technology, membrane pervaporation, is found to increase the environmental sustainability of the process while maintaining the economic constraints. However, improving the reaction conversion is not considered because of a lack of feasible technologies.
Khan and co-workers applied their multicriteria GreenPro methodology and software to design a VCM plant [38]. Again, conventional processing alternatives are considered, including the use of air and an improved wastewater purification stripping process. A multicriteria model consisting of 125 environmental and economic constraints is solved for each alternative and compared to determine which is the most sustainable. Applying this approach, the use of air is both environmentally and economically favored. However, the optimal solution for all constraints is a trade-off between the optimal cost and environmental scenarios.
These examples do not draw heavily on green chemistry, but they still illustrate how sustainable design will lead to the need for applying the principles of green chemistry to achieve economically viable environmental solutions. After applying the three different methodologies, only marginal gains in sustainability for the VCM process can be achieved using conventional technologies. None of the alternatives put forth in these case studies looked at developing new benign catalytic pathways that increase the atom efficiencies or alternative synthesis pathways. Such an alternative could reduce the material and energy cost and consumption and result in a truly greener process. A better case for this point can be seen when considering the role of green design and solvents.
Ionic liquids (ILs) are an interesting development in the pursuit of green solvent technology. On the one hand, they offer a low vapor pressure, are nonflammable, and can dissolve a number of organic, inorganic, and polymeric materials. These attributes can lead to reduced emissions, improved reaction kinetics, and cheaper solvent recycling for chemical processes, all which are attributes of green chemistry. On the other hand, typical IL synthesis involves alkylation of a suitable organic compound followed by anion exchange. Thus, the synthesis of these materials is costly because of a need for large quantities of organic solvents and energy-intensive processes to recover them. In addition to the adverse environmental impacts arising from their synthesis, many ILs have been shown to possess some level of toxicity, making them a questionable choice for sustainable design. For these reasons, Kralisch and co-workers have applied their ECO methodology to the synthesis of ILs to try to identify greener alternatives for the alkylation step based on varying the temperature, organic solvent, reactant concentration and molar ratio, and reaction time [40]. The optimized solution involved a moderate-temperature, solvent-free synthesis, which reduced the EF by 78%, EHF by 98%, and CF by 87%. These improvements can be associated to the proposed green processes involving ILs to help offset the potential toxic risks the ILs themselves may present. In a more fundamental sense, these improvements provide a clear example of how the principles of green chemistry can lead to enhanced economic benefits. Interestingly, this example also suggests that there will always be room for improvement in green processes until true sustainability is achieved.
ILs are just one type of solvent system which have shown promise for green processing. Dunn and Savage examine the use of high-temperature water (HTW) during the synthesis of terephthalic acid, the precursor for polyethylene terephthalate (PET) used in injection-molded plastics [43]. Commercially, terephthalic acid is produced via the catalytic reaction of p-xylene with oxygen, and a bromide initiator in an acetic acid solvent in the presence of a Co-based catalyst. The reaction is carried out at high temperature and pressure, uses large amounts of solvent, and involves the formation of several undesirable by-products, including methyl bromide. Further complicating the process, one of the by-products, 4-carboxybenzaldehyde, prevents polymerization to PET and requires intensive downstream processing steps to separate. In addition, the azeotropic separation of acetic acid from the by-product water requires large quantities of energy. HTW is a promising solvent alternative because it is has minimal environmental impact, offers tunability based on the adjustability of its properties, and is relatively cheap. Its use for the proposed synthesis will provide a completely recyclable solvent requiring no azeotropic separation and eliminate the formation of hazardous by-products such as methyl bromide. However, these environmental benefits will only be a viable option if the process economics are favorable. Dunn and Savage have simulated four alternative HTW processes and assessed their economic and environmental impact based on capital cost and the sustainability metrics for energy intensity and pollutant intensity. Environmentally, the results show that the use of HTW reduces the associated environmental footprint, provided the water can be recycled with little or no makeup volume needed. Economically, the HTW process has roughly the same capital cost as the current acetic acid process, making it appear to be a viable alternative. An evaluation of operating costs is needed to fully understand the economic impact. Dunn and Savage concede that the operating conditions necessary for the HTW process (300–380 o C, 150–250 bar) could result in significant operating costs because of larger energy demands. However, they neglect to consider the potential cost savings arising from factors such as reduced waste management or reduced worker protection costs and insurance when making this point. Also, no comparison of productivity (conversion/yield and processing time) for the HTW and conventional processes is given, which will greatly impact the profitability of the processing plant. These factors are important when trying to understand and assess the economic impact of green chemistry and design.
Perhaps the most recognized application of green chemistry and design is the biorefining industry. As such, extensive work has been published examining the benefits associated with the conversion of renewable feedstocks into fuels and chemicals. For example, Zhang and co-workers have evaluated the acid-catalyzed transesterification of waste cooking oil into biodiesel fuel [44]. As opposed to petroleum-based diesel, biodiesel is renewable, has a low-impact emission profile, and is biodegradable. It is typically produced via the transesterification of triacylglycerols in virgin vegetable oils and animal fats using alkali catalyst in the presence of methanol. This alkali-catalyzed process has a much faster reaction rate than the acid-catalyzed mechanism, but is sensitive to the presence of free fatty acids in the feedstock and requires pretreatment of the feed if low-grade feedstocks (i.e., waste oil) are used.Thus, biodiesel production using alkali-based catalysis is limited commercially because the high cost associated with obtaining the raw feedstock (70–95% of the total production cost) translates to noncompetitive product pricing and with biodiesel costing up to one and a half (1.5) times that of petroleum-based diesel. The ability to use cheaper feedstocks such as waste cooking oil could greatly increase the economic viability of biodiesel (concept of materials and waste management) due to the price of waste cooking oil being two to three times cheaper than virgin materials. Also, additional cost savings could be realized by avoiding costly pretreatment of the feed to control the fatty acid content. With this in mind, Zhang and co-workers conducted their assessment of utilizing the acid-catalyzed process. The economic analysis included fixed capital cost, total manufacturing cost, after-tax rate of return, and the break-even price of biodiesel. On the basis of capital cost, alkali-catalyzed plants are cheaper. However, the high price of feed materials greatly offsets this advantage through elevated manufacturing costs. Ultimately, the break-even biodiesel price is reduced from US$857/tonne for the alkali-catalyzed plant to US$644/tonne for the acid-catalyzed alterative. The reduced break-even price is still more than the US$500–600/tonne of petroleum diesel, but close enough that the potential environmental benefits could offset this minor economic disadvantage. This is another example of how green chemistry and design can lead to environmental gains without disrupting the economic flow of society.
Biodiesel represents only one area of interest in a much larger market of biofuels and biochemicals. Henrich and co-workers have studied the economics for converting biomass into liquid synthetic fuels in a manner analogous to coal-to-liquid (CTL) and gas-to-liquid (GTL) technologies [45]. Such technologies first convert a carbon source into syngas (CO/H2 mixture), which can then be reformed into a number of desired hydrocarbon-based products (fuels, solvents, plastics). For biomass-to-liquid (BTL) conversion, this technology offers a renewable, clean alternative to petroleum. Potential drawbacks include energy-intensive processing supply issues for the feedstock based on the rather large quantity of solid biomass that would need to be transported to supply daily biorefinery demands. The latter issue can be resolved if lignocellulosic biomass (wood, straw, etc.) is used. This is because these materials can be pretreated locally using pyrolysis and converted to a suitable liquid form that is much easier to transport in bulk quantities. However, the issue of energy usage makes this technology economically viable only for plant sizes where product throughput is high enough to overcome operating costs. This is a good example for illustrating how traditional economic factors may not capture the full benefits of green chemistry and design. For example, a technology that is not reliant upon petroleum has the potential for tremendous savings in the future given the uncertainty and volatility of the crude market. In addition, it is possible that tax incentives and other financial benefits will be made available to companies offering “cleaner” fuels and services as societal concerns for environmental issues escalate. This really illustrates the greater point that economic assessment of sustainable technologies needs to be expanded to the national or global level to gain a better understanding.
High operational costs are a prevailing theme with biochemical production, prompting researchers to look for ways to increase both the profitability and sustainability of biorefineries. Eckert and co-workers undertake this problem by looking at the recovery of additional high-value products using a novel solvent extraction process [46]. During bioethanol fermentation, as much as 40% of the biomass is unconverted and typically burned as fuel for its heat value. However, the chemicals that make up this fraction are considered high value and could provide additional profit for a biorefinery. Some of the more prominent chemicals include vanillin and syringol, which are used in food and fragrances and syringaldehyde used in dyes and cancer pharmaceuticals. These materials can sell for anywhere from $5 to $25 per pound. Eckert and co-workers have demonstrated the ability to recover these compounds in high purity using methanol containing dissolved CO2, a gas-expanded liquid. Gas-expanded solvents are relatively green and offer tunability based on the reaction conditions, content, and properties of the gas. For vanillin and syringaldehyde, this simple extraction can replace the multistep syntheses, often involving toxic chemicals, used in the past. Syringol has not been synthesized commercially. This added option to recover these high-value chemicals contributes to the economic and technologic viability of biorefining. Discussions about the use of process intensification and green solvent technology to enhance the economic benefits of biorefining are readily available [47, 48].
A growing application for green chemistry that can provide insight about its benefits is the manufacture of pharmaceuticals. For example, Steffens and co-workers have applied a multicriteria design methodology to the production of penicillin to find a more sustainable alternative [49]. Classic penicillin production involves fermentation, filtration, solvent extraction, crystallization, and drying. There are known issues with this process, including significant water usage, generation of large quantities of solid waste, and discharge of an extraction solvent, butyl acetate, in the process wastewater stream. The applied design methodology combines the total annualized plant cost, LCA results, and sustainable process index (SPI) to optimize and improve the classic penicillin process. The resulting best set of optimized solutions require only 50 kg of water per year represented as critical water mass index (CTWM), a yield and SPI between 2 and 3.5 × 107 m2/y, and an annualized cost savings between US$1.5 and US$1.8 M, at the time the study was completed. The reduced environmental impact observed is the result of a process substitution that uses filtration in place of the organic solvent-based extraction steps. No improvement to the solid waste generation is possible unless new methods for penicillin fermentation are developed. In the end, this example required no radical changes to realize the benefits of green chemistry and design. Instead, it illustrates the benefit of using sustainable design practices early in the design process.
A final application worth mentioning is the use of modular process intensification. Lier and Grünewald have performed a net present value (NPV) analysis to compare the performance of a modular chemical plant with a conventional large-scale plant of the same capacity [50]. The NPV calculations found the modular plant was able to achieve profitability quicker than the large-scale plant because it can be set up and made operational in a much shorter period of time. The other advantages of modular plants are reduced R&D time and cost, configurability to control output (avoid overproduction), staggered module construction, faster installation, reduced operating costs, and a shorter time to reach profitability.
The focus of this chapter has been to provide a better understanding of how green chemistry and green engineering should be construed within the economic structure of chemical manufacturing. The benefits of green technologies are only fully realized by considering economic factors at the microscale, corporate scale, and macroscale. This knowledge will help corporate decision makers embrace green chemistry and green engineering during the product or process development phase by transcending the misguided conventional belief that investment in environmental technologies cannot support economic growth and profitability. The key challenge facing deployment of green technologies is the risk of investment in R&D given the changing societal preferences regarding sustainability. Given the holistic view that must be adopted to best understand the benefits of green chemistry and green engineering in achieving sustainability, chemical assessment must transition from traditional cost–benefit and NPV calculations to larger sustainability assessments. Several methodologies and subsequent software packages have been developed with the goal of guiding decision makers through the process of multicriteria decision support. The successful evaluation of these tools using real-world examples illustrates the value of green chemistry and green engineering when pursuing sustainable development.
1. Anastas, P. T.; Warner, J. C. Green Chemistry: Theory and Practice, Oxford University Press, Oxford, 1998.
2. Anastas, P. T.; Zimmerman, J. B. Environ. Sci. Technol., 2003, 37, 94A–101A.
3. U.N. GAOR, Agenda Item 21. In 46th Session, 1992; p UN Doc A/Conf.151/26.
4. U.S. EPA. EPA Mission Statement. Available at http://www.epa.gov/aboutepa/whatwedo.html, last (accessed September 10, 2012).
5. Huisman, G. H.; Van Rens, G. L. M. A.; De Lathouder, H.; Cornelissen, R. L. Biomass and Bioenergy, 2011, 35, S155–S166.
6. Pacheco, D. F.; Dean, T. J.; Payne, D. S. J. Business Venturing, 2010, 25, 464–480.
7. Murovec, N.; Erker, R. S.; Prodan, I. J. Clean Production, 2012, 37, 265–277.
8. American Wood Protection Association (AWPA). Available at www.awpa.com (accessed september 10, 2012).
9. Matus, K. J. M.; Xiao, X.; Zimmerman, J. B. J. Cleaner Production, 2012, 32, 193–203.
10. BCC Research. Market Research Report — Rare Earths: Worldwide Markets, Applications, Technologies, 2009.
11. Ruiz-Mercado, G. J.; Gonzalez, M. A.; Smith, R. L. Expanding GREENSCOPE beyond the gate: a green chemistry and life-cycle perspective, Clean Technology & Environmental Policy, 2014, DOI 10.1007/s10098-012-0533-y.
12. Pike Research. Green chemistry: biobased chemicals, renewable feedstocks, green polymers, less-toxic alternative chemical formulations, and the foundations of a sustainable chemical industry (Executive Summary Reprint), Ind. Biotechnol., 2011, 7, 431–433.
13. Iles, A. Bus. Strat.Environ., 2008, 17, 524–535.
14. Harmsen, J. Chem. Eng. Process., 2010, 49, 70–73.
15. Geng, Y.; Fu, J.; Sarkis, J.; Xue, B. J. Cleaner Production, 2012, 23, 216–224.
16. Walmart. Available at www.walmart.com, (accessed September 10, 2012).
17. The Sustainability Consortium (TSC). Available at www.sustainabilityconsortium.org (accessed September 10, 2012).
18. Lapkin, A; Joyce, L.; Crittenden, B. Environ. Sci. Technol., 2004, 38, 5815–5823.
19. Azapagic, A.; Millington, A.; Collett, A. Chem. Eng. Res. and Des., 2006, 84, 439–452.
20. Carvalho, A.; Gani, R.; Matos, H. Process Safety and Environmental Protection, 2008, 86, 328–346.
21. Ruiz-Mercado, G. J.; Smith, R. L. Gonzalez, M. A. Ind. Eng. Chem. Res., 2012, 51, 2309–2328.
22. Constable, D. J. C.; Curzons, A. D.; Cunningham, V. L. Green Chemistry, 2002, 4, 521–527.
23. Bare, J. C.; Norris, G. A.; Pennington, D. W.; McKone, T. J. Ind. Ecol., 2002, 6, 49–78.
24. Baumann, H.; Tillman, A. The Hitch Hiker’s Guide to LCA: An Orientation in Life Cycle Assessment Methodology and Application, Studentlitteratur, Sweden, 2004.
25. Gani, R.; Jiménez-González, C.; Constable, D. J. C. Comp. Chem. Eng., 2005, 29, 1661–1676.
26. Folic, M.; Gani, R.; Jimenez-Gonzalez, C.; Constable, D. J. C. Chinese J. Chem. Eng., 2008, 16, 376–383.
27. Capello, C.; Fischer, U.; Hungerbuhler, K. Green Chem., 2007, 9, 927–934.
28. Andraos, J. Org. Proc. Res. Dev., 2009, 13, 161–185.
29. Halim, I.; Srinivasan, R. Chem. Eng. Res. Des., 2008, 86, 809–822.
30. Charpentier, J. Comput. Chem. Eng., 2009, 33, 936–946.
31. Charpentier, J. Chem. Eng. Res. Des., 2010, 88, 248–254.
32. Ruiz-Mercado, G. J.; Smith, R. L.; Gonzalez, M. A. Ind. Eng. Chem. Res., 2012, 51, 2329–2353.
33. Young, D. M.; Cabezas, H. Comput. Chem. Eng., 1999, 23, 1477–1491.
34. Diwekar, U. Resources, Conservation and Recycling, 2005, 44, 215–235.
35. Hugo, A.; Ciumei, C.; Buxton, A.; Pistikopoulos, E. N. Green Chemistry, 2004, 6, 407–417.
36. Li, C.; Zhang, X.; Zhang, S. Chem. Eng., Res. Des., 2006, 84, 1–8.
37. Li, C.; Zhang, X.; Zhang, S.; Suzuki, K. Chem. Eng. Res. Des., 2009, 87, 233–243.
38. Khan, F. I.; Natrajan, B. R.; Revathi, P. Journal of Loss Prevention in the Process Industries, 2001, 14, 307–328.
39. Khan, F. I.; Sadiq, R.; Husain, T. Environmental Modeling & Software, 2002, 17, 669–692.
40. Kralisch, D.; Reinhardt, D.; Kreisel, G. Green Chemistry, 2007, 9, 1308–1018.
41. Yu, Q.; Zhixian, H.; Zhiguo, Y. A. N. Chinese J. Chem. Eng., 2007, 15, 81–87.
42. Yan, W.; Chen, C.; Chang, W. Comput. Ind. Eng., 2009, 56, 1617–1626.
43. Dunn, J. B.; Savage, P. E. Green Chemistry, 2003, 5, 649–655.
44. Zhang, Y.; Dubé, M. A.; McLean, D. D.; Kates, M. Bioresource Technol., 2003, 90, 229–240.
45. Henrich, E.; Dahmen, N.; Dinjus, E. Biofuels, Bioproducts and Biorefining, 2009, 3, 28–41.
46. Eckert, C.; Liotta, C.; Ragauskas, A.; Hallett, J.; Kitchens, C.; Hill, E.; Draucker, L. Green Chemistry, 2007, 9, 545–548.
47. Cardona, C. A.; Sánchez, Ó. J. Bioresource Technol., 2007, 98, 2415–2457.
48. Gutiérrez, L. F.; Sánchez, Ó. J.; Cardona, C. A. Bioresource Technol., 2009, 100, 1227–1237.
49. Steffens, M. A.; Fraga, E. S.; Bogle, I. D. L. Comput. Chem. Eng., 1999, 23, 1455–1467.
50. Lier, S.; Grünewald, M. Chem. Eng. Technol., 2011, 34, 809–816.