The ancient Greek idea of atoms was very different from the one we have today. They suggested that the atoms of each different kind of matter had a shape and texture that matched the properties of the substance itself. They expected that water atoms would be round and smooth, while the atoms in rock would be hard and sharp or gritty.
Unlike Dalton, they never considered testing their atomic theory by observing and experimenting. That was not because atoms were too small to see (although they are). It was because testing ideas through observation, a cornerstone of modern science, had not yet become part of human culture. In fact, Greek philosophy took an opposite approach, valuing pure human thought above all else. Logical thinking and reason alone were considered sufficient to deduce the truth. Great thinkers such as Socrates, Aristotle, and Plato used their powerful minds and logic to deduce what they believed to be the truth about the world around them.
Aristotle was considered so brilliant that people would rarely question his ideas, even long after his death. For nearly two thousand years, people simply accepted Aristotle’s explanation that all the world’s matter was made of four elements: earth, air, fire, and water. The idea of atoms all but disappeared. Today we know that both Democritus and Aristotle were right in one way but wrong in another.
There is a limit to how small a piece of matter can be cut and still remain the same substance. Leucippus and Democritus were right about that. But since most substances are compounds, the smallest possible piece is a molecule instead of an atom. Furthermore, that piece is not indivisible. Molecules of compounds can be divided into atoms, which contain even smaller (subatomic) particles.
Aristotle’s concept of elements was correct, but not the ones he wrote about. The number of natural elements is nearly one hundred, and Aristotle’s four are not among them. Water is a compound of hydrogen and oxygen. Both earth and air are mixtures containing both elements and compounds. Fire is not matter— it is energy produced by a rapid chemical reaction.

Seventeenth-Century Image of Aristotle. This drawing in Thomas Stanley’s 1655 book, The History of Philosophy, illustrates the high esteem scholars of that period had for Aristotle and his ideas.
The Rise of Chemistry
Between ancient Greek natural philosophy and today’s atom-based chemistry came a practice called alchemy, in which people tried to make certain substances out of other substances. Most often, alchemists were searching for ways to turn less valuable metals into gold. We now know that the techniques they used–which often produced real chemical changes–were doomed to failure. Both ancient alchemy and modern chemistry can rearrange the way atoms are combined, but they can’t change one kind of atom (such as lead) into another (like gold).

An Alchemist at Work. In this circa 1660 engraving based on a painting by David Teniers the Younger, an alchemist is tending a flame as he attempts to turn a less valuable metal into gold.
Many of the most famous alchemists were frauds, but others developed a rudimentary knowledge of matter and techniques that they used to extract or purify many useful elements and compounds from natural minerals and ores. By the beginning of the eighteenth century, alchemy had become chemistry. Chemists of that time investigated many important phenomena in their laboratories including the behavior of gases at different pressures and temperatures, processes of combustion and corrosion, and the relationship between electricity and matter. None of these were fully understood, but the chemists collected a great deal of valuable information through systematic scientific measurement and study.
The turn of the nineteenth century marked a change in John Dalton’s life. He had been working in meteorology, but he realized that he could understand the weather better if he knew more about the gases of the air. So he changed his scientific focus to chemistry. It did not take long before he figured out that the old idea of atoms would explain many properties of gases and chemical processes. In 1810, Dalton published A New System of Chemical Philosophy, a book that revolutionized his new field.
Dalton’s “new system” began with the statement that all matter is made of atoms. He went on to explain that each element is made of a particular kind of atom, that all of its atoms are identical to each other, and that atoms join together to form compounds. This is always in small whole numbers; no fractions allowed. The properties of atoms, such as weight, distinguish one element from another.
Applying those simple rules, he determined the atomic weight of different elements. He set the atomic weight of hydrogen, the lightest element, at one unit, and he calculated the atomic weight of other atoms from that. He wasn’t always correct, but it was clear he was making progress. For instance, he knew that water had eight times the oxygen as hydrogen by weight. But he mistakenly assumed that a water molecule had one atom of each element, and therefore he concluded that the atomic weight of oxygen was eight. When later research showed that water molecules had two atoms of hydrogen for each oxygen atom, scientists corrected the atomic weight of oxygen to sixteen.
Organizing the Elements
Dalton had given chemistry a new basic vocabulary. Scientists everywhere spoke of elements and compounds, atoms and molecules, and, of course, they asked questions. How many elements are there, they wondered, and how could they classify the growing number?
Several properties provided hints of similarities and patterns among the elements. These included melting or boiling points, densities (how much each cubic centimeter weighs), the way one element combines with others, and the properties of the resulting compounds. Still, no one had successfully turned those hints into a classification scheme—until Russian chemist Dmitri Ivanovich Mendeleyev (1834–1907), a professor at St. Petersburg University, had a flash of insight in 1869.
Mendeleyev was a walking encyclopedia of the sixty-three known elements, with detailed knowledge of their properties. He decided to make a set of cards, one for each element, listing the known properties of each, and arranged them in order of increasing atomic weight. For three days and nights, he would lay the cards on his table, grouping and regrouping them, hoping to discover a classification scheme before leaving on a long-scheduled train trip to the countryside, where his family owned an estate. Just before the time came for him leave, the weary professor fell asleep and dreamed of playing solitaire with his deck of element cards.

Dalton’s Table of Atomic Symbols. John Dalton produced this table for a lecture at the Manchester Mechanic’s Institution on October 19, 1835. It includes symbols for individual elements and shows how they combine to form common compounds of as many as ten atoms. Note that he thought water, listed first among the compounds, had only one hydrogen atom for each oxygen atom.
How Do We Know that Water Is H2O?
Have you ever heard people call water “good old H-2-O”? That term comes from the chemical formula for water (H2O), which means it is a molecule formed from two hydrogen atoms and one oxygen atom. Scientists first discovered that and many other chemical formulas in experiments with combining gases.
French chemist Joseph Louis Gay-Lussac (1778–1850) was among the first to do so. In 1808, he described what happened when two gases reacted to form another gas. From performing numerous experiments, he found the volumes of the reacting gases at the same temperature and pressure were always in the ratio of simple whole numbers. For instance, when hydrogen burned in oxygen to form water, for every two cubic meters (21.6 cubic feet) of hydrogen burned, it took one cubic meter (10.8 cu. ft.) of oxygen. The result was two cubic meters (528 gallon) of water vapor. He proposed that discovery as a law of nature.

Joseph Louis Gay-Lussac. Gay-Lussac discovered that the volumes of combining gases and their compounds were always in small, whole-number ratios. That principle is now known as Gay-Lussac’s Law.
Italian scientist Amadeo Avogadro (1776–1856) read about Gay-Lussac’s proposed law of gases, and he tried to understand why nature behaved that way. He thought about the nature of gases in containers, and he envisioned lots of molecules colliding with each other and with the container walls. Because the molecules were so far apart, it didn’t matter what kind of molecules they were. In 1811, he published his conclusion that any gas having the same temperature and pressure and occupying the same volume had the same number of molecules—another law of nature for gases.
Combining the two laws, it became clear that Gay-Lussac’s law of whole-number volumes was also a law of whole number of atoms or molecules. Thus a molecule of water had to contain two hydrogen atoms and one oxygen atom. Other experiments showed that hydrogen molecules were two hydrogen atoms combined, or H2, and, likewise, oxygen molecules were O2. Therefore Gay-Lussac’s experiment showed this reaction:
2H2 + O2 → 2H2O
(2 hydrogen molecules and 1 oxygen molecule produce 2 molecules of water)

Dmitri Ivanovich Mendeleyev. Mendeleyev is considered one of the greatest chemists in history for his creation of the periodic table of the elements.
Awake on the train, Mendeleyev played element solitaire. By the time he arrived at his destination, an arrangement of elements in rows and columns had begun to take shape. With the elements ordered by increasing atomic weight (down the columns from top to bottom), he discovered that the horizontal rows of elements aligned themselves to match a chemical property known as valence. That property relates to the numbers of atoms of two combining elements. For example, the alkali metals—lithium, sodium, potassium, rubidium, and cesium—which all have a valence of +1, fell into alignment across one row. Likewise, the nonmetals called halogens— fluorine, chlorine, bromine, and iodine—with a valence of -1, lined up in another row. Because of its repeating pattern, Mendeleyev called this arrangement the periodic table of the elements. (Today, the usual arrangement of the periodic table is increasing atomic masses across rows, with the valences aligned in columns.)
The table was not perfect—most notably, it had gaps. But those did not bother Mendeleyev, who stated boldly that those would be filled by undiscovered elements. He was right. When those elements were discovered, their atomic weights, densities, and other properties were just as he predicted! But even though he predicted what the atomic weights would be, he had only a pattern but not a principle to explain those values. The full explanation would not come for several decades, because it required the discovery of particles of matter even smaller than an atom—including neutrons.
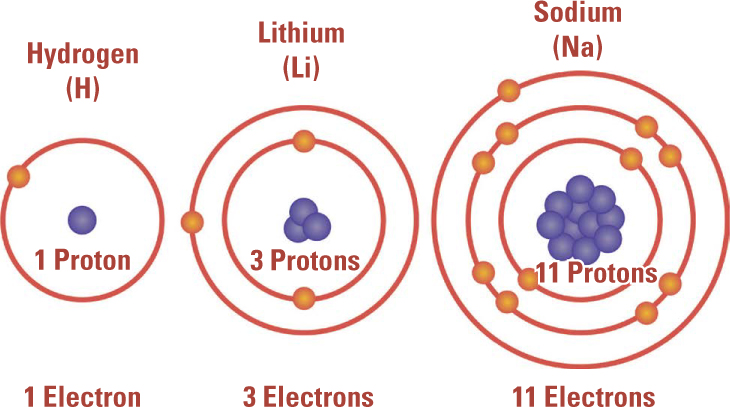
Three Atoms with the Same Valence. In the modern periodic table of the elements, the atoms in the same column have the same valence, a quantity that describes how they form compounds with other atoms. It took scientists more than fifty years and the discovery of subatomic particles to understand the physical basis of valence, which turns out to be related to the number of electrons outside of the atoms’ filled inner “shells.” Hydrogen, lithium, and sodium, as illustrated here, all have one valence electron.
Some Elements Discovered by 1869
Mendeleyev’s periodic table was built on many centuries of discovery. Here, in order of increasing atomic weight, are some of the elements he knew well.
Hydrogen (H): Discovered in 1766 by Henry Cavendish in London, England.
Lithium (Li): Discovered in 1817 by J. A. Arfwedson in Sweden.
Boron (B): Discovered in 1808 by J. L. Gay-Lussac and L. J. Thenard as well as Sir Humphry Davy.
Carbon (C): Discovered in prehistoric times.
Nitrogen (N): Discovered in 1772 by Daniel Rutherford in Edinburgh, Scotland, as well as in the early 1770s by Carl Wilhelm Scheele in Sweden, Henry Cavendish, and Joseph Priestly in England.
Oxygen (O): Discovered independently around 1772 by Carl Wilhelm Scheele in Sweden and 1774 by Joseph Priestley in England.
Aluminum (Al): Discovered in 1825 by Hans Christian Oersted in Denmark.
Calcium (Ca): Discovered in 1808 by Sir Humphry Davy in London, England.
Iron (Fe): Discovered by ancient civilizations.
Zinc (Zn): Known in India and China before 1500 and to the Greeks and Romans before 20 bce.

History on a Page. Mendeleyev’s periodic table in his own handwriting from 1869 listing the elements down the columns in order of increasing atomic weight, and across rows with atoms of the same valence.