The discovery and success of the periodic table led scientists to new questions, including these: What makes the periodic table periodic? What is responsible for the differences between the elements? The answer begins with this fact: depite the meaning of atomos, atoms are not indivisible. Science has revealed that they contain subatomic particles.
The first subatomic particle to be discovered was the electron. J. J. Thomson (1856—1940) of Cambridge University in England was studying an interesting electrical phenomenon in glass tubes from which most of the air had been removed. When scientists inserted pairs of electrodes into the tubes and connected them to the opposite ends of a source of electricity such as a battery, they observed a glow near the negative electrode, or cathode. They didn’t know what was causing the light, but they called it a cathode ray. Some scientists thought cathode rays were probably waves of energy; others said they were streams of particles.
In 1897, Thomson demonstrated that cathode rays were streams of the smallest particles ever known. He called them “corpuscles,” but today we call them electrons. By his measurements, a single corpuscle had less than a thousandth of the mass of the lightest atom—hydrogen. A more precise measurement later showed that it is even smaller, about 1/1800 as massive. Yet it had as much negative electrical charge as that atom might carry in positive charge.

Joseph John Thomson in 1900. J. J. Thomson was the first scientist to discover a subatomic particle, the electron, in 1897.

Ernest Rutherford. This picture shows Ernest Rutherford in 1908, the year he won the Nobel Prize in Chemistry for his work with radioactive materials. His greatest discovery lay ahead. In 1909, he and his students Hans Geiger and Ernest Marsden began experiments that led to the discovery of the atomic nucleus two years later.
It wasn’t long before Thomson and others recognized that electrons were contained within all atoms. Furthermore, they were involved not only in electrical phenomena but also in the relationships between electricity and chemistry—including valence. Since atoms are electrically neutral, they must contain an amount of positive electricity to balance the negatively charged electrons, and that positive charge had to carry most of the atom’s mass. But what was that positively charged subatomic matter, and what was the internal structure of atoms?
J. J. Thomson suggested a model of atoms that resembled a popular British dessert, plum pudding, with tiny electron plums scattered throughout a positively charged bulk. Thomson’s plum pudding atoms seemed sensible, but scientific models must be tested, and Ernest Rutherford (1871–1937) created a method: he probed the inside of atoms with radioactive beams.
Rutherford had left his native New Zealand in 1895 to study the recently discovered phenomenon of radioactivity at Cambridge in Thomson’s Cavendish Laboratory. By the time he left to become a professor at McGill University in Montreal, Canada, in 1898, he had discovered that radiation comes in two distinct forms: in the form of particles or in the form of waves. He named them “alpha rays” and “beta rays” after the first two letters of the Greek alphabet. At McGill, he and colleague Frederick Soddy (1877–1956) discovered a third form of radioactivity in 1902, which they designated “gamma rays.” They also discovered that alpha radiation was a stream of energetic positively charged particles, while beta radiation consisted of high-speed negatively charged particles.
In 1907, Rutherford returned to England, becoming a professor at the University of Manchester and bursting with ideas about how to use radioactive beams. He planned to begin by shooting alpha particles through thin metallic foils. He realized that observing the way the alpha particles deflected, or scattered, would reveal the arrangement of the atoms in the foil—their size and spacing, perhaps even their shape. He and his student Hans Geiger (1882–1945) developed an instrument to detect and count the alphas. They also demonstrated, as Rutherford had suspected, that alpha particles were helium atoms without their electrons.
By 1909, they were ready to begin alpha-scattering experiments. Nearly all the alphas passed straight through the foil or deflected only slightly. That result fit Thomson’s model, except for one puzzling result: the counters were very accurate, and a few alpha particles were missing. Rutherford and Geiger had measured alpha scattering in every direction that seemed likely, but now they had to consider unlikely directions far off to the side. Intrigued, but not wanting to divert Geiger from his main task, Rutherford turned to Ernest Marsden (1889–1970), a young student just learning the techniques of research. Marsden found the missing alpha particles. Astonishingly, not only had some alphas scattered far to the left or right of the original detector positions, but a few had even scattered backward.
Rutherford’s Lucky Break
Rutherford was a brilliant student and researcher, but he almost missed his chance to study and work with J. J. Thomson. In New Zealand, he had been studying how radio waves—then called Hertzian waves after German physicist Heinrich Hertz (1857-94), who first produced them—interacted with small magnetized needles. That led him to develop one of the first radio receivers, a major invention in the fast-growing field of telegraphy.
That success made him a strong candidate to receive a major research scholarship to study in England in 1895. Unfortunately, the scholarship committee ranked him second, behind a chemist. Since only one scholarship was awarded, it was offered to that chemist. Fortunately for Rutherford, that chemist had recently married and decided to stay in New Zealand. Rutherford was next in line, and he eagerly accepted the prize. His choice was Cambridge University’s Cavendish Laboratory, and the rest is history.
What did the result mean? Rutherford realized that the atom was very different than anyone had imagined up to that point. In 1911, he explained his results with a new atomic model. He envisioned atoms as miniature solar systems held together by the electric force instead of gravity. (Opposite electric charges attract each other.) He called the positively charged central body the nucleus. Rutherford noted that his results showed that the positive charge was concentrated in a region about 1/10,000 of the size of the whole atom. The rest of the atom was empty space, except for the lightweight electrons, which he visualized as being like planets in orbit around that minuscule but massive nuclear sun.
That structure explained why most alpha particles would pass through the foil with little scattering: They rarely came close enough to a nucleus to be deflected very much. Only on those rare occasions when a fast-moving alpha particle made a nearly direct hit on a much heavier nucleus would it scatter, and then it would be jolted so much that a sideways or even backward deflection would be possible.
Overweight Nuclei
The nuclear model of atoms was quickly accepted, but that immediately raised new questions. For example, what was in the nucleus? Rutherford performed more experiments with alpha particles. He also became convinced that the hydrogen nucleus was a basic subatomic particle. He first called those nuclei “H particles” but renamed them protons in 1917 when he detected them following alpha bombardment of boron, fluorine, sodium, aluminum, phosphorus, and nitrogen.
That discovery changed scientific thinking about the meaning of atomic number, which was at first defined as the number of an atom’s electrons. Since it was not difficult to turn most atoms into ions by adding or taking away an electron or two, atomic number was redefined as the total positive charge in the nucleus. Hydrogen, the simplest atom with atomic number one, has a nucleus with a single proton. Helium, with atomic number two, has two protons, and so forth. But things are not quite that simple when atomic weight— or atomic mass, the term physicists prefer—is added to the picture.
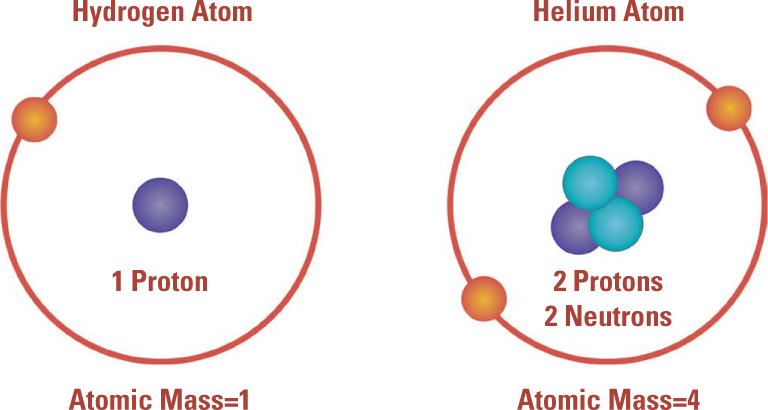
Extra Mass. Once Rutherford discovered the atomic nucleus, he ran into a different problem. The mass of larger nuclei grew faster than their electrical charge. For example, helium nuclei had twice the charge but four times the mass of hydrogen nuclei. Rutherford proposed that nuclei were made up of positively charged protons plus neutral particles of approximately the same mass as protons (later called neutrons), leading to the model shown here.
The atomic mass of hydrogen is one, and the hydrogen nucleus has one proton. But the helium nucleus (the alpha particle), which has two protons, carries an atomic mass of four. The discrepancy gets worse as atomic numbers increase. For atomic number 82 (lead) the atomic mass is approximately 207. (The atomic masses turn out not to be exact whole numbers, and that will be explained later.) Protons do not account for even half the mass of most nuclei. Are there more protons, or might other subatomic particles exist?
A Problem With the Planetary Model
The planetary model explained the results of Rutherford’s scattering experiments, yet it had one serious problem that came from the motion of the electrons. Whenever an electrically charged object is changing its velocity, either by changes in speed or direction of motion, the equations of electricity and magnetism state that it must radiate electromagnetic waves (such as light or radio waves), which carry energy. Orbiting electrons are always changing direction, which means that they should constantly lose energy. That would cause them to spiral inward toward the nucleus. But if that actually happened, every atom in the universe would have collapsed long ago.
Nature’s electromagnetic laws guided the orbits of electrons in atoms. Yet the same laws did not seem to be operating when it came to radiating energy when those electrons changed direction. Rutherford recognized the problem, but could not fully explain it. He could only guess that something was special about electrons in atoms. The planetary model of the atom was clearly imperfect or incomplete. Still, it was an excellent start to discovering the subatomic world.
Rutherford believed that the extra mass led to a different, and very important, question about nuclei: What held them together so tightly? Electrical force increases very rapidly as the separation of charged bodies decreases. Cutting the separation in half multiplies the force by four (two times two). At one-third the separation, the force is nine times as great (three times three). Since the nucleus is about 1/10,000 the size of an atom, and since two positive or two negative electric charges repel each other, two protons in the nucleus would push apart with a force millions of times as great as the attractive force between a proton and an orbiting electron. Such powerful forces would surely blow the nucleus apart—unless there was a stronger force within the nucleus to hold it together.

Holding the Nucleus Together. Rutherford thought his neutral particles did more than solve the mass problem. They might also serve as the glue to hold the protons of a nucleus together even though they repel each other electrically.
Rutherford realized that whatever gives the nucleus more mass is probably also responsible for holding it together. That led to his next important subatomic idea, the neutron, and to the discovery of two previously unknown forces within the nucleus.