Before Rutherford discovered the nucleus he was already famous. He won the 1908 Nobel Prize in Chemistry for his studies of radioactivity. He was responsible for discovering alpha and beta radiation and, with Frederick Soddy, gamma rays. They and other scientists soon learned how to identify each radioactive element by the energy of its alpha, beta, and gamma rays. Remarkably, radioactive decay achieved the kind of changes that alchemists had sought— transforming one element into another—a process that came to be called transmutation.
Rutherford and Soddy were particularly interested in tracking the elements from their original form to their new forms. Since they had not yet discovered the nucleus, they did not yet know that the chemical changes they were observing were the result of nuclear events. But they could describe the changes in terms of atomic mass and atomic number. For instance, when an atom emits an alpha particle, its atomic mass decreases by four units and its atomic number decreases by two. Likewise, the emission of a beta particle increases the atomic number by one unit but doesn’t change atomic mass.
Both emissions result in the transmutation of an atom of a “parent” element into an atom of a different element, the “daughter.” The new atom is frequently more radioactive than its predecessor, so there is a chain of alpha or beta decays from one atom to another to another, and so on. A gamma ray is pure energy, with neither mass nor electric charge, so the emission of a gamma does not cause transmutation. However, a radioactive atom emits a gamma ray only following an alpha or beta emission, so a gamma is a sign that transmutation has recently occurred.
Rutherford and Soddy studied different radioactive decay chains. They discovered in some cases that the daughter atoms in different chains behaved the same way chemically, which meant that they were the same elements. Yet they found that the two chemicals had different atomic masses. Soddy called such atoms with the same chemical behavior but different atomic masses “isotopes,” and he quickly realized that many nonradioactive atoms also had more than one isotopic form as well. To distinguish one isotope from another, scientists began denoting atoms by their chemical symbol and their atomic mass. For instance, natural chlorine is a mixture of two isotopes, Cl35 and the less common Cl37, with an average atomic mass of 35.47.
The Search for the Neutron
By 1920, the year after Rutherford had replaced the retiring J. J. Thomson as leader of the Cavendish Laboratory at Cambridge University, no one questioned that the hydrogen nucleus was a single proton or that alpha particles were helium nuclei with two protons and an atomic mass of four units. The mass that didn’t come from protons remained a puzzle.
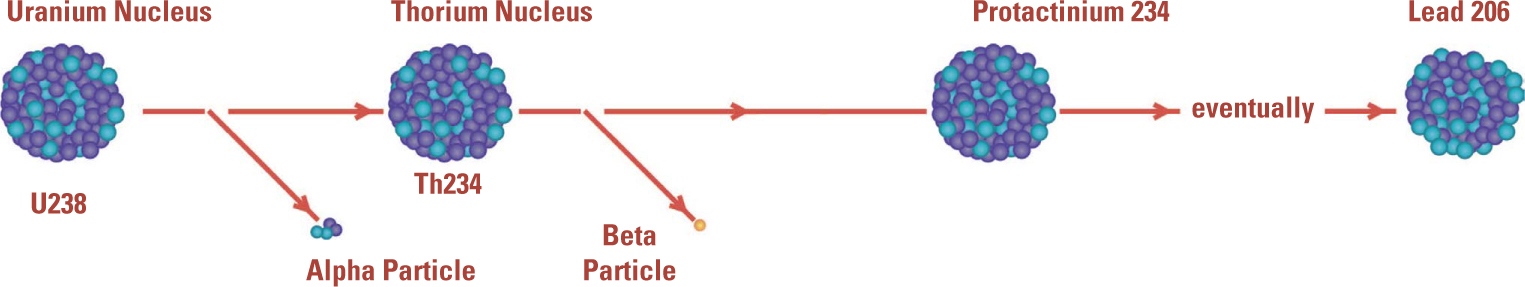
A Radioactive Series. Rutherford and Soddy discovered that one radioactive decay was often followed by another until the sequence led to a stable isotope. Although the atomic nucleus was not understood, this diagram shows their discovery of the sequence of nuclear changes that takes place over billions of years, transforming a uranium-238 nucleus to lead-206.
Some scientists suggested it was due to extra protons plus an equal number of electrons. Rutherford disagreed. His argument was that the nucleus “is so small that any electron inside it would experience a powerful electrical attraction to any proton, and the pair would immediately bind together.” The result would be an electrically neutral subatomic particle he called a neutron. Alpha particles, for example, consisted of two protons, each with one unit of positive electric charge, and two uncharged neutrons.
Beta Rays and Neutrinos
Rutherford’s explanation of beta rays was flawed. When he stated that beta particles came from the transformation of a neutron within the nucleus into a proton and an electron, he left out one very important piece of the puzzle.
When the nucleus of a particular radioactive isotope nucleus emits an alpha particle, that alpha always carries the same exact amount of energy. That allows scientists to identify the isotope by the energy of its alpha particle. The same is true of gamma rays. But beta rays are different. They can have any amount of energy from nearly zero up to a certain maximum.
That caused a problem, because the scientific principle of conservation of energy was never violated in any other circumstance. Energy could change form, but it was never gained or lost. The famous equation E=mc2 showed that mass was a form of energy. When a neutron transforms into a proton and an electron, the nucleus loses a small amount of mass that transforms into energy. The proton stays in place, but the electron leaves the nucleus as a beta particle and carries some of that energy with it.
Experiments showed that the energy the beta particle carried was never more than the lost mass. But it could be much less. To conserve energy, Wolfgang Pauli (1900–1958) proposed in 1930 that another particle was also emitted. That particle could not carry electric charge or have much mass, which makes it very hard to detect. He called it the neutrino, for “little neutral one.” The neutrino was a central part of a theory of beta decay published by Enrico Fermi (1901–1954) in 1933, and it was finally detected in 1956.
Enrico Fermi. One of the greatest physicists of the mid–twentieth century, Fermi developed a theory of beta decay that included the emission of a very light, electrically neutral particle, which he called the neutrino, meaning little neutral one in his native Italian.
Rutherford now explained that alpha radiation occurs when two protons and two neutrons come together within a large unstable nucleus and burst free. Likewise, he claimed that beta emission results from the splitting of a neutron in an unstable nucleus, further resulting in a proton and an electron. Since electrons are so light, the new nucleus would have approximately the same atomic mass, but the added proton would increase its atomic number by one. Rutherford was right about alpha emission and almost right about beta emission. Later research showed that every beta particle has a tiny partner, a subatomic sprite called a neutrino with no electric charge and so little mass that no one has succeeded in measuring it.
Of course, Rutherford knew that any theory, no matter how good, still required proof. So he set out to find neutrons, and that was no easy task. During the 1920s, a number of scientists developed instruments that enabled them to see the paths of subatomic particles. These devices depended on the interactions between the subatomic particles and matter, especially their ability to ionize gases that they passed through. That worked fine for charged particles such as protons and alphas, but not neutrons.
Finally in 1932, James Chadwick (1891–1974), one of Rutherford’s colleagues at the Cavendish Laboratory, discovered a way to detect neutrons indirectly but convincingly. In 1930, German researcher Walther Bothe (1891–1957) and his student Herbert Becker discovered that when a beam of particles bombarded beryllium metal, which had four protons and five neutrons in the nuclei of its naturally occurring isotope Be9, the result was a powerful beam of neutral radiation. They assumed these beams were gamma rays because of the ease with which they penetrated matter.
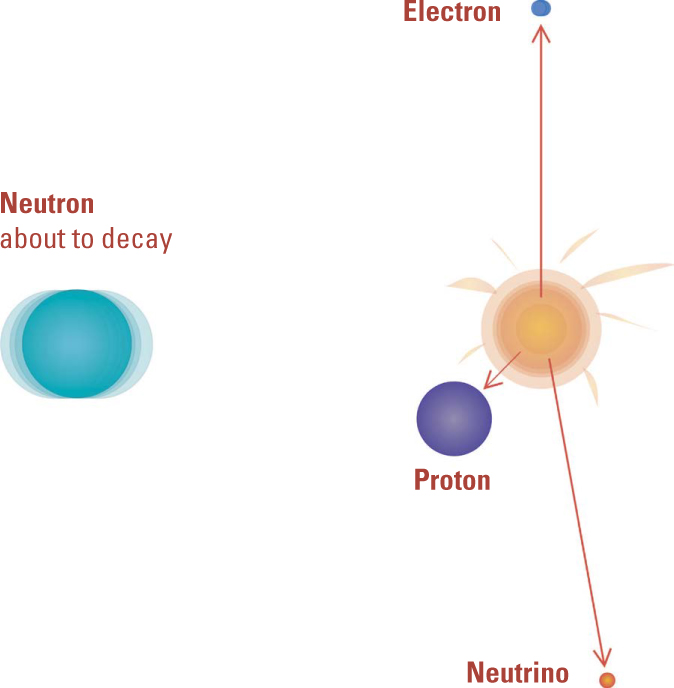
Beta Rays Explained. Once the neutron was discovered, physicists recognized that Rutherford’s explanation of beta decays by the transformation of a neutron into a proton and electron was on the right track—except for some missing energy. It took the addition of the neutrino to the theory to complete the picture.
Irene Curie (1897–1956), the daughter of the famous Pierre and Marie Curie, and her husband Frederic Joliot (1900–1958), later discovered that the neutral radiation would knock protons out of paraffin wax, which is rich in hydrogen. That was a surprising result for gamma rays, which could knock light electrons loose but had never been observed to eject heavier particles such as protons. When Chadwick heard of that result, he knew right away that the neutral beam had to be composed of neutrons.
Chadwick performed a series of experiments in which he allowed the beam to collide with a variety of gases. By measuring the scattering of the nuclei of those gas atoms, he was able to measure the mass of the particles in the beam, which turned out to be almost exactly the same mass as a proton, just as Rutherford had predicted for neutrons. That result established the basic atomic structure we now know: A tiny but massive nucleus of positively charged protons and electrically neutral neutrons, occupying only about a ten-thousandth of the atom’s diameter, surrounded by light electrons in equal number to the protons.
Neutrons and Nuclear Forces
At the time Rutherford was probing the atomic nucleus, two other revolutions in physics were also underway: quantum mechanics and relativity. Albert Einstein (1879–1955) had a hand in both. You have probably heard about Einstein’s famous equation E = mc2. This equation comes from his 1905 work on the theory of relativity and expresses the unexpected idea that mass (m) and energy (E) are two aspects of the same property of a physical system. Since they are measured in different units, we need a conversion factor to match them up, just as you might change a measurement in inches to centimeters by multiplying by 2.54. To convert mass to energy, you multiply by the speed of light (c) times itself (or squared).
The power of that simple equation shows up in radioactive decay. When a radioactive nucleus emits an alpha particle, you might expect sum of the mass of the alpha particle and the mass of the daughter nucleus to add up to mass of the parent nucleus. But that is not true. The total mass after the decay is less than the original mass. The missing mass, when converted to energy by Einstein’s famous equation, is exactly the amount of energy carried by the alpha particle.

Albert Einstein in 1919. This colorized photo shows the great physicist Albert Einstein at the height of his career. In 1905, he changed the way physicists viewed space and time, matter and energy, and the meaning of the speed of light with his special theory of relativity. That same year, his explanation of the photoelectric effect launched the quantum revolution.
Einstein solved another puzzle in 1905. The puzzle concerned the photoelectric effect, in which light could knock electrons free from metals—but only if its color was far enough toward the ultraviolet end of the spectrum. The color of the light was a measure of the frequency of light waves. That frequency had to reach a threshold before the light freed electrons.
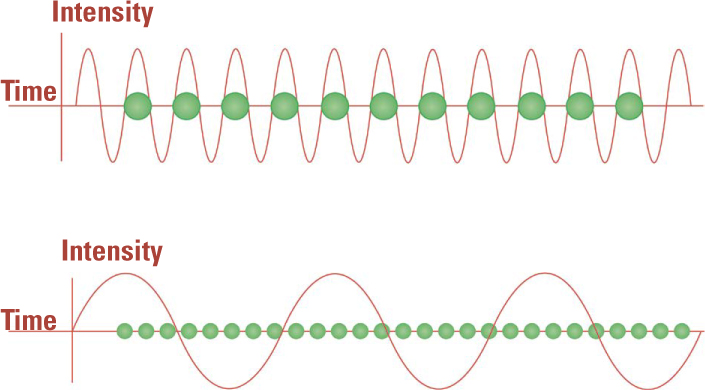
Trick or Treat? Planck considered his idea of light quanta—packets that contained an amount of energy equal to a constant multiplied by the frequency of the waves—to be nothing more than a mathematical trick. But Einstein’s analysis of the photoelectric effect showed that they were real. A single high-frequency light quantum from the dimmest light source had enough energy to knock an electron loose from a metal, but low frequency quanta from the brightest sources produced no electric current.
Einstein recognized a similarity between that threshold and an odd idea developed a few years earlier by Max Planck (1858–1947) in his calculations of the spectrum produced by a hot body. Planck’s equation depended on having light energy coming not in smooth waves like water, but in a stream of packets called quanta (singular, quantum). In that equation, the energy of a quantum was equal to a constant times the frequency of the light wave. That constant came to be called Planck’s constant.
Planck didn’t believe that quanta actually existed, but they made his calculations work. Einstein’s breakthrough was to recognize that the photoelectric effect was evidence that light quanta, which eventually came to be called photons, were real.
That was the beginning of a new field called quantum mechanics, which states that all subatomic particles are quanta, and, just like photons, they act like waves under certain circumstances and particles at other times. For example, electrons in atoms have certain wavelike states that are allowed, each corresponding to a certain energy level specified by four quantum numbers. The quantum numbers create a pattern as atoms increase in atomic number. Certain numbers of electrons act as filled “shells,” while the remaining electrons are available for atoms to interact. The pattern is the same one discovered by Mendeleyev. The table of elements is periodic because of quantum mechanics.
Quantum mechanics has been a spectacularly successful theory. It has changed the way physicists look at the basic forces of nature. Applying the laws of electromagnetism at the atomic level required a new mathematical approach called quantum electrodynamics, in which attraction and repulsion are the result of an interchange of photons between electrically charged quanta, such as electrons and protons— and that takes us back into the nucleus. Since protons repel one another by exchanging photons, what keeps the nucleus from blowing itself apart? Another force must act inside the nucleus, and it must have something to do with neutrons.
That force is called the strong nuclear force, or simply the strong force. (Another nuclear force is called the weak force, and it explains beta decay.) The strong force has unusual properties compared to electromagnetism. For example, despite its power within the nucleus, the strong force must have a short range, quickly becoming weaker than electromagnetic forces as you move away from the nucleus. Otherwise, nuclei of different atoms would be drawn together and the universe would be one giant atom. Yet within the nucleus, there must be a limit to the power of the strong force when particles get too close. Otherwise, nuclear matter would crush itself to nothingness.
Quantum Mechanics and Orbiting Electrons
Quantum Mechanics solved the problem of orbiting electrons in Rutherford’s model of the atom. Danish physicist Neils Bohr (1885–1962) took the first steps when he described a set of special electron orbits could exist without radiating electromagnetic waves. For those orbits, a physical quantity called the electron’s angular momentum had to be equal to a whole number times Planck’s constant. Bohr didn’t have an explanation for why those orbits were special, but they successfully predicted the spectrum of light produced when electricity made hydrogen gas glow.
Once the idea of wavelike properties of electrons came into being, it turned out that the circumference of each of Bohr’s allowed orbits was equal to a whole number of electron wavelengths. That is what made them special. Instead of orbiting, those electrons were like the standing waves of a violin string (which produces a fundamental tone and certain overtones, but no notes in between). Since the electron waves didn’t rotate, they could obey the laws of electromagnetism without radiating energy away. Rutherford’s problem had disappeared.
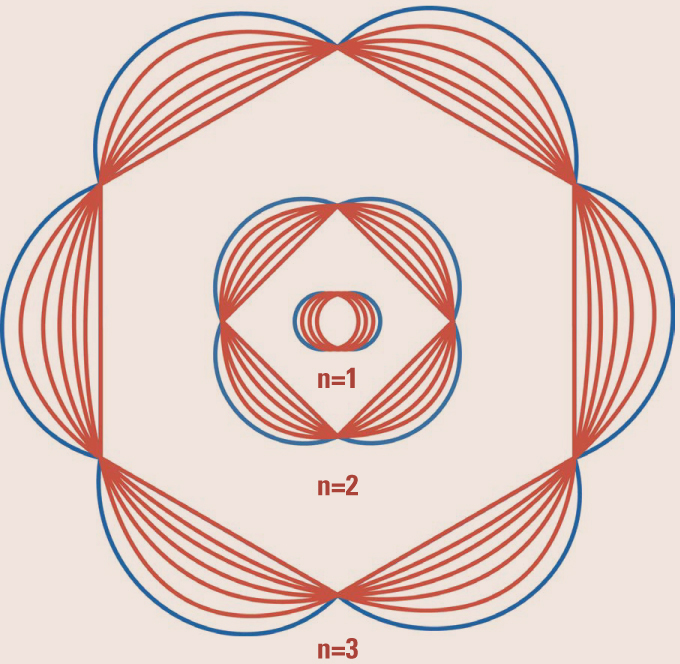
Electron Waves. In quantum mechanics, just as light waves can have particle-like properties, particles can have wavelike behavior. This drawing shows the three lowest energy levels of an electron in an atom. In the lowest level, the circumference of the orbit is one electron wavelength. The second level has a circumference of two electron wavelengths, and so on for higher energies.
In the quantum world, nuclear forces can be explained by a theory called quantum chromodynamics. It is similar to quantum electrodynamics, with a few differences. Chromo refers to the Greek word for “color,” but it has nothing to do with colors of light. Instead, physicists have borrowed the word to describe a property that nucleons—protons and neutrons—have through which they interact with the strong force, just as electric charge is the property that enables particles to interact electromagnetically. Instead of trading photons, nucleons attract each other by exchanging quanta called pi mesons, which have a mass of about 250 times that of an electron.
Because the strong nuclear force has such a short range, it does not always completely succeed in holding a very large nucleus together. Its nucleons are constantly rearranging, and they sometimes form smaller clusters that are more stable than the large nucleus itself. The clusters repel each other electrically, and the larger nucleus breaks apart.
Most commonly, one of the smaller clusters is an alpha particle, and the result is a familiar form of radioactivity. But in some large nuclei, the clusters are both medium sized nuclei, and the result is a process called nuclear fission that blows the nucleus to pieces and releases lots of energy. Remarkably, people have figured out ways to harness that fission energy. How that happens and what can be done with the energy is the main topic of this book’s final chapter.