28
The Wolf
When a person goes to a doctor, he or she starts with the symptoms. My throat hurts, my leg aches, I’ve got fever, there’s this rash.
The doctor starts with the symptoms too. The first question: What’s bothering you?
With much of disease, the medical questions then move into the cause of the symptoms. You have a cold, pneumonia, a virus or bacteria, a parasite, cancer.
The thing about autoimmunity is that the questions and answers sometimes don’t get any further than focusing on the symptoms. My joints ache, I have fever, I’ve got this rash, I have diarrhea, constipation, mind-numbing fatigue.
And the doctor says: I believe you, but I can’t find anything wrong.
Something is wrong, all right. But there is nothing to point to. There is no pathogen. There is no infection. There is no foreign disease.
No aspect of the immune system story is as pointed or pure as that of autoimmunity.
The mystery began with the werewolf.
As early 963 AD, one history notes, scientists observed an unusual condition that left people looking as if they’d been bitten by an animal. Hippocrates was the first to describe symptoms consistent with the skin disease, and Hebernus of Tours is thought to be the first to apply the term lupus to it. The word derives from the Latin word meaning wolf. Its sufferers showed sores, “ill-favored lesions,” and various other colorful descriptors—gnawing dermatosis—that I read in accounts of the medieval history of the illness. These “grotesque” lesions appeared on the face, lower limbs, all about. These symptoms—some caused by lupus and some not—were considered the product of a wolf bite and even a sign that someone had turned into a werewolf, according to the Lupus Endeavor, an advocacy project.
The vernacular and diagnosis were equaled in their primitive nature only by the treatment: “cutting away diseased tissue or burning it with caustic chemicals. These interventions rarely provided a cure, and patients suffered gradual disfigurement over decades,” reads a case history of lupus that appeared in 2016 in the vaunted medical journal The Lancet.
In 1872, the Vienna School of Medicine employed a doctor named Moritz Kaposi, who associated lupus with other conditions in the body, including arthritis. In the latter half of the nineteenth century, a Canadian physician, Sir William Osler, connected lupus lesions with even more conditions, including impacts on the heart, lung, and liver. Dr. Osler gets credit for the name systemic lupus erythematosus.
The key word here is systemic. The condition was not just about the skin. Something bigger was going on.
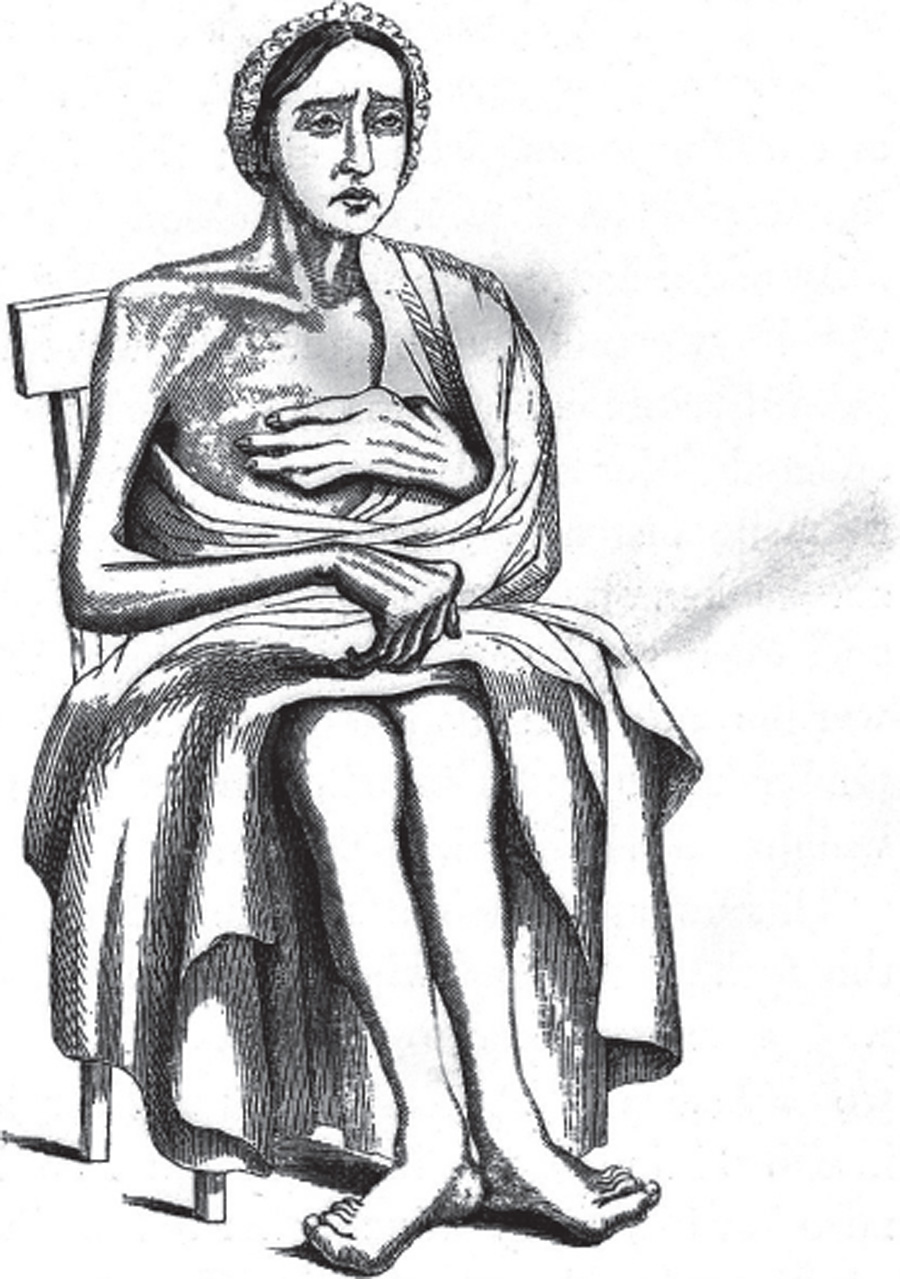
A nineteenth-century woodcut depiction of a woman suffering from arthritis, long before the agony of women like Linda and Merredith was taken seriously by the medical community. (Wellcome Collection)
On a parallel path, scientists had begun identifying and exploring an unusual condition that led to pain in the joints. In Paris in 1800, a doctoral student assessed nine patients and determined that the joint pain they were suffering was different from the overarching diagnosis of gout that many people suffered from. The student initially called it asthenic gout. Then in 1859, at University College Hospital in London, a pioneering doctor and researcher, Alfred Garrod, gave this condition its modern name: rheumatoid arthritis.
This was a disease characterized by inflammation, typically impacting the joints. Remember that inflammation is the body’s own response to disease. Inflammation is not “other.” It is “self.”
Did that mean the disease was caused by self?
The very idea that the body would attack itself was still relatively new. The pioneering immunologist Paul Ehrlich introduced the term horror autotoxicus right around 1900. Autoimmunity. The body attacking itself.
As immunology spun forward into the twentieth century—a relatively tiny community in a field thought by many to be a backwater—the people exploring these unusual inflammatory conditions were an even smaller subset. One research hub was the Mayo Clinic in Rochester, Minnesota. In 1926, according to a Mayo history, 574 patients were admitted to the rheumatology service with joint swelling and pain. The presumption was that the cause was chronic infection—something foreign was sparking it. This was, of course, wrong. Vaccines were tried. They led to serious side effects, even death.
Imagine: an overheated immune system getting a boost from medicine and vaccine.
Other patients were treated with “fever” treatments—meaning that fever was induced to try to reverse the symptoms, an effort to stop a mysterious condition by literally igniting the immune system.
Then, in 1929, there came a revelation.
Doctors who work on joint pain are called rheumatologists. A pioneering rheumatologist named Dr. Philip Hench, working at the Mayo Clinic, noticed an oddity with one particular rheumatoid arthritis patient. Her joint pain and stiffness seemed to get better when she developed acute jaundice. She got a disease and her joint pain got better, not worse.
The doctor also noticed that other rheumatology patients saw their symptoms recede after surgery and during pregnancy. Dr. Hench theorized that the patients under duress had secreted a compound that countered whatever was attacking their joints, explains a history in the journal Clinical Chemistry.
Dr. Hench had a hunch. When patients experience stress and are under duress, it typically means that they are secreting adrenaline. Dr. Hench theorized that joint pain and inflammation were being dulled by a secretion from the adrenal gland, a small, triangular-shaped nub located atop each kidney that produces essential hormones. Fueled by the hypothesis, Dr. Hench and a biochemist named Edward Calvin Kendall made one of the most important discoveries in the history of autoimmunity.
In an effort to discover the substance that had improved the condition of these Mayo patients, Kendall started trying to isolate secretions from the adrenal glands of cows. The biochemist took regular shipments of adrenal tissues from Chicago slaughterhouses, according to the history published in the journal Clinical Chemistry. The biochemist discovered a handful of hormones that were labeled with letters of the alphabet: A, B, C, etc. The one that changed science was called Compound E.
It was studied initially because it seemed relatively simple. It also made patients feel better, sometimes euphoric.
It took many years to refine and isolate. Then in 1948 at the Mayo Clinic, the very scientists who had begun working there in 1929 gave Compound E to a twenty-nine-year-old woman immobilized with severe rheumatoid arthritis. “Two days and two more injections later, the patient could walk and left the hospital to enjoy a three-hour shopping spree,” reads a recounting of the story published in the same 2010 scientific article.
“This startling result stunned people throughout the world,” another history recounts. The two Mayo researchers won the Nobel Prize for this in 1950.
You might know Compound E by a different name, cortisol. Cortisol is a steroid that suppresses the immune system. Steroids are the first line of defense against many autoimmune disorders. They are a mixed blessing, as you’ll see later on. For the moment, though, the discovery of steroids in the field of immunology and medicine was analogous to the discovery of a vaccine or antibiotic; they were a tremendous revelation, a response to a vexing problem, but a response that came without an understanding of the underlying mechanism of the disease they were meant to treat—autoimmunity.
As in so much of immunology, other major pieces began to fall into place in the late 1950s, as scientific technology improved. For instance, lupus researchers could now see that the condition involved a patient’s own immune cells eating away at free-floating material in the bone marrow. This was a double whammy of sorts. The bone marrow helps gestate and stimulate the immune system, and it was under attack by the very system it had helped spawn.
Another major break in the autoimmune mystery came in the late 1950s and 1960s from Dr. Henry George Kunkel, widely considered one of the pioneers in the field of autoimmunity. Dr. Kunkel spent his entire career working at the Rockefeller Institute in New York. His patient and research base there included women suffering from liver disease. Many of the women also had arthritis. This was thought to be largely coincidental; after all, arthritis can have many causes, including aging and repetitive physical stress. It is not always an autoimmune issue.
In studying these liver patients, Dr. Kunkel isolated some of the women’s antibodies—those large specialized molecules on cell surfaces that help our bodies target what to attack. Among the molecules he collected, Dr. Kunkel observed and isolated nineteen antibodies that did something quite disturbing. These antibodies, rather than picking up on and reacting to signals from foreign cells, reacted to the patient’s own white blood cells.
Now he understood rheumatoid arthritis. He’d found a key test to prove the body was attacking itself, using the very properties that other immunologists had begun to understand as essential to defending ourselves against invaders. It was a brilliant and essential insight.
In 1948, a related test was developed to probe for the presence of antinuclear antibodies. These antibodies can bind to the nucleus of a normal cell and had been shown to be present in virtually everyone with systemic lupus. (Complicating matters, the antibodies also appear in people who don’t have lupus, so initially, the test worked only about half the time; by the mid-1960s, the effectiveness of the test rose to 95 percent.)
Thus, at the dawn of the nuclear age, there were somewhat effective tests for only two of nearly a hundred known autoimmune disorders. And there was little in the way of treatment.
This was largely the state of affairs in the late 1960s when a patient in her forties came to Johns Hopkins suffering terrible joint pain, sobbing, trying to hold it together. Among others who tended the woman was a medical student named Bevra Hahn, who would go on to become a prominent specialist in this area.
The woman’s story captures the reality of autoimmunity during the period. Despite all the fantastic science by Dr. Kunkel and others, autoimmunity remained difficult, if not impossible, to diagnose and treat. This challenge was compounded by the sexist way that women were viewed in society at that time. When women complained—whether about physical or emotional duress—they were often deemed “hysterical.” Society could be quick to dismiss the work of women solely as caretakers of children and the home, employment deemed second class and not particularly taxing. In reality, this work could be brutal on the joints and compound the pain.
“Women had very defined roles. The husband never did the laundry. The husband never made a meal. Diapering a baby is really hard when your joints are swollen and painful,” Dr. Hahn explained. This patient, a white woman from a middle-class family, wore pants, not skirts, to hide swollen joints.
Dr. Hahn didn’t have much to give her. Steroids didn’t work. “All I had was aspirin and gold shots,” she explained. There was a theory, she told me, that compounds with gold in them could kill tuberculosis germs, and there was another theory that TB was related to autoimmunity. The treatment, as Dr. Hahn pointed out, “was very primitive.”
In 1975, Carolyn Wiener, a behavioral scientist at the University of California at San Francisco, wrote a research paper that captures the reality of living with autoimmunity. The article is painful to read. It gives shape to the emotional side of living with a disease, rheumatoid arthritis, that is difficult to diagnose, with “no cure available.”
The paper starts with a journal entry from a twenty-nine-year-old woman suffering from RA:
Being physically comfortable
And doing a simple chore
Can raise one’s spirits to
Levels of supreme joy.
Persistent pain and wretched
Tiredness brings one to
Near despair
In the next forty years, I
Wonder how many variations thereof
I shall experience.
“Rheumatoid arthritis patients learn, along with their diagnosis, that the disease is not only incurable but that its specific manifestations are unpredictable. As often as not, they hear the physician say, ‘You are going to have to learn to live with it,’” the paper reads.
Among the “self-doctoring” strategies that the paper describes for coping are “ingestion of celery juice, or massive doses of vitamin E or plastic bags filled with powered sulphur wrapped around the feet at night . . . a poultice of ginger root steeped in vodka and an alloy.”
Another tactic in the paper is referred to as “covering up.” Autoimmune sufferers would pretend they weren’t suffering, try to look as if nothing were wrong. It was a mixed blessing. Friends and family would then assume nothing was wrong and expect full activity from the afflicted.
I was privileged to hear the intimate medical and personal narratives of two autoimmune sufferers, Linda and Merredith—two of the stories I share in this book. Their stories also provide insight into some of the key factors that impact the balance of everyone’s immune system—namely, sleep, stress, hygiene, family history, and the ecosystem of our gut, known as the microbiome.
And they tell us about the fight of this ever-increasing group of patients to move out from the shadows.