Pennsylvania
118
Joseph Priestley House, Northumberland, PA
![]()
Oxygen, Soda Water, and More
The British clergyman and scientist Joseph Priestley is widely credited as the person who discovered oxygen. He also discovered the gases nitric oxide, nitrogen dioxide, nitrous oxide, hydrogen chloride, ammonia, sulphur dioxide, carbon monoxide, nitrogen, and silicon tetrafluoride. And he put the bubbles in soda water.
Prior to Priestley’s work, three “airs” were known: air (as we know it); carbon dioxide (called, at the time, fixed air); and hydrogen. Priestley had observed carbon dioxide in a brewery in Leeds, where it settled over the fermenting beer.
Carbon dioxide is a basic byproduct of fermentation. When yeast ferments, sugar is converted into ethanol and carbon dioxide. Priestley determined that bubbling “fixed air” through water resulted in bubbly water with a pleasant taste. Soda water is simply water mixed with carbonic acid (H2CO3), which is a combination of water (H2O) and carbon dioxide (CO2). It’s carbonic acid that gives soda water its characteristic “bite” when it mildly burns your tongue.
Priestley went on to develop a method of making soda water by mixing water, sulphuric acid (H2SO4), and chalk (calcium carbonate: CaCO3). The acid reacts with the chalk to produce carbon dioxide, calcium sulphate (CaSO4), and more water. The carbon dioxide is then forced through water to make carbonic acid (see Equation 118-1). Priestley described this process in his 1772 book Directions for Impregnating Water with Fixed Air.
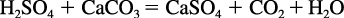
Equation 118-1. Sulphuric acid and chalk reaction
But his greatest success came in 1774 with the discovery of oxygen. Priestley used a lens to focus sunlight onto mercuric oxide (HgO), heating it to over 400°C, at which point it broke down into mercury and oxygen. He noted that the gas, which he termed dephlogisticated air, made candles burn brighter, that mice lived longer in a fixed quantity of the gas than in the same quantity of air, and that breathing the gas gave a pleasant feeling.
He called oxygen “dephlogisticated” air because he believed that it was air from which the “phlogiston” had been removed. This mythical element was, at the time, thought to be the cause of flammability in materials. It was believed that when things burned, phlogiston was released; Priestley could explain that oxygen prolonged burning because more phlogiston could be released than in ordinary air (which he assumed contained a quantity of phlogiston already). He hung on to the phlogiston theory even after the Frenchman Antoine Lavoisier had demonstrated that it was not true. (Lavoisier also gave names to hydrogen and oxygen, and demonstrated that humans breathe oxygen to live.)
Priestley was an English Dissenter, a minister who broke away from the Church of England, and a political theorist who strongly supported the French Revolution. In 1791, his opinions culminated in the Birmingham Riots in which his home, church, and other buildings were burned to the ground. In 1794 Priestley emigrated to the United States of America and made his home in Northumberland, Pennsylvania, where he died in 1804.
Today, the Joseph Priestley house is a museum to his life and work. Although his original books and equipment were dispersed when he died, the house has been restored to the state in which it would have been found when he lived there, and his laboratory has reproductions of his equipment.
From time to time, Heritage Days are organized at the house, with costumed performers playing the role of the Priestley family and demonstrations of Priestley’s experiments. At all other times, guided tours of the house bring to life Priestley’s chemical experiments and his time in the U.S.
The house is also a site of pilgrimage for the American Chemical Society, which uses it to commemorate special moments in chemical history.
Practical Information
The Joseph Priestley House’s website is at http://www.josephpriestleyhouse.org/.