Chapter 15
The Kidney and HIV
J. Booth and J. Connolly
UCL Centre for Nephrology, Royal Free Hospital, London, UK
Overview
Acute renal failure remains common in the HIV population, usually due to pre-renal causes, acute tubular necrosis or drug injury
Chronic kidney disease is a growing problem as patients with HIV live longer; causes are both specific to HIV e.g. HIV-associated nephropathy or more general e.g. hypertension
All patients with HIV should be screened for kidney disease by urinalysis for protein and measurement of estimated glomerular filtration rate
Antiretroviral drugs should be dosed according to renal function, and some, e.g. nucleotide reverse transcriptase inhibitors, have serious renal adverse effects that require monitoring
Haemodialysis and renal transplantation are now being used with increasing success in end-stage renal disease in HIV
Introduction
The dramatically improved patient survival afforded by combination antiretroviral therapy (cART) has served to unmask chronic kidney disease as a major determinant of health in the HIV population. Up to 30% of patients with HIV show evidence of chronic kidney disease (Table 15.1) with either proteinuria on urinalysis or an elevated serum creatinine, and these abnormalities are associated with an increased risk of death.
Table 15.1 Classification of chronic kidney disease according to estimated glomerular filtration rate (eGFR, mL/min/1.73 m2).
| 1 |
eGFR >90* |
|
Normal GFR |
| 2 |
eGFR 60–90* |
|
Mild renal impairment |
| 3 |
eGFR 30–60 |
|
Moderate renal impairment |
|
Frequently asymptomatic |
| 4 |
eGFR 15–30 |
|
Severe renal impairment |
|
Anaemia, bone disease, fluid, electrolyte and acid-base disturbances common |
| 5 |
eGFR <15 |
|
Established renal failure. Impending need for renal replacement therapy |
Effective treatment for HIV has also led to a shift in the spectrum of renal diseases affecting those with HIV, and while conditions such as HIV-associated nephropathy (HIVAN) remain important, new problems such as the renal toxicity of antiretroviral drugs have also emerged.
This chapter focuses on the important renal disorders encountered in HIV along with approaches to their diagnosis and management.
Evaluating renal disease in HIV
The finding of an elevated serum creatinine or abnormalities of urine dipstick are often the first pointers towards the presence of renal disease in HIV. A targeted history should follow to seek the presence of urinary symptoms, pain or oedema, relevant drug exposures, including cART, and pointers towards the presence of hypovolaemia (e.g. diarrhoea). Blood pressure should be checked and volume status assessed clinically.
Urine dipstick will detect the presence of protein, blood or glucose, whereas microscopy may show red cell casts, suggesting glomerulonephritis. Protein levels should be quantified by measuring a urinary protein–creatinine ratio, a spot urine measurement that correlates well with formal 24-hour measurements of protein excretion.
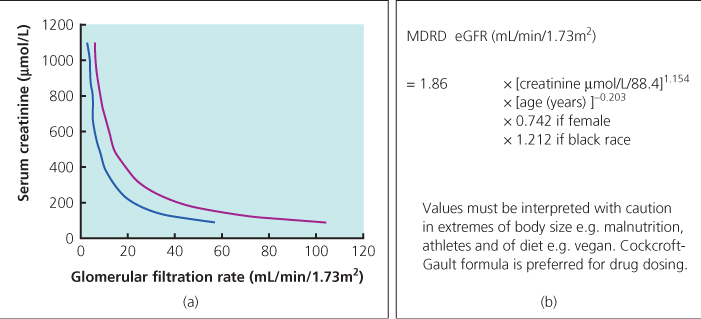
When taken alone, serum creatinine is a poor indicator of the absolute glomerular filtration rate (GFR) as baseline levels vary widely between patients of differing age, sex or race (Figure 15.1). Various equations have been developed that incorporate such variables to give a more accurate prediction of GFR (so-called estimated or eGFR) and the four-variable modification of diet in renal disease (MDRD) formula is preferred in the UK. Chronic kidney disease has recently been reclassified into five stages of severity on the basis of eGFR. Caution should be exercised when interpreting eGFR in patients with HIV in whom wide variation in muscle mass or the use or creatine or protein supplementation may make the MDRD equation inaccurate.
Ultrasound of the renal tract will exclude obstruction and may also point to specific pathology, for instance the enlarged echogenic kidneys often seen with HIVAN. Renal biopsy is frequently required to make a definitive diagnosis in cases where the results of non-invasive tests alone are insufficient.
Acute renal failure
Episodes of acute renal failure are common in the HIV population, the majority due either to pre-renal causes, such as hypovolaemia or severe sepsis, or intrinsic renal events. The latter include ischaemic acute tubular necrosis, drug injury and less common causes such as rhabdomyolysis and haemolytic-uraemic syndrome. Opportunistic infection is a common setting for acute renal failure in HIV, both due to severity of illness and the array of potentially nephrotoxic antimicrobials used in treatment (Table 15.2).
Table 15.2 Common nephrotoxins encountered in the care of patients with HIV.
| Antibiotics |
|
| Co-trimoxazole |
ATN, acute TIN, hyperkalaemia |
| Aminoglycosides e.g. gentamicin |
ATN |
| Sulfadiazine |
Crystalluria, haematuria and nephrolithiasis |
| Antivirals |
|
| Aciclovir |
Crystallisation with tubular obstruction |
| Foscarnet |
ATN, glomerulonephritis, RTA |
| Antifungals |
|
| Amphotericin |
ATN, proximal tubular dysfunction |
| Antiretrovirals |
|
| Indinavir, atazanavir |
Nephrolithiasis, chronic TIN |
| NtRTIs, e.g. tenofovir |
Proximal tubular dysfunction |
| NRTIs, e.g. didanosine |
Lactic acidosis |
Episodes of acute renal failure can generally be managed conservatively with appropriate volume resuscitation and removal of nephrotoxic agents. Treatment should also be directed at the underlying cause where necessary, e.g. opportunistic infection. Complications such as hyperkalaemia, acidosis and fluid overload should be monitored for and temporary recourse to renal replacement may be required in the most severe cases.
HIV-associated nephropathy
Rao and colleagues first described a syndrome of heavy proteinuria with rapidly progressive renal failure among the AIDS population of New York in 1984. Histological appearances, with focal and segmental glomerulosclerosis and marked interstitial nephritis, appeared to resemble heroin nephropathy, but many patients had no history of intravenous drug abuse. Subsequent case series emphasized the frequent and striking histological finding of collapse of the glomerular tuft. In the years to follow, HIV-1 has been intimately linked to the pathogenesis of this condition, which has become known as HIV-associated nephropathy (HIVAN).
HIVAN is the most common cause of end-stage renal disease in HIV, and is almost exclusively seen in people of Afro-Caribbean or Hispanic ethnicity. The prevalence within the African American HIV population is estimated at 3.5–12%. Classically considered a late manifestation of HIV infection, recent evidence suggests that HIVAN may also occur earlier in the course of infection when immune responses are well maintained.
Pathogenesis
HIVAN results from direct infection of renal tissue by the HIV virus although host factors play an important role in expression of disease. Infection of podocytes results in severe cellular dysregulation, with dedifferentiation and proliferation. Podocyte foot processes are lost, which culminates in collapse of the capillary loop producing the typical histological lesion. Animal studies have suggested an important role for the HIV accessory gene nef in initiating these events. Recent studies have confirmed a genetic association with MYH9 and APOL1 genes in African-Americans with HIVAN.
Clinical features
HIVAN is characterized clinically by nephrotic-range proteinuria and marked renal impairment. Despite significant hypoalbuminaemia, patients frequently have little oedema and are often normo- or hypotensive. Ultrasound of the renal tract often reveals enlarged, echogenic kidneys. Renal biopsy is required to confirm the diagnosis.
Histological findings are of focal and segmental glomerulosclerosis, mesangial hyperplasia and variable collapse of the glomerular tuft as the glomerular basement membrane pulls away from the visceral epithelium. Renal tubular atrophy with microcystic dilatation is also seen. Immunostaining of tissue is often negative and characteristic tubuloreticular inclusions are seen within glomerular endothelial cells on electron microscopy.
Treatment
Without intervention, HIVAN progresses rapidly towards end-stage renal disease over weeks to months. cART appears to prolong renal survival and forms the current backbone of treatment. The current recommendation is to commence cART at the time of HIVAN diagnosis, irrespective of the CD4 count or stage of disease. Maximal benefit is obtained if virus is kept persistently suppressed.
Blood pressure should be tightly controlled to a target of 125/75 using angiotensin converting enzyme inhibitors or angiotensin receptor blockers (ARBs). Older data from small non-randomized studies suggest corticosteroids may improve renal function and slow disease progression but this has not been confirmed and steroids are now rarely used.
Immune complex kidney disease
Immune complex-mediated glomerular diseases constitute as much as three-quarters of all chronic kidney disease in HIV. Patterns of disease include those unique to HIV, such as HIV immune complex kidney disease (HIVICK), and those which are also prevalent in the general population, e.g. membranous glomerulonephritis, IgA nephropathy. Immune complexes formed between antibody and HIV antigens such as p24 have been detected in the kidneys of HIV-infected patients with glomerulonephritis and are postulated to be pathogenic. Presenting features include proteinuria, haematuria and renal impairment, but diagnosis requires renal biopsy as differentiation from HIVAN is usually not possible on clinical grounds alone.
HIVICK describes a unique histological pattern of mesangial and subepithelial immune deposits that produce a characteristic ‘ball in cup’ appearance on light and electron microscopy. Immunofluorescence shows variable staining of deposits for IgA, IgM, IgG and C3.
Few organized clinical trials have addressed the treatment of immune complex disease in HIV. Small retrospective studies have produced conflicting data on the efficacy of cART in this setting. Most clinicians choose to initiate cART on diagnosis, as this seems rational if HIV accounts for the immune dysregulation causing the disease. Case reports support the efficacy of corticosteroids, but their use is limited by the increased risk of infection.
Antiretroviral drugs and the kidney
cART has revolutionized HIV care but at the expense of a broad range of adverse effects. Although many antiretrovirals have been linked in case reports to renal adverse events, it is the drugs indinavir, tenofovir and atazanavir that have received most attention. A large European cohort analysis showed that these three drugs were associated with an increased risk of developing chronic kidney disease for each year of exposure. Careful monitoring for renal toxicity is required for individuals taking HIV medication (Table 15.2).
Tenofovir
Tenofovir is an acyclic nucleotide reverse transcriptase inhibitor (NtRTI) and is excreted unchanged in the urine. Initial prospective trials with tenofovir showed no evidence of nephrotoxicity, but since becoming widely available in clinical practice more than 50 reports have been published linking tenofovir to the development of Fanconi syndrome.
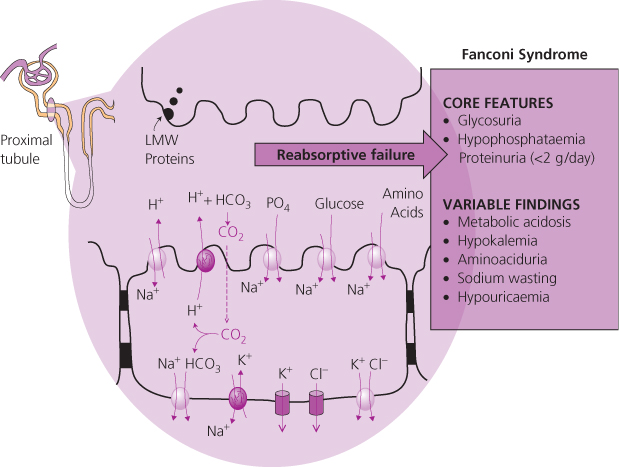
Fanconi syndrome is characterized by the triad of normoglycaemic glycosuria, hypophosphataemia and tubular proteinuria and is underpinned by a failure of reabsorptive mechanisms in the proximal tubule (Figure 15.2). Symptoms may include lethargy, polyuria, polydipsia and severe muscular weakness. Osteomalacia, on occasions severe, may develop as a result of prolonged hypophosphataemia. A rise in creatinine is an insensitive marker of the Fanconi syndrome and usually occurs late in disease.
The finding of glycosuria with a normal plasma glucose, hypophosphataemia and proteinuria (generally less than 2 g per 24 hours) in a patient receiving tenofovir are highly suggestive of Fanconi syndrome and should prompt discontinuation of the drug. Where necessary renal biopsy will exclude most alternative pathologies, and may show the typical tubular cell changes of thinning and vacuolation. The biochemical abnormalities associated with tenofovir toxicity usually resolve completely within 1–2 months after treatment is discontinued.
Indinavir
Nephrolithiasis has been reported to occur with all ritonavir-boosted protease inhibitors but none more so that indinavir. Indeed, it is the renal complications of indinavir which have led to it rarely being used in the UK.
Indinavir is excreted in the urine after metabolism by the liver, where it has a tendency to precipitate at pH values above 4.5 and form rectangular plate or fan-shaped crystals. Sludging of crystalline aggregates within the renal tract can lead to flank pain, haematuria and dysuria as well as classical renal colic. A provoking factor, such as dehydration is often present. Microscopic cystalluria is present in up to 65% of patients, but the majority remain asymptomatic. The stones are radiolucent and thus not visible on plain radiographs. Renal tract ultrasound or CT imaging is required for diagnosis.
Indinavir therapy has also been linked with insidious loss of renal function in the absence of urological symptoms or evidence of renal obstruction. Biopsy typically reveals a tubulointerstitial nephritis with marked fibrosis and evidence of crystals within the distal tubules. Improvement in renal function is variable after stopping treatment. This pattern of renal impairment appears to be associated with a persistent leucocyturia on urinalysis, and periodic monitoring of urine for white cells and serum creatinine is recommended in the first 6 months of indinavir treatment, with biannual checks thereafter.
Atazanavir
Cases of renal stones linked with atazanavir have been reported and the drug has been confirmed to be present in the stones using infrared spectroscopy.
Renal monitoring in HIV infection
Careful monitoring is important to detect renal toxicity at an early stage (Table 15.3). A baseline urinalysis, serum phosphate and renal function should be obtained at diagnosis and prior to starting treatment, and these parameters should then be checked regularly thereafter. More frequent monitoring is recommended if other risk factors for chronic kidney disease (CKD) are present or the patient is taking nephrotoxic drugs. Ideally, proteinuria should be assessed by formal measurement of urine protein creatinine ratio as dipstick analysis detects primarily albuminuria and is insensitive for the low molecular weight proteinuria characteristic of tenofovir toxicity.
Table 15.3 Recommendations for monitoring of patients receiving the anti-retroviral medications indinavir and tenofovir.
| Tenofovir |
Urinalysis (for glucose or protein), serum calcium, phosphate and creatinine pre-treatment |
|
Periodic (optimum frequency) checks for development of glycosuria, proteinuria, or hypophosphataemia; Fanconi syndrome may not develop until months or years after starting tenofovir. |
|
Increased surveillance if: |
|
Receiving a ritonavir-boosted protease inhibitor; pre-existing renal impairment; diabetic or hypertensive; receiving other potential nephrotoxic medications e.g. aciclovir; older age e.g. >50 years |
| Indinavir |
Urinalysis (for leucocytes and blood) and serum creatinine pre-treatment |
|
Periodic urinalysis and serum creatinine during first 6 months; twice per year thereafter |
|
Patients to drink 1.5L water per day to reduce chance of stone formation |
Renal replacement therapy
Survival rates for HIV-positive individuals with end-stage renal disease now rival the general population. Haemodialysis and peritoneal dialysis are both viable options for renal replacement in HIV, although the former tends to be preferred. Native arteriovenous fistulae are preferred to artificial grafts or tunnelled lines for haemodialysis access as the infection and thrombosis rates are significantly lower. Dialysis units operate strict infection control protocols, minimizing any risk of transmission to other patients.
Renal transplantation has the potential to transform the quality of life and improve survival of patients with end-stage renal disease but until recently was considered contraindicated in HIV due to the burden of exogenous immunosuppression required. Studies now suggest that with careful patient selection and planning of immunosuppression regimens, graft and patient survival rivals the general population for both cadaveric and live-related transplants. Patients must have an HIV viral load of <50 copies/mL for 6 months with good adherence to a stable antiretroviral regimen in order to be considered, with no active infections or AIDS-defining illnesses.
Antiretroviral dosing in the presence of CKD
Dose alteration of nucleoside and nucleotide reverse transcriptase therapy is required in the presence of mild to moderate CKD: no adjustment is usually required for other antiretroviral agents. Careful consideration of dosing of all HIV drugs is required during renal replacement therapy and the use of therapeutic drug monitoring may be required.
Further reading
EACS guidelines http://www.europeanaidsclinicalsociety.org/images/stories/EACS-Pdf/eacsguidelines-v6_english.pdf.
IDSA. Guidelines for the Management of CKD in HIV Infected Individuals. (www.idsociety.org/assets/0/18/312/924/D35A41AB-4B98-4314-A57D-0A3C339B719A.pdf).