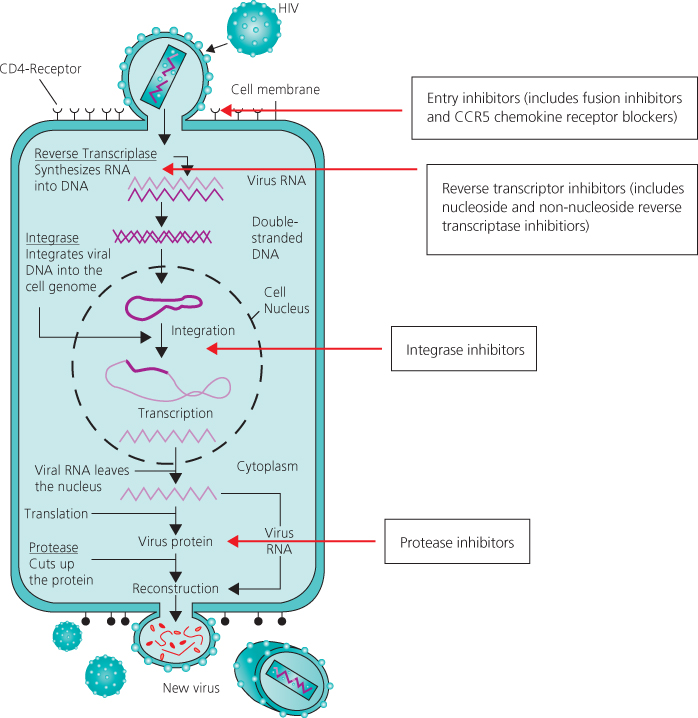
Figure 19.1 Targets for combination antiretroviral therapy in HIV life cycle.
Chapter 19
Antiretroviral Drugs
Overview
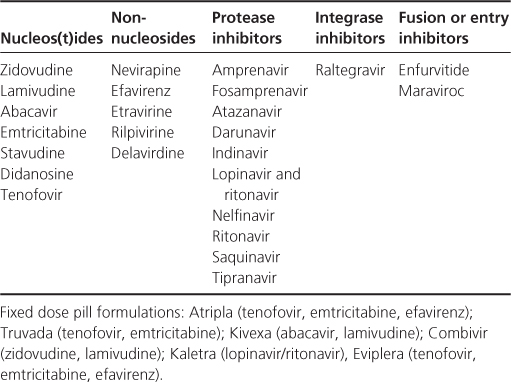
There are more than 25 different drugs within six classes which interfere with the HIV life cycle
First-line treatment should include three antiretroviral agents, which usually consists of two nucleoside/tide analogues and either a non-nucleoside or a protease inhibitor as the third agent. This is often referred to as highly active antiretroviral therapy (HAART) or combination antiretroviral therapy (cART).
Life-long continuous treatment is recommended, as intermittent therapy has been shown to be associated with increased rates of complications (HIV and non HIV)
Resistance testing is recommended prior to starting treatment due to the risk that patients may have acquired, or developed, drug-resistant virus
Co-formulations of drugs commonly used to treat HIV infection have helped to reduce pill burden and improve adherence
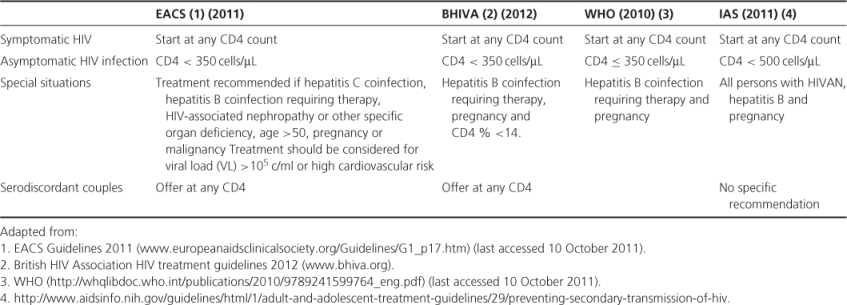
The choice, tolerability and clinical effectiveness of cART has improved markedly over the last decade. This has led to guidelines recommending earlier initiation of therapy
Since 1996 in the developed world there have been dramatic falls in the incidence of new AIDS cases and AIDS-associated deaths. Published data in the late 1990s estimated the mortality rate in patients with CD4 counts of less than 100 × 106/L had fallen by nearly two-thirds to <8 per 100 person years. Although the long-term clinical efficacy of the current antiretroviral treatment regimens remains uncertain, the biological rationale for maintaining a clinical response has been established. Sustained inhibition of viral replication results in partial reconstitution of the immune system in most patients, substantially reducing the risk of clinical disease progression and death. Strategies to sustain suppression of viral replication in the long term will be necessary. There are several potential targets for antiretroviral drugs in the viral replication cycle (Figure 19.1). Six classes of antiretroviral drugs are currently licensed for use in combination for the treatment of HIV infection. Once initiated, life-long continuous treatment is recommended as intermittent therapy has been shown to be associated with increased rates of HIV and non-HIV disease complications. New therapeutic agents and treatment strategies are constantly being evaluated.
Figure 19.1 Targets for combination antiretroviral therapy in HIV life cycle.

The first drugs made available for clinical use were inhibitors of the HIV reverse transcriptase. Before the virus can be integrated into the host cell genome DNA, a copy of the viral RNA has to be formed (proviral DNA). This is regulated by the specific HIV DNA polymerase: reverse transcriptase. If a DNA copy is not formed, the viral RNA genome becomes susceptible to destruction by cellular enzymes. The nucleoside reverse transcriptase inhibitors are both competitive inhibitors of reverse transcriptase and DNA chain terminators. The normal 2′ deoxynucleosides, which are substrates for DNA synthesis, link to form a chain by phosphodiester linkages bridging the 5′ and 3′ positions on the five-carbon sugar molecule. The 2′,3′-dideoxynucleoside analogues are formed by the replacement of the 3′-hydroxy group by an azido (zidovudine), hydrogen or other group. These nucleoside analogues as substrates will bind to the active site of the HIV reverse transcriptase and will be added to the growing HIV proviral DNA chain. However, once inserted, the normal 5′ to 3′ links will not occur, resulting in HIV proviral DNA chain termination. Genotypic mutations at various codons in the reverse transcriptase gene result in decreased susceptibility of HIV to inhibition by the nucleoside reverse transcriptase inhibitors. Several nucleoside reverse transcriptase inhibitors are currently licensed (Table 19.1).
Table 19.1 Antiretrovirals licensed for use in UK: 2011.
The primary mechanism for toxicities of NRTIs is via mitochondrial damage due to the incorporation of NRTIs into mitochondrial DNA by DNA polymerase. This can manifest clinically as peripheral neuropathy, myopathy, pancreatitis, hepatic steatosis and rarely lactic acidosis. Consequently, dideoxynucleosides are no longer recommended treatment options. Newer agents such as tenofovir and abacavir are thought to cause less mitochondrial damage. Thymidine analogues such as D4T and AZT are usually avoided as they have been shown to be the main cause of facial and limb fat loss (lipotrophy).
The use of abacavir should be limited to those who are HLA B*5701 negative as they are significantly less likely to experience a hypersensitivity reaction, which occurs most commonly within the first 6 weeks of therapy. Owing to recently published data, caution should be applied when prescribing abacavir in patients with a high cardiovascular risk and/or patients with a plasma viral load greater than 100 000 copies/mL.
The non-nucleoside reverse transcriptase inhibitors are a group of structurally diverse agents that bind to reverse transcriptase at a site distant to the active site resulting in conformational changes at the active site and inhibition of enzyme activity. These agents show high antiviral activity in vitro and have relatively low toxicity. They are also highly specific, inhibiting the reverse transcriptase of HIV-1 but not HIV-2. In monotherapy, rapid emergence of resistant strains associated with single point mutations of the reverse transcriptase gene, high level phenotypic resistance and loss of antiviral effect occur. The drugs therefore need to be combined with other antiretroviral agents, usually two nucleoside/tide reverse transcriptase inhibitors, to achieve and maintain an effective long-term treatment response. Rash and hepatitis can occur with all non-nucleoside therapy. A greater risk of hepatotoxicity has been reported with nevirapine, particularly in patients with higher CD4 counts. Vivid dreams, insomnia and other CNS side effects are well described with efavirenz.
The protease inhibitors (PIs) bind competitively to the substrate site of the viral protease. This enzyme is responsible for the post-translational processing and cleavage of a large structural core protein during budding from the infected cell. Inhibition results in the production of immature virus particles that are unable to infect other target cells. Pharmacokinetic enhancement of PIs by concomitant administration of low-dose ritonavir permits lower pill burden and a higher genetic barrier to the development of resistance. This is due to the ability of ritonavir to inhibit cytochrome P450-mediated metabolism of PIs. Metabolic complications such as insulin resistance, hyperlipidaemia and truncal fat accumulation (lipohypertrophy/lipodystrophy) are well described with protease inhibitors. Other important complications include an increased cardiovascular risk and nephrolithiasis.
The integration of the viral DNA into the host chromosome is achieved through a process of DNA splicing and recombination. First, two nucleotides are removed from each 3′-end of the viral DNA, a process termed 3′-end processing. In the second step, termed DNA strand transfer, the processed viral DNA ends are inserted or joined into the host DNA. The HIV-1 integrase catalyses these first two steps of integration. In the third step, cellular enzymes repair the single gaps in the DNA chain by removing the two unpaired nucleotides at the 5′-ends of the viral DNA. The integrase inhibitors raltegravir and elvitegravir both impede viral replication by inhibiting strand transfer. They are well tolerated and recent studies have confirmed similar efficacy to other first-line regimens with fewer potential drug–drug interactions as they are not metabolized via the cytochrome P450-mediated pathway. These properties have led to their inclusion as first-line therapy in recent guidelines.
Chemokine receptors CCR5 and CXCR4 act as co-receptors to facilitate HIV viral entry into host cells. HIV isolates can be divided into R5 and X4 strains depending on which co-receptor they use. The majority of strains utilise CCR5 but X4 using strains may develop in patients who have had a low CD4 count or longer duration of HIV infection. Resistance testing using tropism assays is recommended prior to initiating therapy (see Chapter 3).
Enfurvitide (T20) is a fusion inhibitor that prevents entry of HIV into the cell by binding to the viral protein gp41, preventing binding of gp120 to the CD4 receptor. T20 is administered twice daily by subcutaneous injection and its use is currently limited to those with multidrug-resistant HIV. T20’s main side effect is local injection site reactions.
Trials have shown that although treatment with cycles of the cytokine interleukin 2 results in substantial increase in CD4 counts, there is no clinical benefit when used with combination antiretroviral therapy (cART). Therapeutic vaccines have had similar disappointing results. Research is ongoing to develop drugs that can activate latent HIV which, if possible, offer the potential to eradicate HIV infection.
In the mid-1990s, several large clinical endpoint studies demonstrated a strong association between falls in plasma HIV RNA levels (plasma viral load) in the first few weeks on therapy and clinical outcome at 1 year. It is now accepted that falls in plasma viral load combined with increases in CD4 count are predictive of the clinical treatment response on different combination regimens at 1–2 years, although changes in the markers probably do not fully predict the observed clinical effect. Where possible an objective of cART is to reduce and sustain plasma viral load levels to below the level of detectability of the current ultrasensitive viral load assays (<50 copies/mL). If patients are adherent to therapy, the likelihood of a viral load rebound and drug resistance is minimal. Despite inhibition of viral replication in plasma, lymph nodes and at other sites, reservoirs of HIV infection in latently infected resting T lymphocytes remain. Continued activation of these cells will theoretically result in the reduction of this reservoir; however, new cells probably continue to be infected as a result of either localized small bursts of viral replication or loss of the antiretroviral effect of the treatment regimen. Even in patients who have sustained, undetectable levels of plasma viral load (<50 copies/mL) for 3 years or more, discontinuation of cART results in rapid rebound of plasma viral load to pre-treatment levels with an increased risk of HIV and non-HIV-related clinical disease. The optimal time to initiate therapy with the current antiretroviral drugs has not been established in clinical studies. CD4 count and plasma viral load are predictors of the estimated risk of progression to AIDS, which is a factor in determining when to start treatment. The motivation of a patient to start and adhere to therapy and the known effectiveness of current regimens are also important.
Clinical practice across Europe and North America varies, but most clinicians would consider initiating therapy at some point when the CD4 count is less than 500 × 106/L and in all patients who have symptomatic HIV disease. There is emerging data highlighting benefit of cART during primary HIV infection (PHI). UK guidelines (BHIVA, 2012) recommend initiating cART if PHI is severe (neurological involvement, the presence of an AIDS-defining illness, or a low CD4 count (<350 cells/µL). In addition, it is suggested that cART should be considered if the HIV diagnosis is made very soon after HIV acquisition (<12 weeks).
Table 19.2 When to start: summary.
Treatment of HIV has been shown to reduce HIV transmission. Specific situations where the initiation of cART may be considered for this purpose include PHI and serodiscordant couples.
Preferred first-line therapies cited in guidelines worldwide are varied and dependent on date of publication. Most guidelines recommend that first-line treatment should consist of three antiretroviral agents, which usually consists of two nucleoside/tides and either a non-nucleoside or a protease inhibitor as the third agent. This is often referred to as HAART or cART. Resistance testing is recommended prior to starting due to risk that patients may have acquired, or developed, drug-resistant virus. Co-formulations of drugs commonly used to treat HIV infection have helped to reduce pill burden and improve adherence.
There are various international antiretroviral treatment guidelines, the British HIV Association Guidelines for treatment of HIV-infected adults are among the most recent at the time of publication are summarized in Table 19.3.
Table 19.3 Initial combination regimen for antiretroviral-naïve patients.
| Select NRTI backbone | ||
| and either a boosted | ||
| protease inhibitor, | ||
| NNRTI or an integrase | ||
| inhibitor | Preferred | Alternative |
| NRTI backbone | Tenofovir & emtricitabine | Abacavir & lamivudine |
| 3rd Agent | Atazanavir/ritonavir
Darunavir/ritonavir Efavirenz Raltegravir |
Lopinavir/ritonavir
Nevirapine Rilpivirine Fosamprenavir/ritonavir |
BHIVA. Treatment Guidelines 2012. (www.bhiva.org).
EACS HIV online treatment guidelines. (www.europeanaidsclinicalsociety.org/Guidelines/index.htm).
Priority interventions HIV/AIDS prevention, treatment and care in the health sector (2010 version). (www.who.int/hiv/pub/guidelines/9789241500234/en/index.html).