2
Tribes
The Strange World of Granular Materials
History is a child building a sand castle by the sea, and that child is the whole majesty of man’s power in the world.
PLAYING WITH SAND
Watch kids digging at the beach, their concentration and intimacy with their material. Scooping, scraping, molding, patting, sculpting, repairing, they are lost in the cycles of excavation, construction, and destruction. Their hands are adapted to different purposes, working both industrially and artistically. It’s not just children who enjoy playing with sand. Sitting on the beach, we adults also make patterns, furrows, and heaps, or sift it through our fingers spontaneously, subconsciously, as we talk or watch the kids. Sand, whether in its dry or wet mood, is a compelling substance, somehow seductive and sensuous. Its topography conforms warmly to ours; it flows through our hands, yet when we stand up, we can walk on it. There is something therapeutic about sand, and indeed it has long played a role in healing ceremonies of widely different cultures. Today, sandplay therapy is a common tool of psychiatry for both adults and children.
Playing with sand can be serious. Make a complex sand castle or simply a pile on the beach, and you are conducting experiments that reveal some of the fundamentals of nature’s character and materials in our everyday lives. Engineers, physicists, space scientists, chemists, mathematicians, and biologists all play very seriously with sand, and the results are often of considerable commercial importance. Why?
SOCIAL INTERACTIONS
Sand grains are gregarious, gathering together on a small or a gigantic scale, in your shoes or in desert dunes. When they congregate into communities, however, they exhibit strange social behaviors and interactions, often surprising, sometimes definitely counterintuitive, and always interesting.
Sand is a granular material, like rice, sugar, salt, coffee beans, the nuts in your cocktail assortment, cereal in its box, grain in storage silos, tablets in a medicine container and the contents of capsules, lawn fertilizer in your spreader, and countless other components of our daily lives. Granular materials behave very strangely. They are not really solids, they are not really liquids, but in many ways they behave more like the latter. Sometimes they behave more like gases, and some physicists believe they should be regarded as a type of matter all of their own. There are more questions than answers about their behavior. For example, why does sand form dunes of all shapes and sizes? Why doesn’t it just spread itself around evenly? Watch the beach as the waves and tides move in and out. Why does the sand sometimes form itself into an undulating surface like a rippled cloth? Ralph Bagnold had a behavioral definition for sand that was based on a peculiar characteristic not shared by coarser or finer materials—the power of self-accumulation, sand’s skill in using the energy of the wind to collect all its scattered grains and build them into heaps, separated by areas free of sand. In many areas of desert, for example, the sand is piled into dunes with bare rock in between, as if some giant combination of a vacuum cleaner and a bulldozer is constantly at work. We shall look more closely at this phenomenon in chapter 6.
Granular materials are everywhere in our everyday lives, and our lives often depend on how they behave. More than a billion tons of granular materials of one kind or another are produced annually in the United States alone. Pharmaceutical products often rely for their effectiveness on the proper mixing of granular components. In agriculture, mining, construction, and other industries, safely storing and working with granular materials in mountainous piles and heaps is vital. In North America each year, more than one thousand silos containing granular materials collapse—suddenly, spontaneously, and for no apparent reason. Landslides, after hurtling down a mountainside, will often turn themselves into a kind of dry fluid, flowing out over level ground for long distances. We simply do not understand how and why they do this, but it is urgent that we learn. It is estimated that natural landslides cause a minimum of $1.5 billion worth of damage and kill at least twenty-five people each year in the United States.
Reflecting its potential fluidity and fickleness, sand, as the quintessential granular material, has become a symbol of instability and impermanence. The biblical admonition against building a house on sand may be exaggerated (see chapter 9), but the imagery is powerful. Albert Einstein described the left-hand side of the equation for his general theory of relativity as built on granite, the right-hand side on sand. Think of the symbolism of “ropes of sand” and “written in the sand.”
Understanding granular materials is vital to countless aspects of our lives, from making good cement to building sand castles, from ensuring that our medicines work to preventing breakfast cereals from jamming up in the box, from constructing sand traps on the golf course to laying the foundations of buildings. It plays a key role in military operations too: the black sands of Iwo Jima, made up of the ash from the island’s volcano, have treacherous granular properties, one Marine describing the sand as “so soft it was like trying to run in loose coffee grounds.”
SAND PILES
The next time you pile up sand on the beach, consider, however briefly, two things. First, the sorites paradox, a philosophical problem originated by Eubilides of Miletus in the fourth century B.C. Eubilides loved paradoxes (the classic “liar’s paradox” was his), and perhaps he loved the beach, or at least playing with sand. For he became concerned about what is, and what is not, a “heap” (sorites derives from the Greek for “heap”). If a single grain of sand is removed from a heap, it clearly remains a heap, as it does after the removal of the next grain. So, asked Eubilides, when does it become a non-heap? The reverse is the same problem—a single sand grain is obviously not a heap, but when does a non-heap become a heap? The problem, philosophically, is one of vagueness and the imprecision of word usage. Though you might not be able to solve the philosophical problem, you can learn a great deal about the physics of nature by experimenting with making avalanches down the side of your heap. As you add grains to the pile, causing periodic avalanches, large and small, which look for all the world like flowing liquid, you are opening a window on how nature works. Sand piles are of intense interest to physicists.
However hard you try, there are clear limitations to what you can do with a pile of dry sand (or any other granular material). You can build it up until the sides reach a certain steepness and then, however many grains you add, it retains the same slope; a couple or a handful of grains will spontaneously tumble down the slope to maintain the same steepness. Dunes are gigantic piles of sand, constantly moving by avalanching (Figure 6). The physics of how sand piles behave was introduced by Bagnold, but their true weirdness and fundamental importance began to be demonstrated in the last years of Bagnold’s life by a Danish physicist, Per Bak, and his colleagues. Their pioneering work was done at the Brookhaven National Laboratory on Long Island, New York, itself a great pile of sand.
Bak ultimately experimented with sand mathematically, but you can do on your kitchen floor the kinds of experiments that he started with. Take a variety of ingredients that are granular materials of different characters. Try using normal table salt (it may declare “fine flowing” or “it never rains but it pours” on the package), granulated sugar, assuming it is somewhat coarser-grained than the salt, and “sea salt,” the big grains of angular salt to be put in a mill. On three different plates, pour out a good-sized pile of each. The three piles will have quite different appearances.
The granulated sugar makes a steeper pile than the fine salt, and the coarse sea salt is steeper still, with a more rounded peak. The steepness of each pile is a reflection of the material’s natural angle of repose, the maximum slope at which the grains are stable. Try to steepen the slope and grains will tumble down the flank of the pile, reestablishing the angle of repose. The angle of repose is shallower, the slope gentler, for finer and rounder grains. Conversely, it’s steeper for larger and more angular grains—the sea salt shows this clearly. The balance of the frictional relationships between individual grains versus the pull of gravity governs the angle of repose.
Salt and sugar aren’t common sand, but they are granular materials, and all granular materials behave in the same way. A pile of real sand may show an angle of repose steeper or shallower than fine salt, depending on the size of the grains and how round they are. To emulate the work of Bak, take a spoonful of sugar and drop a few grains at a time onto the pile, watching what happens. Some grains will roll down the side to the bottom of the pile, but not all. Some grains will set off miniature avalanches of sugar grains down the slope; some of these avalanches will continue to the bottom, while some will come to a halt on the way. But the angle of repose is maintained. However, it’s next to impossible to predict what behavior will result from your next addition of grains.
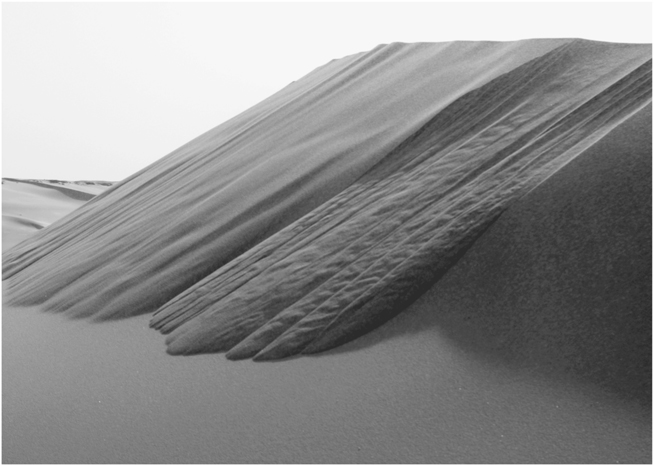
FIGURE 6. An avalanching dune in Egypt’s Western Desert. Sand blowing over the crest is causing avalanches to sweep across the face of the dune; the rippled surface is caused by “liquid” sand in motion as the photograph was taken. (Photo by author)
Strictly speaking, there are two key angles for a pile of any particular material: the angle of repose is its slope after avalanching and coming to rest; the angle of stability is the slightly steeper slope that it can adopt as grains are added before avalanching. To keep things simple here, the former alone captures the principle of what is going on. The angle of repose of a given granular material is the key to its stability, and it is therefore of fundamental importance to us. A wall of sand seeking its angle of repose can create human tragedy: around the world, significant numbers of people, generally children and young adults, often at the beach, are killed by the collapse of excavations in the sand.
In detail, the slope of a pile of grains is irregular, the average being the angle of repose. As grains are added, the irregularities change their shape by the sliding of one or a few or many grains. The irregularities are, in a way, places of marginal stability, poised for instant change. Bak demonstrated in detail what you could see with your pile of sugar: the addition of a single grain anywhere on the heap can set off an avalanche of any magnitude. So is this whole process truly unpredictable? Yes and no. Yes, because predicting what will happen with each grain added is indeed impossible. No, because the general behavior of the pile over time can be predicted. Truly massive avalanches, those that affect large parts of the pile, are very rare. Far more common are small avalanches, and the smaller they are, the more frequent. If you plot a graph of the frequency of avalanches involving different numbers of grains, it will be overwhelmed by the huge number of very small events. But analyze the numbers a little differently and some remarkable characteristics show up, a pattern, an order to things—nature at work. To see the importance and applications of this work, we need to take a moment for a little mathematics.
PLOT LINES
Statistics has a long history as an important but somewhat opaque and difficult area of endeavor. From the early data collection on the range of height of individuals in a population, any number of successful efforts have been made to squeeze order out of what seems to be natural randomness. The “bell-shaped curve” has become a standard, qualitative expression of this. In any population of natural occurrences, whether humans, sand grains, or tosses of a pair of dice, there is a peak, the most common occurrence of whatever is being measured—height, diameter, or added numbers. The peak is always around the middle of the range, with fewer and fewer of successively bigger or smaller measurements. If these kinds of results, say people’s heights, are plotted on a graph that shows the heights set out in group increments against the number of people in each group, then the graph looks like a bell: at the top is the most frequently occurring height, which is flanked by the successively decreasing frequencies of taller or shorter people. This is the so-called normal distribution, because it crops up so commonly that it seems to be nature’s way of organizing things. And it’s not at all random; it has a very clear shape to it.

FIGURE 7. Sand size distributions, illustrating Johan Udden’s “law of the chief ingredient”: a “normal” histogram (left) and the same data plotted logarithmically (right).
This is true of sand size distributions, as Johan Udden noted with his “law of the chief ingredient.” For a sample of sand from a particular place, a graph of the frequency of grain size occurrences against categories of size often looks like a bell curve, a normal distribution (Figure 7, left). For dune sands, it is a tall and narrow bell—they are well sorted. For river sands, it is a squat bell, showing the great range in size.
Bagnold, however, recognized that the bell curve might not be the most useful visual representation of sand size distribution. The bell curve displays size for the sake of size, the very smallest and the very largest grains disappearing off into the indiscernible extremes of the curve. But those ends of the population have a lot to tell us. The Udden-Wentworth grain size scale was developed on the basis of ratios or multiples of size, giving very small grains equal time with the big boys. Better, surely, to honor the principle of the grain size scale and show size frequencies not in terms of a linear scale but of a ratio scale? This was another of Bagnold’s pioneering approaches, plotting graphs of sizes and frequencies on logarithmic rather than linear scales.
We don’t need to enter into a deep mathematical discussion, particularly about logarithms, but they are critical in discerning what is going on with sand sizes—and piles—so a brief excursion is necessary. All Bagnold was doing by using a logarithmic scale was applying the principle of the importance of ratios or multiples. Each of the ascending major categories on the grain size scale is twice as large as the grain size of the previous category (Figure 1). Plotted on a logarithmic scale, the length of each of these increments is the same. When used to plot a graph of grain size frequencies, a logarithmic scale stretches out the portrayal of grain sizes to give every size range an equal say, and allows us to distinguish as easily among very small grains as among very large.
In order to display the characteristics of his samples, Bagnold not only plotted size in multiples (logarithms) but also plotted frequencies that way. This way, the curving sides of the bell curve graph became dramatically straight lines (Figure 7, right) and the tail ends of the distribution were no longer lost. This was not the first time this had been done, but his work was pioneering in gathering huge volumes of data, developing the details, and interpreting the results. The character of his plots, in particular that of the straight lines, proved to be invaluable in comparing and contrasting different communities of sand grains.
Interpreting the details of these kinds of graphs can quickly become another arcane science. But the principles behind them have a wide-ranging significance. Through detailed statistical analysis (not to be reproduced here), Bagnold argued for the fundamental importance of this kind of distribution in our world. And in thinking this way, that “Nature is concerned with relative rather than absolute magnitudes,” he was among those who anticipated some imminent and extraordinary revelations of physics: the physics, as Bak demonstrated, symbolically enough, of sand piles and their avalanches.
A conventional graph of the frequency of avalanches involving different numbers of grains is overwhelmed by the huge number of very small events, and it seems random, characterless. But bearing in mind nature’s concern with relative magnitudes, it is time to use logarithmic scales again. Bak showed that plotting his avalanches this way resulted in a straight line (Figure 8).
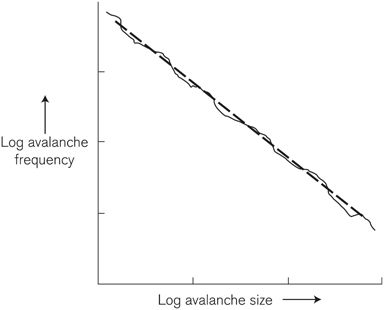
FIGURE 8. The size of sand pile avalanches versus their frequency, plotted logarithmically, is a straight line.
Straight lines in Bagnold’s graphs of sand sizes, straight lines in Bak’s graphs of sand avalanches—what is going on? If a complex natural phenomenon becomes simple when displayed using logarithms, it is proof of nature’s liking for multiplication rather than addition. Lots and lots of small things and only a few big things, whether they are sand grains or earthquakes, is the rule in natural processes, a fundamental way in which nature operates. (Per Bak’s book discussing the behavior of sand piles is titled How Nature Works.) The laws governing such processes are referred to as scaling or power laws: the frequency (or its logarithm) of occurrence of something is proportional to a measure (often size) of that something multiplied by itself a specific number of times (raised to a certain power, mathematically speaking). Newton’s law of gravity is a power law; the pull of gravity on an object decreases with distance to the object squared. Since it decreases, Newton’s law is an inverse power law—and so is that of sand avalanches: the bigger the event, the more rare it is.
But what about the “real world"? Scaling laws show up everywhere—in earth-quake magnitudes (each successively larger magnitude on the scale is a multiple of the previous), population distributions, city sizes, the brightness of the Sun, and music (the structure of rock music, classical music, and the spoken word all obey scaling laws). And the real world of sand and other granular materials is, unsurprisingly, full of examples. Bak was more interested in the underlying mathematics than with messy reality; he wrote in How Nature Works that “the experiments on sand turned out to be much more complicated and tedious than we had anticipated.” But then, “Don’t get me wrong. I have the deepest respect for the type of science where you put on your rubber boots and walk out into the field to collect data about specific events.” Fortunately, many scientists, particularly geologists, have been willing to do exactly that. They have amassed a substantial body of data on landslides in the Himalayas, documenting that the slides range in size from a few wheelbar-rowfuls to ten thousand dump truck loads: they obey a power law. Flushes of sand and mud down into the deep ocean also follow a power law in their frequency versus size. And, if you are on a beach with sand dunes behind it, look for the avalanches down the sand slopes or make some yourself: they are obeying power laws.
The more you look, the more interesting sand avalanches and their laws become. As sand grains are added to a pile and avalanches maintain the slope, it seems as if the system, the pile, is poised in a constant state of almost instability. Bak termed this self-organized criticality, a state in which a very small event can trigger huge consequences—or very small ones. A pile of sand is a self-organized critical system, disturbed by single grains moving down its side or large-scale avalanches—it is a simple model of many seemingly complex natural systems.
Sand piles have even stranger characteristics, some of which may explain why grain silos spontaneously collapse. You would think that the greatest weight of a sand pile, and therefore the greatest pressure exerted at its base, would be directly under the tallest part of the pile, below the greatest weight of sand. But this is not always the case. The weight and pressure distribution within a pile of any granular material is determined by the way in which the individual grains contact each other and distribute the stress. Quite commonly, grain shapes and sizes mean that there are microscopic chains and networks of grains that are oriented and in contact with each other in such a way that they carry most of the pressure from the weight of the material above them. These chains seem to behave like the soaring arches of Gothic cathedrals, which serve to transmit the weight of the roof, perhaps a great dome, outward to the walls, which bear the load. In a sand pile, particularly one that is confined in a container of some sort, these chains perform the same function—they carry the stress outward to the container, rather than directly downward to the base of the pile. If grains of wheat or rice in a silo organize themselves in this way, the resulting stress may cause sudden and catastrophic failure of the structure if it’s not designed to withstand it. It’s this same mechanism that allows sand-filled timers, or hourglasses, to work so well (see chapter 9) and helps support your weight at the beach.
The behavior of sand, or any granular material, is strange enough even when the grains are more or less uniform and the only external influence is dropping an additional grain or two onto a pile. When the grains are different sizes and different densities, and when they are poured or shaken a little, things become seriously bizarre.
SHAKING AND STIRRING, MIXING AND UNMIXING
In Russian folklore, there are many stories of Baba Yaga, a terrifying ogress who eats children and flies around in a mortar using the pestle to steer. One of the tales stars Vasalissa the Beautiful, a merchant’s only daughter who is sometimes, for reasons that will become obvious, referred to as “the Russian Cinderella.” On her deathbed, Vasalissa’s mother gave her a doll that would look after her, and when the father remarried—to, yes, a cruel stepmother with two ugly daughters—the doll helped Vasalissa complete all the menial tasks that were forced on her. One day, the two sisters dispatched Vasalissa to fetch fire from Baba Yaga’s house (a log cabin that moves around the forest on chicken legs), but the girl was kept captive by the ogress and required to perform tasks in return for fire. In addition to the inevitable domestic chores, Vasalissa was given a number of seemingly impossible tasks, one of which was to pick out grains of flour from a bucket of sand (the ingredients vary in different versions of the tale, but it is always a granular materials problem). Fortunately, Vasalissa had her doll to help her, and the job, with great combined effort, was done. She eventually escaped with the fire, the stepmother and the ugly sisters burned to death, and Vasalissa married the tsar.
But had Vasalissa (or her doll) known a little of the physics of granular materials, she could have accomplished the task of separating the flour from the sand with ease. Some years ago, when the mysteries of the physics of granular materials were beginning to be revealed, a group led by Hernán Makse at Boston University demonstrated that a mixture of sand and sugar would separate into its components if you simply poured it slowly and evenly into a pile. “A miracle occurs,” said Eugene Stanley, one of the collaborators. “It’s like throwing a deck of cards on the table and having all the blacks fall on one side and the reds on the other.” Makse, Stanley, and their colleagues conducted a series of experiments using different materials, including sand, glass beads, and sugar crystals, and the results were clear. Typically, larger grains will have a steeper angle of repose than smaller ones, and they will roll down the slope more energetically. The smaller grains tend to get stuck at the top of the pile, the larger ones at the base—they spontaneously segregate (Figure 9). But things become more complicated. As the different angles of repose of different grains are reached and exceeded, successive avalanches will be made up of different-sized grains. The cascades of smaller grains will stop first, to be then covered by a layer of the larger grains still on the move. The process repeats itself over and over, creating a layered pile.
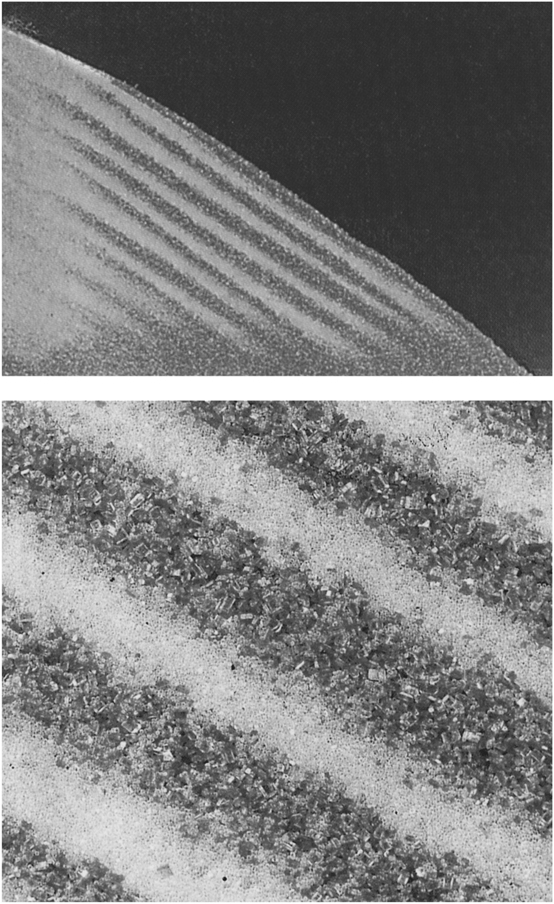
FIGURE 9. Segregation of small glass beads and larger sugar grains (dark) as a result of the simple act of pouring; below, a detailed view. (Photo reprinted by permission of Hernán Makse and Macmillan Publishers Ltd. © 1997 Nature Publishing Group)
Different (and unpredictable) results can be achieved by varying the size, density, and shape of the grains (and therefore their angle of stability or repose), but spontaneous segregation is a common phenomenon. As we shall see, all natural sands and sandstones are layered in one way or another, regardless of whether they were formed underwater or by wind, and clearly understanding the kinds of spontaneous segregation and layering demonstrated in Makse’s experiments is fundamental to understanding how these processes work. Figure 9 is an experimental version of what is happening in the sand dune avalanches of Figure 6. Furthermore, pouring of granular materials is a common industrial activity, and this research begins to hint at how critical an understanding of these strange behaviors is to ensuring that undesirable things don’t happen in the process.
But pouring is only the start of the story. I travel with a container of assorted vitamins and other pills. If I want one of the small items—say, a daily aspirin—it becomes quite frustrating because all the small pills have settled at the bottom of the container and all the larger ones at the top. The same thing occurs with a jar of assorted cocktail nuts—open it up, and the contents have sorted themselves out, with the Brazil nuts all at the top (particularly irritating to me, since I am violently allergic to them). Cocktail nuts have lent their name to a phenomenon that has come to symbolize the strange sorting behavior of granular materials: the Brazil nut effect. The same thing can be observed on opening a box of breakfast cereal where the components are different sizes or weights—the big bits are all at the top. The effect was first documented in the 1930s, but explanation was a challenge.
Today, research into granular behavior takes place at universities all over the world, but in the 1990s it was Sidney Nagel and his colleagues at the University of Chicago who brought the science to the world’s attention when they demonstrated some of the bizarre origins of the Brazil nut effect. In a 1 999 Scientific American Frontiers program on PBS, it was suggested by the interviewer, Alan Alda, that Nagel really loved sand. His response was, “Of course. It’s one of the best substances there is.” Sand, glass beads, sugar, salt—it doesn’t matter what the granular material is, Nagel loves playing with it. To examine the Brazil nut effect, he buried a large glass bead (the Brazil nut) in a jar of small beads (the peanuts) and shook it. After each shake, students were required to meticulously pick out each bead in order to see what was going on; Heinrich Jaeger, who is now a leading researcher at Chicago, was one of those students and described it as a painful experiment. But it was worthwhile. While previous explanations had suggested that it was simply a matter of small things filling in the holes under larger things (percolation), Nagel’s work demonstrated that convection was occurring—convection, as in a genuine liquid, where hot liquid rises, cools, and falls, setting up circulating cells—but this convection was happening in a jar of glass beads. As shaking occurs, the beads at the center move upward, but the larger ones, the Brazil nuts, become marooned at the top because the downward movement takes place along the sides of the container and they are too big to participate. Today, techniques of medical imaging have been borrowed that, combined with time-lapse photography, save tedious hours of student labor and enable detailed documentation of convection in granular materials. Bob Hartley and Bob Behringer, at Duke University, have spent a lot of time vibrating piles of sand and filming their behavior. Figure 10 dramatically illustrates convection patterns in one of their piles: it looks like a liquid.
These are startling, gravity-defying results, but they don’t mean that we understand fundamentally what is going on. This would require the ability to model and construct the mathematics for the behavior of each individual grain, which even with today’s computing power is impractical. The Brazil nut effect may well result from a conspiracy of percolation and convection. Mysteries remain, but at least we can experiment with the strange behaviors of granular materials and apply this knowledge when we need to mix efficiently or transport safely such things as breakfast cereals, medicines, coal, or fertilizer. And do these behaviors account for the annual crop of stones and boulders that appear on the surface of farmers’ fields each spring? Or is that simply the result of freezing and thawing? Fragments of meteorites that may have fallen thousands of years ago remain on the surface of the great Saharan dunes—extraterrestrial Brazil nuts? And while, as we saw in the previous chapter, many modern and ancient sands show graded bedding, where the large grains are deposited first, the smaller ones later as the current wanes, occasionally we find reverse grading. Layers where the largest grains are on the top are typical of rapid and violent depositional events (chapter 5). Sand-covered slopes in the shallow ocean may become unstable, perhaps shaken by an earthquake, and the sand and mud hurtle down into deeper water as debris flows; when they settle out, the larger grains are often at the top—deep-ocean Brazil nuts?
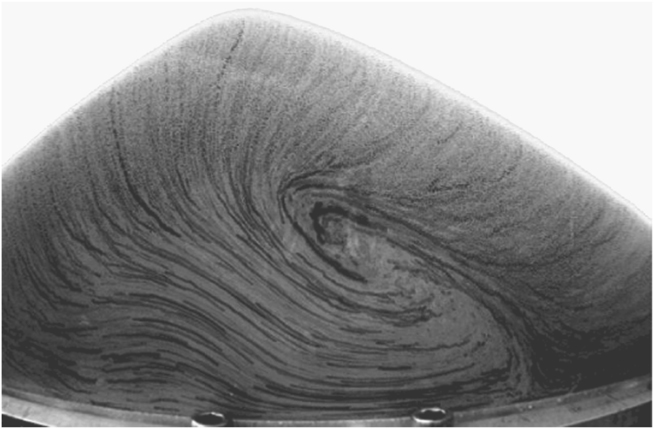
FIGURE 10. Convection in a vibrating sand pile. This view is of a vertical slice through the pile, seen from the side. Some grains have been colored (dark) to highlight the convection pattern. (Photo courtesy of R. R. Hartley and R. P. Behringer, Duke University)
The story continues. You will by now be sufficiently familiar with the strange behavior of granular materials to expect the unexpected, so the fact that a “reverse Brazil nut effect,” the RBN, has been documented will not come as a surprise: large lightweight objects can, counterintuitively, sink through a bed of smaller, denser grains. A whole range of behaviors are sensitively dependent on densities of the grains, speed of vibration, and other variables.
The more research plays with sand, the more complex granular materials appear to be. Grains of different sizes and materials really seem, given the slightest provocation, to prefer the company of their own kind, separating themselves from the others. It seems easier to unmix things than to mix them. Put well-mixed rice and split peas into a horizontal glass drum, start the drum rotating slowly, and go away for a couple of hours; when you come back, the components will have organized themselves into alternating bands of gregarious peas and socially bonding rice along the length of the container. Repeat the experiment with other materials and look lengthwise along the axis of the drum: there will be one of any number of exotic geometrical patterns of self-segregated granular materials.
Why does this happen? Troy Shinbrot, a researcher at Rutgers University who has worked on segregation in drums and the RBN, often in collaboration with Jaeger, has written: “The RBN is sure to provide fruit for further exploration and debate. We find ourselves facing the situation anticipated by Mark Twain: ‘The researches of many commentators have already thrown much darkness on this subject, and it is probable that, if they continue, we shall soon know nothing at all about it.’ Although farmers can count on continuing harvests of heavy boulders under any segregation model, it remains to be seen whether—and how—pharmaceutical engineers should expect their granular formulations to mix or separate.”
MUSIC OF THE SPHERES
If you play in a rock band, try putting a jar filled with a mixture of granular materials from the kitchen in front of the bass amplifier during a gig. Strange patterns may appear, or components may separate in response to the musical frequencies—it’s happened in laboratories. By the same token, nothing at all may happen.
In the late eighteenth century, a lawyer, musician, and scientist in Leipzig, Ernst Chladni, was determined to make sound waves visible. He succeeded in doing so by covering glass or metal plates with sand and drawing a violin bow across the plates’ edges. The vibrations provoked the sand grains into a frenetic, leaping dance, not just randomly: they arranged themselves like Scottish dancing groups into formations. The patterns were highly variable—stars with different numbers of points, crosses, complex intersecting arcs (Figure 11). The science of acoustics and the physical manifestation of sound was born.

FIGURE 11. A Chladni pattern. (Photo courtesy of Paul Koza, Department of Physics, Ludwig-Maximilians-University, Munich)
Over the years, Chladni’s experiments generated increasingly complex patterns, their shapes animated according to frequency and amplitude. In the nineteenth century, Michael Faraday, the British scientist who revealed the fundamentals of electricity and magnetism, first observed convection in granular materials (although not, presumably, in a jar of cocktail nuts). Faraday also demonstrated that vibrating a fluid generates complex standing wave patterns on its surface. Similar to Chladni’s patterns, these were referred to as Faraday waves or, more poetically, crispations. Once again, the similarity in behavior between granular materials and real liquids is disturbingly apparent.
The patterns produced in Chladni’s experiments were as much about the material on which they were produced as about the sand itself, but the patterns seem to relate to underlying natural behaviors. All granular materials indulge in some extraordinary pattern making. Faraday’s crispations appear on the surface of a container of vibrated sand, just as they do in a conventional liquid. Heinrich Jaeger and Troy Shinbrot have worked on this phenomenon too, together with researchers elsewhere, such as Paul Umbanhowar, documenting standing waves in a vast variety of stripes, squares, hexagons, and interlocking, fractal-like patterns—not to mention structures that resemble the stitching on a baseball (Figure 12, left).
The patterns, as Chladni found, respond dramatically to changes in frequency and amplitude, but this relationship is complex. Umbanhowar and others at the University of Texas first demonstrated, accidentally, what happens at low frequencies: clumps of sand grains bounce up and down, alternately leaping into spouts and subsiding into hollows (Figure 12, right). Termed oscillons, these clumps move slowly around the surface of the sand, sometimes combining with and sometimes repelling others, but generally remaining stable for long periods of time. This is yet another example of the bizarre behavior of granular materials. Considerations of particle interactions, energy transfer, impacts, and other aspects of classical physics shed some light on these patterns, but what exactly is going on is not entirely clear. The patterns’ significance goes further than aesthetics and oddball behaviors, however: there is a startling resemblance to patterns in atomic lattices, quantum physics, the weather, and a host of other natural forms. The team at Chicago led by Nagel and Jaeger has recently reported that a jet of sand fired at a small circular target mimics structures interpreted as characteristic of the birth of the universe.
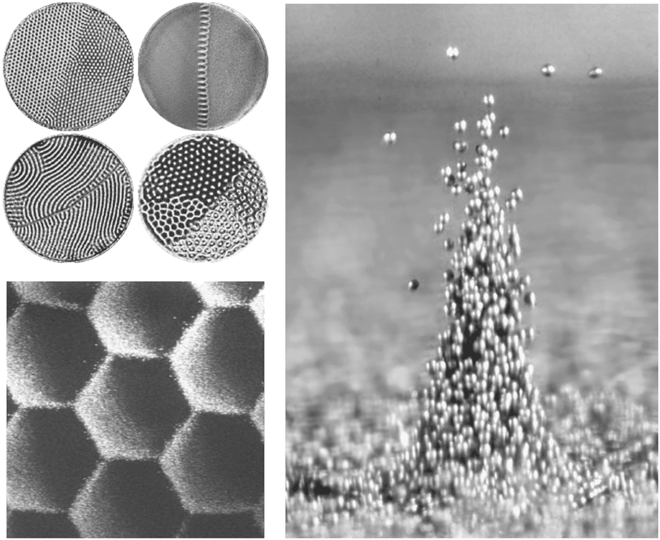
FIGURE 12. Patterns formed on the surface of vibrated sand, viewed from above (left, top and bottom); an oscillon in action, with metal beads mimicking very fine sand grains (right). The four dishes at top left are each 12.6 cm (5 in) across; the hexagons in the image below are each approximately 0.6 cm (¼ in) across, and the oscillon is 2.7 millimeters (1/10 in) across at its base. (Photos courtesy of Paul Umbanhowar)
In November 2000, Jaeger, Shinbrot, and Umbanhowar published a short article in Proceedings of the National Academy of Sciences, titled “Does the Granular Matter?” Their answer is hardly surprising. It is quite possible that dancing, as well as avalanching, sand grains will help us understand some of the fundamental ways in which nature works.
Much of sand’s behavior is a result of the spaces in between the grains and what those spaces contain, as well as of the grains themselves. The air in dry sand contributes to avalanching and the Brazil nut effect—if the same experiments are done in a vacuum, the results are not always the same—and fundamentally affects its properties. Golfers should be especially concerned about the air in the spaces between sand grains and may find the experiment shown in Figure 13 somewhat distressing. Physicists at the University of Twente in the Netherlands (and Jaeger’s group, again) simply dropped a ball bearing into loose sand and generated a new kind of matter—a fluid of dense material and air. The air driven into the sand by the impact, together with the air between the grains, is compressed and erupts, forming a delicate geyser that, very briefly, reaches higher than the level from which the ball was dropped.
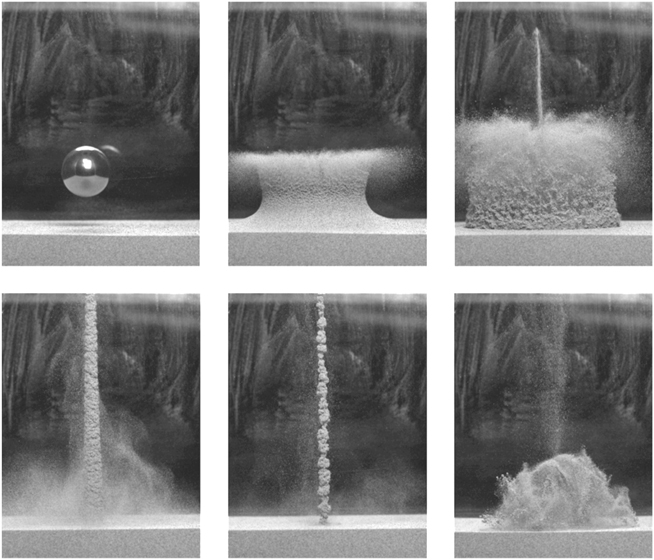
FIGURE 13. A sequence of high-speed photographs showing the impact of a ball bearing in loose sand causing the eruption of a “grainy geyser.” (Photos courtesy of Detlef Lohse and the Physics of Fluids Group, University of Twente, The Netherlands; see Lohse et al., Phys. Rev. Lett. 93, article 198003 [2004])
For a golfer, the lie of the ball would be soul-destroying, but this is why a great deal of attention (and science) is devoted to the character and properties of the sand in bunkers and sand traps. One of those properties, the factors that determine the all-important lie and “playability” of an errant golf ball on landing in sand, is the firmness—how well packed together the sand is. The size, sorting, and shapes of the grains are critical—the applied science of sand in golf course design is a whole discipline in its own right. And, of course, the sand trap plays very differently when it’s wet.
So far, we have covered some of the extraordinary ways in which dry sand grains behave when they get together, but add a little water and everything changes. Dry sand itself behaves eerily like a liquid, but wet sand behaves more like a solid—as long as it’s not too wet.
JUST ADD WATER-ANOTHER MIRACLE OCCURS
Why can sand castles only be built with wet sand? Why is how wet the sand is so important? Why do our footprints on the beach leave a pale, drier “halo” around them? Why is wet sand darker than dry sand? This last question is the simplest to answer—the multiple refractions caused by water in the sand cause light to spend more time bouncing around the sand, and less light escapes, so the sand looks darker. The other questions are a bit trickier. As Alan Alda remarked in his interview with Sidney Nagel, “The beach is a great place for thinking up trivial questions”—but the answers and their implications are far from trivial.
As every kid knows, you need wet sand to build a sand castle. Sand sculpture is an old art form and a global one. Today, festivals, exhibitions, competitions, corporate events, advertising, and therapy feature the art of sculpting sand into often huge—and very often beautiful—representational and abstract forms. The material that, when dry, runs between our fingers, avalanches, and blows away becomes robust and workable when it is wet.
Larry Nelson is a sand sculptor based in Venice Beach, California, and his work is extraordinary. He achieves forms with sand that seem impossible: fragile soaring arches, interlocking curves (Figure 14). To achieve these exquisite results, he has developed an intimate relationship with his material, and his description of the character of wet sand eloquently summarizes its magic:

FIGURE 14. Examples of Larry Nelson’s sand sculptures. Each is a little over a meter (3.5 ft) in height. (Photos courtesy of the artist)
It doesn’t take long to learn that water mixed with sand changes both completely. Two fluids become a formable solid. It may be counterintuitive, but anyone who has spent time in the playground knows this in the fingers.
Water tends to pull in on itself. This makes raindrops, rainbows, and flying sparks from every sprinkler. Anything in contact with the water feels this pull, imparting a tiny tensile characteristic to damp sand. It sticks together.
This adhesion is the essence of sand sculpture. It’s also the bane, because damp sand stickily resists being compacted beyond a certain point, no matter how hard it’s hit. Granular materials naturally form arch structures, tiny but powerfully resistant to compaction. Smack it on top, and your pile simply spreads sideways.
The closer the sand grains, the better they stick together. The sand has to be wet, but how do you get it to pack well if the constituent grains hang up on each other, leaving relatively large air spaces? What you want is grains fitting closely together, filling space as densely as possible so that surface tension embraces as many as possible.
The answer is in the water. It is a lubricant. Not a very good one, but effective if there’s enough of it to give the grains a little time, as they sink, to become neighborly. In the final milliseconds of their fall through the water, you give them some help by rapidly striking the pile with a stick. This shakes the whole assembly, helping the grains snuggle in next to each other, minimizing gaps, giving the water remaining after most has drained away the strongest possible handle on the sand.
As Nelson says, the answer is in the water. Surface tension is a force that operates at the interface between water and air, causing the slight bulge on the water in a full glass, pulling liquid slightly up a straw by capillary action, and allowing insects to walk on the surface of a pond. Surface tension is incredibly strong—the microscopic attraction between the capillary films of water coating two neighboring sand grains is greater than gravity; the grains are very effectively stuck together. Even with the addition of a very small amount of water, a network of sticky bridges is built up within the wet sand and its character changes completely; its angle of stability increases dramatically. You can illustrate the strength of surface tension by wetting two ping-pong balls and putting them on a table: touch them together and they stick, allowing you to pull one with the other across the surface.
We probably know less about the physics of wet sand than we do about its dry version, but the food and pharmaceutical industries, never mind landslide investigators, need the information. Only recently has it been appreciated how little water is needed to effect a dramatic transition (although sand sculptors have known this empirically for a long time). Sand becomes a miraculously solid material with the addition of as little as 1 percent water—and it preserves this character even when the water content is more than 10 percent. The key lies in the strength of surface tension, which depends on the boundary between the water and the air in the spaces between the grains. As more water is added, the total length of that boundary decreases and the adhesion between the grains diminishes. Eventually, you end up with a slurry (sand becomes a liquid again), and, of course, the total absence of that boundary is why you can’t build a sand castle underwater. Unless, that is, you are using magic or space sand, which behaves in exactly the opposite way as normal sand: the grains have been specially coated and treated so as to be water-repellent (hydrophobic); this causes them to clump and cluster together underwater but become loose when brought into the air.
Nelson is an expert and unique sand sculptor. He devotes a lot of attention to the water he uses and the careful, continuous packing of the sand in order to be able to sculpt vertical and overhanging surfaces into his signature floating, hollow sculptures. Often he packs the sand inside a fabric or plastic form, which provides a containing pressure as the grains get themselves organized. An alternative technique is to build with layers of dripping wet sand, using no form. But as he says: “No matter how you make the pile, the truth comes out as soon as you start to carve. The nature of the pile comes from the sand of which it’s made, the way you pack it, the shape of the grains, and the type of water you used. It takes time to know sand.”
The way in which sand packs itself is an area of investigation in its own right. Even if all the grains are the same size and shaped as perfect spheres, there are still heated arguments about how packing works, in spite of the fact that scientists and mathematicians have been studying it for centuries. Put marbles in a jar and gently shake it: they will jam themselves into “random close packing,” the marbles occupying around 64 percent of the total packed volume and air occupying the other 36 percent. To pack the marbles more efficiently, or more densely, the packing needs to be organized rather than random. Shake the jar vigorously so that the marbles bounce up and down a little, and they will settle into an ordered hexagonal arrangement exactly like oranges stacked at the vegetable store. They now occupy 74 percent of the volume. That this is the maximum density for spheres was first proposed by Johannes Kepler four hundred years ago, as he mapped the universe and sought its inner harmony and structure. But “Kepler’s conjecture” took on a similar stature to “Fermat’s last theorem” and was proved mathematically only in the closing years of the twentieth century. Continuing work shows that the state of packing achieved by any group of spheres that may be poured, stirred, or shaken depends very much on how they are poured, stirred, or shaken. Larry Nelson knows a lot about that, and he is handling grains that are not all the same size and not all perfect spheres. In his comment quoted above, about giving “the grains a little time, as they sink, to become neighborly,” he’s describing one of the processes that researchers are curious about. They are also worrying about another detail that helps sand sculptors—the tendency of grains in certain packing structures to jam up, to become jam-packed. Magicians have performed a dramatic trick based on this. The performer produces a pot of sand, into which he inserts a knife and withdraws it, repeating this several times to show how loose the sand is. Plunging the knife in one last time, the magician gives the pot a fairly vigorous shake and swings it around his head by the handle of the knife: the shaking caused the sand to enter into this peculiar jammed state, as everyday granular substances seem to do with irritating frequency.
QUICKSAND
But let’s return to the beach. It is the conspiracy of grains with water and air in the spaces in between that weaves the magic—and explains why our footprints appear the way they do. For the magic mix has yet another extraordinary property. Sand settled through water below the high-tide line is extremely well organized and closely packed. When we walk on it, this structure is disturbed and our weight actually causes the spaces in between the grains underfoot to expand—allowing water to drain into the increased volume and creating the dry halos around our footprints. This effect is known as Reynolds dilatancy, after Osborne Reynolds, the nineteenth-century scientist who pioneered our understanding of fluid dynamics. Fill a balloon with well-packed sand and attach it to an open-ended glass tube with a piece of cloth over the balloon end so as to allow the movement of water but not sand. Hold this contraption upright with the open end of the tube uppermost; pour water into the tube until the sand in the balloon is saturated and water appears in the tube. Now squeeze the balloon—contrary to expectations, the water level in the tube drops. You have demonstrated Reynolds dilatancy and explained the footsteps-on-the-beach phenomenon.
But this habit of dilating is not simply an amusing and counterintuitive property of sand. It’s extremely important—and dangerous. For a start, it causes quicksand. A hapless character being sucked in by quicksand is a movie classic: the character is perhaps saved, perhaps not, and a hat, a Stetson, or a pith helmet floating on the surface is all that remains. The Hound of the Baskervilles, Ice Cold in Alex, Lawrence of Arabia, and The Jungle Book are just a few movies that feature a dramatic—but completely unrealistic—quicksand scene. It’s simple to demonstrate that it’s impossible to sink completely in quicksand, never mind being “sucked in.” But it’s also essentially impossible to pull someone out—there is a real risk that the victim will be torn apart. Quicksand forms when there is sufficient water in between the grains to separate them—to push them apart through dilatancy—but the water is prevented from draining; the sand is in suspension. This can happen when an incoming tide scours large holes in the sand that are rapidly filled by the outgoing tide, trapping water and air in the sand. Or a subsurface spring or other source of water percolates upward through a body of sand, dilating it. The result is a slurry, delicately balanced between solid and liquid, switching instantly but briefly between the two states with the slightest disturbance. But being a mix of water and sand, quicksand is more dense than water, and the human body floats well in it; this was demonstrated effectively in an episode of the highly entertaining television series Mythbusters, where the presenters bobbed around like corks in a huge tub of quicksand.
The problem arises when a person floating in quicksand tries to move too quickly; the movement destroys the dilatancy of the slurry and the grains reconvene and jam back into a solid, effectively cementing the unfortunate person in place. It has been estimated that the force needed to pull your foot out of jammed quicksand is about that needed to lift a medium-sized car. The key is to wiggle, allowing water to fill the space created around you, and then swim, very slowly. Quicksand is lethal because lone individuals die of exposure, starve, or drown when the tide comes in, not because they are sucked under.
Dry quicksand is something else altogether. Golfers will be relieved to know that the sand in Figure 13 was prepared by blowing air through it to create a very loosely packed bed, with the result that the ball bearing disappeared completely. There are tales, as in the stories of T. E. Lawrence, of camels and vehicles disappearing completely in dry desert quicksand, but it has never been documented as being lethal. (The dry quicksand scene in Indiana Jones and the Kingdom of the Crystal Skull is relatively realistic—at least Professor Jones has time to deliver a short lecture on the physics of their predicament.) It has been suggested that there is no such thing as dry quicksand, but that is also incorrect. Ralph Bagnold, in his autobiography, Sand, Wind, and War, observed in the Egyptian desert that “there were other places, pools of ‘liquid’ sand, in appearance just like the rest of the surface, but so soft one could plunge a six-foot rod vertically into them without effort.” And ask Keith Tabscott. In July 2007, he lowered himself into a sand hopper at an industrial plant in Florida in order to try to free a blockage. The sand collapsed on him up to his chest, and the more he struggled, the more the sand locked around his legs. Fortunately, he was wearing a safety harness; news helicopters were kept away in case their vibrations caused further avalanching and settlement of the sand, and it took two hours to free him, unharmed. He was lucky: fatal accidents have occurred in sand hoppers. Perhaps this was not truly “dry quicksand,” but don’t tell Keith that.
SHAKING EARTH
Quicksand may be a problem, but it’s a relatively small-scale one. Dilatancy can also cause problems on a devastating scale, when what is going on in the balloon experiment causes sand and soil to dilate and liquefy under houses, office blocks, and entire towns. Shake waterlogged sand, and liquefaction occurs: the grains move apart and the friction and adhesion between them is lost, destroying the stability of the material, which then flows and compacts, expelling the water. Earthquakes are particularly good at doing this, with catastrophic consequences. The earthquake that devastated the region around Bhuj in northwestern India in 2001 was one of the most damaging in the country’s history. Twenty thousand people were killed and the havoc caused more than $3 billion of damage. Because the vibrations provoked the sand and soil into liquefying, buildings no longer had a foundation, causing them to sink, tilt, and collapse. Huge cracks in the earth appeared, and water and sand erupted in volcanic fountains as the ground collapsed back on itself. This is a common result of earthquakes: more damage is done by liquefaction than by the tremors themselves. This was the case in the great San Francisco earthquake of 1906, in the Anchorage event in 1964, and in the Loma Prieta earthquake in 1989, when some buildings in San Francisco sank until their third floors were at ground level, and sand volcanoes erupted from the ground (Plate 5). Liquefaction was also amply demonstrated in the most powerful series of earthquakes to strike the lower 48 in recorded history—not in California, but in New Madrid, Missouri, between 1811 and1812.
New Madrid at that time was, fortunately, a small town of four hundred inhabitants, none of whom were aware that they lived above an ancient system of deep fractures in the Earth’s crust beneath the Mississippi River valley. The fractures do not exercise themselves very often, but when they do it is with extreme violence. In the early hours of December 16, 1811, the terrified residents were awoken by violent shaking, accompanied by an appalling roaring sound. They later reported that the surface of the Earth moved in waves and that cracks opened in the ground from which water and sand erupted. One resident, Eliza Bryan, wrote that “the surface of hundreds of acres was, from time to time, covered over in various depths by the sand which issued from the fissures, which were made in great numbers all over this country, some of which closed up immediately after they had vomited forth their sand and water” (quoted in Lorenzo Dow’s Journal, 1849).
We are often tempted, for dramatic purposes, to compare the destructive power of natural events such as earthquakes to the biblical demise of Sodom and Gomorrah. Ironically, work by David Neev of the Geological Survey of Israel and K. O. Emery of Woods Hole Oceanographic Institution, together with research by engineers and geologists in the United Kingdom, has suggested that, assuming the twin cities existed at all, they may well have been destroyed by earthquakes and liquefaction. The area around the Dead Sea, the likely location for Sodom and Gomorrah, lies across the boundary of two rapidly shifting segments of the Earth’s crust and has experienced significant earthquakes over recorded history. Lying below sea level, it is also the ultimate destination of vast volumes of sand and mud, and in many of these ancient layers are the telltale signs of violent water expulsion from the sediments. Large-scale slumping is also evident. Altogether, these forensic clues point to an obvious culprit for the destruction. But what about the fire and brimstone? Bitumen, natural asphalt or tar, was much prized during ancient times for medicine, the caulking of boats, and the preservation of mummies, and sulfurous bitumen, together with lighter oil, has for thousands of years been leaking from fractures around the Dead Sea, seeping into the ground and the water. All that would be required would be gas leaking along with the oil and a spark as the ground catastrophically gave way—fire and brimstone?
As we have seen, waterlogged sand can disastrously lose all of its strength as a result of liquefaction, and it is therefore no surprise that this is the focus of intense research by engineers, physicists, and lovers of granular materials. Because liquefaction is a triumph of gravity over friction and surface tension, considerable effort has been put into eliminating the effects of gravity in order to concentrate on what’s going on between the grains and the water. How? By conducting experiments on granular materials in space. By testing the behavior of sand, wet or dry, in the microgravity environment aboard a space shuttle, not only can we prepare for building on the Moon or Mars, but we can better understand the quirks of granular materials here on Earth, including the potential for better construction engineering in earthquake areas. Such experiments have been an ongoing feature of shuttle missions. Tragically, a series of such experiments was on the agenda for the crew of the ill-fated Columbia in 2003; some of the results had been downloaded to Earth during the mission, and readable data were recovered from the debris.
The results of orbiting and earthbound scientific investigation of granular materials contribute in a practical and potentially life-saving way to our understanding of how to handle them. Among the outcomes is better engineering to counteract, or at least mitigate, the process of liquefaction. Is one solution to try to glue the grains together so that they can’t liquefy? In spite of the scale of the challenge, the answer is yes. Engineers can inject chemicals, such as epoxies, into sandy soil; however, the chemicals may be toxic. Fortunately, we may be able to seek help in this from a seemingly unlikely source. The unexpected aid comes from Bacillus pasteurii, a natural bacterium that lives between sand grains. Research at the University of California at Davis has shown that the bacterium causes calcite, or calcium carbonate, to precipitate, which glues the grains together. Inject sand with cultures of these bacteria, feed them well, and provide them with oxygen, and they will turn loose sand into solid rock. It’s not just water and air that inhabit the spaces in between grains of sand.
AN UNDERGROUND CITY, A WORLD ON A GRAIN OF SAND
In The Edge of the Sea, Rachel Carson wrote:
Walking back across the flats of that Georgia beach, I was always aware that I was treading on the thin rooftops of an underground city. Of the inhabitants themselves, little or nothing was visible. . . .
In the intertidal zone, this minuscule world of the sand grains is also the world of inconceivably minute beings, which swim through the liquid film around a grain of sand as fish would swim through the ocean covering the sphere of the earth. Among this fauna and flora of the capillary water are single-celled animals and plants, water mites, shrimplike crustacea, insects and the larvae of certain infinitely small worms—all living, dying, swimming, feeding, breathing, reproducing in a world so small that our human senses cannot grasp its scale, a world in which the micro-droplet of water separating one grain of sand from another is like a vast, dark sea.
In the great hierarchy of living things on our planet, it is generally accepted that, at the head, there are five kingdoms, one of which—animalia—contains all multicelled animals. Within each kingdom are a number of phyla: all mammals, birds, reptiles, amphibians, and fish belong to the phylum chordata. Depending on which biologist you speak to, between thirty-six and forty total phyla are recognized on our planet. Rainforests, our flagships of biodiversity, are known to contain sixteen phyla. The spaces in between sand grains are home to twenty-two.
Bacillus pasteurii, our potentially helpful construction worker, is only one of many bacteria that dwell on and in between sand grains. This way of life goes back a long way—in fact, it could go back to the very beginning of life itself. Zachary Adam, an astrobiologist at the University of Washington, has recently suggested that radioactive sand grains in beaches of the early Earth could have provided the chemical energy to assemble the building blocks of cells into the complex molecules of life. Certainly, the oldest known structures built by life on Earth were stromatolites, the reef-like apartment blocks of cyanobacteria (blue-green algae), which built successive layers by trapping sand grains washed across them by the primordial ocean. It was cyanobacteria that provided all-important oxygen to the Earth 3.5 billion years ago, and some forms of stromatolites survive today. As do multitudes of cyanobacteria, whose sticky filaments bind sand grains together in the soils of arid regions, forming a crust that provides nutrients and stabilizes the soil, making the task of erosion more difficult.
The dark, watery world beneath the surface of the intertidal zone of most beaches is home to many kinds of bacteria—and a jungle of other minute creatures. Silica, or quartz-based sands, are the most hospitable, since the calcium carbonate in sands composed of shell fragments makes the water too alkaline for many creatures to flourish. Pick up a handful of wet quartz sand on the beach and you are holding a miniature zoo with thousands of inhabitants. Some of these were first observed by Antony van Leeuwenhoek as he peered down his microscope at sand grains and his animalcules, but we owe our understanding of the incredible diversity and importance of this community to the work of Robert Higgins, who since the 1960s has devoted his life to identifying and describing its members. Now retired from the Smithsonian Institution, Higgins is the pioneer of work on meiofauna (“lesser animals”), creatures whose giants are 1 millimeter long; he continues to contribute to the identification of new species, which crop up at the rate of a dozen or so every year. In a 2001 symposium that paid tribute to his discoveries, Higgins modestly described his work as “how the ‘lesser-knowns’ became better-known.”
Life between the sand grains is not easy. The grains move and settle under the pressure of the waves and the incessant flushing of the tides, water occasionally drains out completely, and the ecosystem contains a variety of predators. Life has had to adapt, and it has done so in strange and extraordinary ways that reflect an intimacy with the behavior of granular materials. Many of these creatures have armored or padded bodies designed to withstand abrasion by moving sand grains; their shape is often flat or elongated to enable squeezing between the grains; and they have developed a variety of ways of attaching themselves, using glue or suction, to individual sand grains, clinging on for dear life. Figure 15 is a portrait of some of Higgins’s weird and wonderful creatures. Rotifers, nematodes, mystacocarids, tardigrades, gastrotrichs, turbellarians, and kinorhynchs—it’s tempting to view these little animals as rejoicing in their exotic names. Tardigrades, which live in both marine water and freshwater, can’t swim, so hanging on to a sand grain is vital; some use mechanical suction toes, some claws, some both. A gastrotrich can glue and unglue itself in an instant. A kinorhynch is ungainly, described by Higgins as an umbrella in a canister, but it moves effectively, if slowly, exploring one sand grain at a time. Rotifers, so named because they look like rotating hairy wheels, are represented by 2,500 different species, most of which are freshwater dwellers. Tardigrades have the remarkable ability to suspend operations if the water disappears, remaining dormant and dehydrated for a hundred years, only to spring back to life when rewetted. They seem to do this by replacing the water in their cells with sugar, which renders them immune to freezing and radiation, a talent that is of considerable interest in the worlds of medicine and extraterrestrial biology.
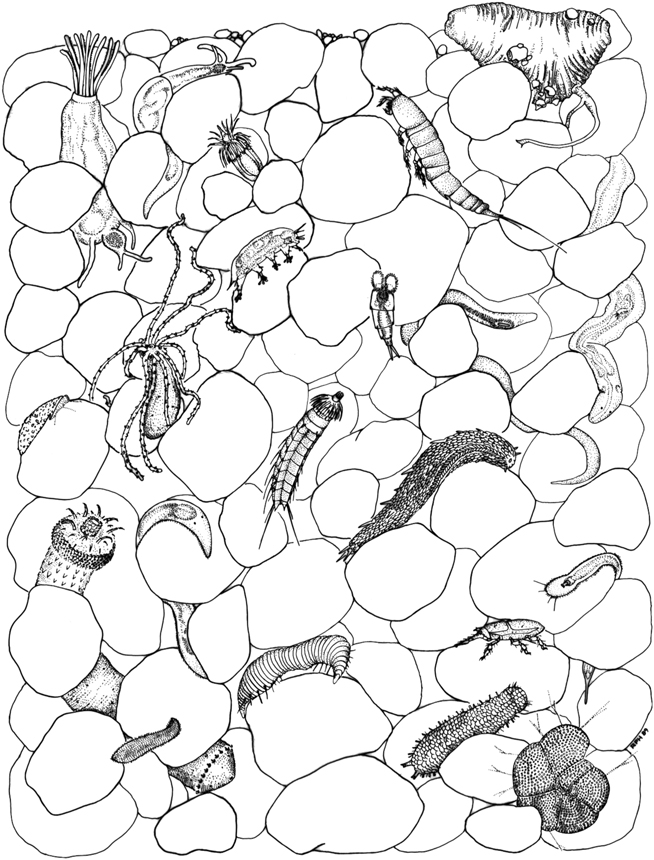
FIGURE 15. Portraits of the meiofaunal community. (Illustration courtesy of the Department of Invertebrate Zoology, National Museum of Natural History, Smithsonian Institution)
In the entire living world, only three new phyla were identified in the twentieth century, and one of them came from the strange world of meiofauna. In the 1970s, Reinhardt Kristensen, a professor at the University of Copenhagen and an old student of Higgins, showed him a collection of creatures from the coast of Brittany that he could not identify. “Oh,” responded Higgins, “I have one of those too.” The animals fit with no known group of living things, and it took several years of meticulous collaborative work to define the character of the creature as a new phylum. It looks rather like a classical amphora with snakes emerging from it, and they named it loricifera, “corset-bearing,” reflecting the appearance of the rings that sheathe the animal. More than seventy species have now been identified, but we know next to nothing about their behavior, since, sadly, they die before reaching the laboratory. One remarkable thing we do know is that loricifera have the smallest cells of any known animal.
Inevitably, the community of organisms growing on or living in sand has its own name: psammon. Among the psammon are psammophiles (or arenophiles, if you prefer Latin to Greek), psammobionts, and psammoxenes. The names are weighty, but the members of the community are not; what they are is wondrous. Most of us don’t know they exist, but we should be grateful for them. Without meiofauna, the sands of our beaches and lakeshores would be stinking, toxic places, with organic debris rotting unconsumed and dangerous bacteria rampant. The microscopic creatures of the meiofauna feed off his debris: they keep our beaches clean.
OUR UNKNOWN WORLD
Contrary to Heraclitus’s claim, sand illustrates for us the limitations of our power in the world: it reminds us constantly that what we know is eclipsed by what we don’t know. Whether we’re concerned with the behavior of a simple pile of sand, the internal structure of a sand castle, the mathematics of criticality, liquefaction, or the daily habits of loricifera, we have barely scratched the surface of the mysteries of this strange material. And these mysteries continue to crop up in our daily lives. The pictures reproduced in Figure 16 were submitted to the New Scientist for its weekly section devoted to readers’ questions. On a beach along the shores of Lake Michigan appeared the mysterious natural sculptures in the sand shown at the top. It seems that something bound the sand grains together and the loose sand around them was then eroded. But what caused the grains to bind is anyone’s guess—suggestions ranged from worm excretions to raindrops, from sand boils to urinating animals. The sculpture on the bottom appeared one morning on a Scottish sand dune; what went on to produce it is again elusive. Another of the simple—and not so simple—mysteries of sand.
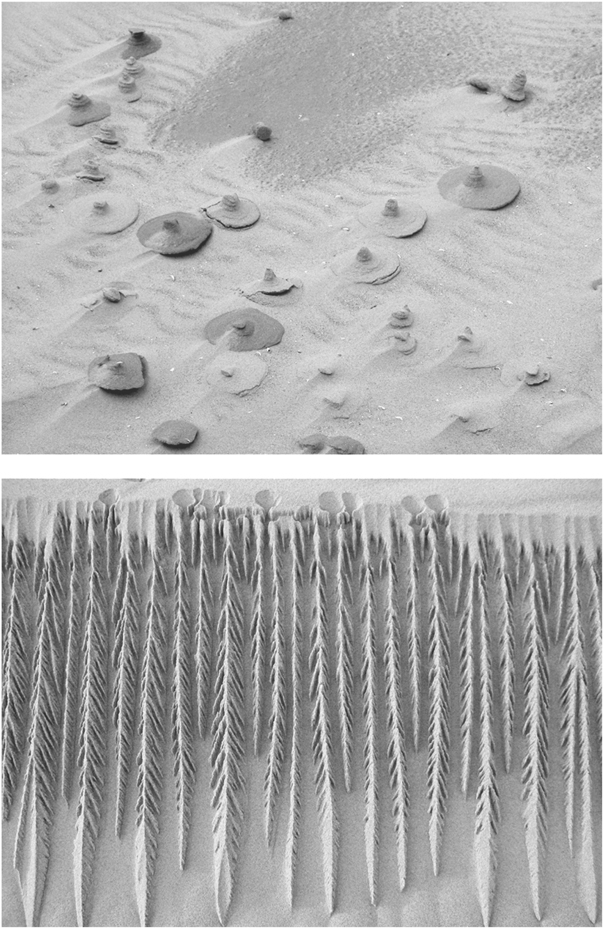
FIGURE 16. Sand mysteries: natural sand sculptures from the shores of Lake Michigan (top) and from a Scottish sand dune (bottom). (Photos courtesy of Steven C. Thorpe and Noreen Slank, top, Martyn L. Gorman, bottom)