Chapter 9
Tackling Technical Information and Science Review
In This Chapter
 Exploring the metric system of measurement
Exploring the metric system of measurement
 Reviewing general scientific fields
Reviewing general scientific fields
 Delving into biology, chemistry, and physics
Delving into biology, chemistry, and physics
 Perusing earth and solar system facts
Perusing earth and solar system facts
 Understanding how the sciences apply to daily life
Understanding how the sciences apply to daily life
Believe it or not, the military flight aptitude tests require you to have knowledge in a wide range of technical and scientific topics, so you need to cover down on those subjects (that’s a military term for getting a grasp of something) to ensure your exam success. In this chapter, we review all the scientific subject matters that you need to know to perform well on your test. We cover the basic fundamentals of biology, physics, anatomy, chemistry, and the earth sciences.
Brushing Up on the Metric System
So, what’s so great about the metric system? The answer is simple, just like the metric system itself. In the metric system, everything operates on a scale of ten, unlike the U.S. customary system — the system you’re probably used to — which is based on a scale of . . . well, who knows. The simple fact is that most folks in the United States know little about the metric system. You were probably exposed to it during your high-school and college math and science classes, but if not, the following sections have you covered.
Reviewing units and abbreviations
Forget about feet, pounds, and Fahrenheit. The metric system uses a different system of units than you may be used to. The following list showcases some common metric units:
 Seconds (s): The second is the main metric unit of time.
Seconds (s): The second is the main metric unit of time.
 Meters (m): Meters are the main metric unit of distance.
Meters (m): Meters are the main metric unit of distance.
 Degrees Celsius or centigrade (°C): These degrees are the metric unit of temperature in everyday use; check out the later section “Taking the temperature: Going from Fahrenheit to Celsius and back” for help converting Fahrenheit temperatures to Celsius and vice versa. Note: We say “in everyday use” because temperature in the metric system is officially measured in Kelvin (K). You find the temperature in Kelvin by adding 273 to the temperature in degrees Celsius. Temperature in Kelvin is useful when describing things in term of absolute zero (0 degrees Kelvin is the coldest temperature possible).
Degrees Celsius or centigrade (°C): These degrees are the metric unit of temperature in everyday use; check out the later section “Taking the temperature: Going from Fahrenheit to Celsius and back” for help converting Fahrenheit temperatures to Celsius and vice versa. Note: We say “in everyday use” because temperature in the metric system is officially measured in Kelvin (K). You find the temperature in Kelvin by adding 273 to the temperature in degrees Celsius. Temperature in Kelvin is useful when describing things in term of absolute zero (0 degrees Kelvin is the coldest temperature possible).
 Kilograms (kg): Kilograms are the metric unit of mass. The first time you order something from a deli that uses the metric system, be careful!
Kilograms (kg): Kilograms are the metric unit of mass. The first time you order something from a deli that uses the metric system, be careful!
 Hertz (Hz): The metric unit of frequency is hertz. If something happens once per second, it happens with a frequency of 1 hertz.
Hertz (Hz): The metric unit of frequency is hertz. If something happens once per second, it happens with a frequency of 1 hertz.
 Joule (J): The Joule is the metric unit of work/energy.
Joule (J): The Joule is the metric unit of work/energy.
 Pascal (Pa): Pascals are the metric units of pressure. There are 101,325 pascals (101.325 kPa) in 1 atmosphere (1 atm). Regardless, inches of mercury is the standard measure of barometric pressure throughout the world aviation community. One inch of mercury is equal to 3,386.389 pascals at 0 degrees Celsius.
Pascal (Pa): Pascals are the metric units of pressure. There are 101,325 pascals (101.325 kPa) in 1 atmosphere (1 atm). Regardless, inches of mercury is the standard measure of barometric pressure throughout the world aviation community. One inch of mercury is equal to 3,386.389 pascals at 0 degrees Celsius.
Preparing for prefixes
The metric system uses prefixes to indicate how a particular quantity relates to its base unit (see the preceding section). Table 9-1 lists some of the more common metric prefixes:
Table 9-1 Prefixes Used in the Metric System
|
Prefix (Symbol) |
Numeric Equivalent |
Example |
|
nano (n) |
10–9 |
0.000000001 meters = 1 nanometer (nm) |
|
micro (μ) |
10–6 |
0.000001 meters = 1 micrometer (μm) |
|
milli (m) |
10–3 |
0.001 meter = 1 millimeter (mm) |
|
centi (c) |
10–2 |
0.01 meter = 1 centimeter (cm) |
|
deci (d) |
10–1 |
0.1 meter = 1 decimeter (dm) |
|
kilo (k) |
103 |
1,000 meters = 1 kilometer (km) |
|
mega (M) |
106 |
1 million meters = 1 megameter (Mm) |
|
giga (G) |
109 |
1 billion meters = 1 gigameter (Gm) |
Covering conversions and equivalents
Need to quickly convert a metric unit to a U.S. customary one? No problem. Here are some fast conversion formulas for your viewing pleasure:
 Seconds to minutes: Number of seconds ÷ 60 = number of minutes
Seconds to minutes: Number of seconds ÷ 60 = number of minutes
 Meters to inches: Number of meters ÷ 0.0254 = number of inches
Meters to inches: Number of meters ÷ 0.0254 = number of inches
 Centimeters to inches: Number of centimeters ÷ 2.54 = number of inches
Centimeters to inches: Number of centimeters ÷ 2.54 = number of inches
 Kilograms to pounds: Number of kilograms ÷ 0.45 = number of pounds
Kilograms to pounds: Number of kilograms ÷ 0.45 = number of pounds
 Pascals to atmospheres: Number of pascals ÷ 101.325 = number of atmospheres
Pascals to atmospheres: Number of pascals ÷ 101.325 = number of atmospheres
 Joules to calories: Number of joules ÷ 4.184 = number of calories
Joules to calories: Number of joules ÷ 4.184 = number of calories
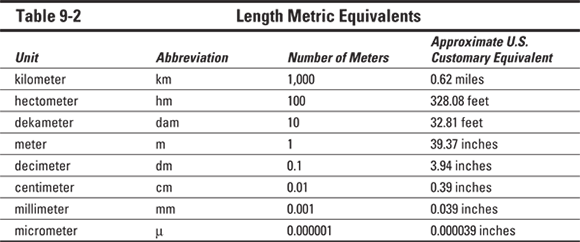
Tables 9-2 through 9-4 provide a handy list of metric equivalents that you can use to get the hang of the various metric measurements.

Taking the temperature: Going from Fahrenheit to Celsius and back
The degree Fahrenheit (°F) is the U.S. customary scale of temperature measurement; the freezing point of water is 32 degrees, and the boiling point is 212 degrees. The formula for converting Fahrenheit to Celsius is
°F = °C × 9/5 + 32
The degree Celsius (which we introduce in the earlier section “Reviewing units and abbreviations”) is an easy system of measurement with the freezing point of water at 0 degrees and the boiling point at 100 degrees. (Of course, pressure has some impact on these figures, but these base figures are for standard pressure.) The formula for converting Celsius to Fahrenheit is
°C = (°F – 32) × 5/9
Wrapping Your Brain around the Scientific Method and Scientific Notation
The scientific method is a formalized method for objectively observing, experimenting with, developing, and further modifying a given scientific idea, or hypothesis. The scientific method is how companies test new drugs and how astronomers figure out whether a new planetary theory is correct. It’s the basis of all experimentation.
You have probably used the scientific method in your lab classes, where you performed a series of experiments to confirm your (or the class’s) idea. Of course, your instructor already knew the outcome in these experiments, but the tests gave you an understanding of the systematic and objective process necessary to understand and develop scientific principles. Newton didn’t just come up with his laws; he developed them through the scientific method.
Scientific notation is a way to express very large or very small numbers in a more concise way without diminishing the accuracy. Writing that something weighs 3.4 × 10–6 grams is much easier than writing 0.0000034 grams. The scientific world uses scientific notation in most content.
To convert a number less than one or greater than ten to scientific notation, follow these steps:
1. Convert the number you’re working with into a number between 1 and 10 by moving the decimal to the left or right.
For example, you convert 35,000 to 3.5 by moving the decimal four spaces to the left. To convert 0.00035 to 3.5, you move the decimal four spaces to the right. Remember: Keep track of how many places you move the decimal point.
2. Write the number that you came up with in Step 1, followed by × 10.
3. Add the appropriate exponent based on the number of spaces you moved the decimal.
If the original number was greater than 10 (that is, you moved the decimal point to the left in Step 1), write the number of moved decimal places as a positive exponent attached to the 10. In the 35,000 example from Step 1, you move the decimal point four places to the left, so your final result would be 3.5 × 104. If the original number was less than 1 (you moved the decimal to the right), attach the number of places you moved as a negative exponent. For the 0.00035 example, your final result would be 3.5 × 10–4.
Getting Physical with the Natural Sciences
Ah, the natural sciences. Natural sciences is really just an encompassing term for all subjects that try to explain life as we know it. From how much force is required to lift a bag of cement to how a plant makes energy, the natural sciences cover a wide range of disciplines. The following sections introduce you to some of the main branches of natural science.
Living it up with life sciences
Life science is just the study of living organisms. Some life sciences you may be familiar with include biology (the basic science concerned with life and living organisms), anatomy (the study of a living thing’s structure), and physiology (the study of living systems’ functions); we cover these topics in more detail later in this chapter. Here are a few other life sciences to be aware of:
 Botany: Botany is the part of biology that concerns itself with the study of plants and their mechanisms: plant structure, growth, reproduction, energy production, and nutrition; plant diseases; the chemical properties of plants; and plant classification relationships. Botany is one of the oldest known sciences; after all, primitive peoples had to learn what was safe to eat. More than 550,000 known species of living plants exist today.
Botany: Botany is the part of biology that concerns itself with the study of plants and their mechanisms: plant structure, growth, reproduction, energy production, and nutrition; plant diseases; the chemical properties of plants; and plant classification relationships. Botany is one of the oldest known sciences; after all, primitive peoples had to learn what was safe to eat. More than 550,000 known species of living plants exist today.
 Ecology: Ecology is the study of the hierarchical relationships living organisms have with their natural environments (ecosystems) and with other species. Ecology spans natural environments from the microscopic to the planetary level.
Ecology: Ecology is the study of the hierarchical relationships living organisms have with their natural environments (ecosystems) and with other species. Ecology spans natural environments from the microscopic to the planetary level.
 Zoology: Zoology is the study of the classification, habits, evolution, health, and social patterns of all species in the animal kingdom, and it deals with both current and extinct animals.
Zoology: Zoology is the study of the classification, habits, evolution, health, and social patterns of all species in the animal kingdom, and it deals with both current and extinct animals.
Digging into the earth sciences
Another division of natural science is earth science, which focuses on the makeup and function of our planet. The earth sciences use tools from all the other sciences (such as studying the composition of particular rocks through chemistry), but earth science is primarily geared toward understanding this and other planets. The following sections cover a few important earth science fields.
Geography
Geography is the science that studies land masses, their structures, and their movement patterns over the ages, as well as the people who inhabit the areas of interest. You can break geographical studies down into two basic subcategories: human geography and physical geography. Human geography looks at the cultures, people, and communities in various regions of the earth and considers their interactions with their environments. Physical geography deals with the structures or functions of the different sections of the earth and its atmosphere.
Geology
Geology is the branch of science that deals with the study of the earth, its structure, and its past and continuing evolution. In modern times, you may find geologists working for energy companies by helping to discover new sources for oil or working to understand the possible impact of underground earthquake faults.
Hydrology
Hydrology focuses on the study of water movement, sources of distribution, and quality on earth and other planets, including the water cycle, water resources, and environmental watershed sustainability. Some projects for hydrologists may include working on planetary water research for NASA or working on a watershed initiative at a local environmental group.
Meteorology
Meteorology deals with the scientific study of the atmosphere and assists in the forecasting of weather patterns. Meteorological phenomena are observable weather events, which are then interpreted by scientists who are experts in meteorology. Natural variables, such as temperature, pressure changes, and humidity, can severely impact the weather of the earth, and the meteorologist’s job is to interpret the phenomena and give predictions based on past observations and computer models. Meteorology impacts everyday life, from planning a social outing outside to hurricane tracking and level prediction to monitoring windspeed at upper altitudes for flying conditions. Meteorological training is a huge part of flight school, and throughout your career, you’ll regularly seek the advice of meteorological experts; flying in a thunderstorm is no fun. As the old aviation saying goes, “It’s better to be down here wishing you were up there than to be up there wishing you were down here!”
Astronomy
Astronomy is the science that studies the celestial sky, including stars, planets, comets, nebulae, different star clusters, and galaxies. Astronomy is one of the oldest sciences; early man used astronomy as both a supernatural predictor of events and as a way to navigate the exploration of the earth. Today, astronomy breaks down into various subcategories. Observational astronomy deals with gathering hard data from observations and using basic laws of physics to clarify those findings. Theoretical astronomy is the section of astronomy that deals with developing computer models to predict and describe astronomical locations and events. Flip to the later section “Exploring Your Solar System” for information on the sun and planets.
Cramming Life Science Essentials
In the earlier section “Living it up with life sciences,” we introduce you to some of the many branches of life science. One we don’t cover in detail there is biology, the basic science concerned with life and living organisms and their structures, origins, classification, functions, and growth. Biologists have agreed to five basic fundamentals:
 Cells, from plants to bacteria to humans, are the foundation of life as we know it.
Cells, from plants to bacteria to humans, are the foundation of life as we know it.
 Evolution results in new species and new traits.
Evolution results in new species and new traits.
 All organisms use and transmit some form of energy.
All organisms use and transmit some form of energy.
 Genes form the basis for heredity.
Genes form the basis for heredity.
 An organism attempts to control its environment to maintain function and stability.
An organism attempts to control its environment to maintain function and stability.
Biology breaks down into smaller disciplines, such as biochemistry, physiology, and cellular biology. The following sections look at some of the biology basics you should be familiar with for your flight aptitude test.
Classifying all living things
Taxonomy is the classification of organisms in a way that corresponds with their relationships to each other. Here’s the standard classification system for bringing together various species into progressively larger levels:
 Species: A category of organisms that has the capability to interbreed and produce fertile offspring
Species: A category of organisms that has the capability to interbreed and produce fertile offspring
 Genus: One or more related species
Genus: One or more related species
 Family: Similar subfamily
Family: Similar subfamily
 Order: Families with similar characteristics
Order: Families with similar characteristics
 Class: Orders with similar characteristics
Class: Orders with similar characteristics
 Phylum: Related species
Phylum: Related species
 Kingdom: Related divisions of phyla
Kingdom: Related divisions of phyla
 Domain: Broadest level of classification
Domain: Broadest level of classification
The most widely accepted classification scheme currently recognizes three domains: Archaea, Eubacteria, and Eukarya. Domain Eukarya (the one humans are part of) is subdivided into four kingdoms: Protista, Fungi, Plantae, and Animalia. All living organisms are given a two-part name; the first name reflects the organism’s genus, and the second name is the species. For example, humans are called Homo sapiens.
All you wanted to know about cells
Cells are the basic structural unit in any living organism; they’re the building blocks of animal, bacteria, fungi, and human life. The following sections highlight important cell considerations to know for your flight aptitude test.
Cell theory
Cell theory puts forth the idea that new cells are created from other cells that already exist. The tenets of modern cell theory include the following:
 Every known living thing is comprised of one or more cells. Unicellular organisms are comprised of just one cell, while multicellular organisms are comprised of more than one cell.
Every known living thing is comprised of one or more cells. Unicellular organisms are comprised of just one cell, while multicellular organisms are comprised of more than one cell.
 In every living organism, the cell is the fundamental unit of structure and function.
In every living organism, the cell is the fundamental unit of structure and function.
 New cells are created by the division of pre-existing cells.
New cells are created by the division of pre-existing cells.
 Metabolism and biochemical energy development occurs within cells.
Metabolism and biochemical energy development occurs within cells.
 DNA (genetic information) is contained in the cells and is passed to new cells during cell division.
DNA (genetic information) is contained in the cells and is passed to new cells during cell division.
 Similar species’ cells have the same basic chemical makeup.
Similar species’ cells have the same basic chemical makeup.
 The total activity of independent cells makes up the net activity of an organism.
The total activity of independent cells makes up the net activity of an organism.
Cell parts
Cells — both plant and animal — have specific parts that serve particular functions, from cell energy production to genetic reproduction and beyond.
In the following sections, we break down the important cell components.
Membrane
The cell membrane (also known as the plasma membrane) is the structure that separates the inside of a cell from the outside of a cell. The cell membrane is selectively permeable to certain items (specifically, ions and organic molecules) and it can control what substances are able to move in and out of a cell. Cell membranes play a vital role in many different processes, including ion conductivity, cell signaling, and cell adhesion.
Cytoplasm and endoplasmic reticulum
Cytoplasm is the material that resides within the cell membrane. It’s a liquid, gel-like substance that contains the cell’s internal structures except the nucleus.
The endoplasmic reticulum (ER) is a eukaryotic organelle within cells comprising an interconnected network of complex structures. (A eukaryotic organism’s cells contain complex structures enclosed within membranes.) The ER is made up of two parts. The rough endoplasmic reticula make proteins. The smooth endoplasmic reticula metabolize carbohydrates and steroids, regulate the concentration of calcium, make lipids (fatty substances) and steroids, and assist in attaching receptors on cell membrane proteins.
Golgi body
The Golgi body (also called the Golgi apparatus) is found in most eukaryotic cells. After proteins are synthesized, the Golgi body processes them as they make their way to their destinations. The Golgi body plays a crucial role in preparing proteins for movement within and out of the cell. Part of the cellular endomembrane system is formed by the Golgi body.
Mitochondrion and nucleus
A mitochondrion (the plural is mitochondria) is an organelle found in most eukaryotic cells. An organelle is a structure within a cell that performs a role in helping the cell function properly. Mitochondria act as the cell’s power plant because they create most of the cell’s chemical energy source, adenosine triphosphate (ATP). Mitochondria are involved in a range of other processes, including chemical signaling and controlling the cell’s growth and eventual death. A number of human diseases, such as cardiac dysfunction and mitochondrial disorders, have potential mitochondria sources. Studies suggest mitochondria play a role in the aging process as well.
Another membrane-enclosed organelle found in eukaryotic cells is the nucleus, where most of the cell’s genetic material appears. This material is mostly comprised of DNA molecules that are organized into chromosomes. The genes (units of heredity, comprised of specific DNA sequences) in these chromosomes create the cell’s genome and an organism’s specific characteristics. For all practical purposes, the nucleus is a cell’s control center, kind of like the bridge of the ship.
Ribosome
Within plant and animal cells, DNA produces RNA, which in turn produces proteins. Genes contain a DNA sequence that is copied into a messenger RNA (mRNA). After this step, the ribosomes read the information in this mRNA, arrange the required amino acid out of the 20 specific ones, and use it to create proteins in a sequence for genetic transfer. This process is known as translation.
How cells survive and thrive
Ever wonder how the foods you eat give you the eventual energy you need to run five miles? Where does the waste that you eliminate (through defecation and urination) come from? This section gives you the basic knowledge you need on these and related topics for the flight aptitude test.
Metabolism
Metabolism is the process by which cells convert nutrient molecules into energy, and it is further divided into two distinctly different processes. Catabolism is the process through which cells derive their energy by breaking down complex molecules, and anabolism is the process by which cells use catabolic energy to build complex molecules and carry out other cellular tasks.
Osmosis
Osmosis is the tendency of fluids (most often water, in the case of cells) to pass through a semipermeable membrane into a solution where the solvent concentration is higher. This movement of fluids ultimately equalizes the concentration of dissolved materials (solutes) on both sides of the membrane. Osmosis is the primary route for transport of water in and out of cells, and is therefore essential for life.
Phagocytosis
Cells have the capability to engulf or eat substances or particles — kind of like a football player with a pizza — by way of a process called phagocytosis. The result of phagocytosis (found only among eukaryotic cells) is a food vacuole, where the engulfed substances or particles are digested by lysosomes. The engulfed substances or particles enter cells through a cell opening called a cytostome. Some cells use phagocytosis to consume pathogens; other cells use it to generate energy (known as phagotrophic energy, which is different from absorption — a kind of osmotrophic nutrition.
Photosynthesis
Plants, algae, and some types of bacteria use the process of photosynthesis to convert light into chemical energy. The actual process uses sunlight and chlorophyll (the green pigment in plants) to transform water and carbon dioxide gas into glucose (which is used for food) and oxygen — a waste product expelled into the earth’s atmosphere (and gladly breathed by humans and other animals).
6H2O + 6CO2 + light → C6H12O6 (glucose) + 6O2
Cellular respiration
The conversion of energy-laden molecules (for example, molecules of glucose) into energy by breaking down their chemical bonds is known as cellular respiration. With the exception of viruses, all life forms on earth use cellular respiration to create the energy they need to function.
C6H12O6 + 6O2 → 6CO2 + 6H2O + energy
There are two categories of cellular respiration: anaerobic and aerobic. Anaerobic respiration, the more primitive of the two, takes place without oxygen and results in less energy than aerobic respiration does. Aerobic respiration is a more recent innovation in the history of life on earth, and it requires oxygen.
Cellular respiration occurs in three steps:
1. Glycolysis
2. Krebs cycle
3. Electron transport chain
A systemic approach to human anatomy and physiology
You may have taken an anatomy and physiology class in your undergraduate training. Basically, human anatomy is the study of the structures or systems of the human body, and physiology studies the function of these living systems; anatomy discusses what you have, and physiology discusses how it works. In the following sections, we highlight the important concepts of each field in enough detail to prepare you for the exam.
No bones about it: The skeletal system
The skeleton serves an essential function: providing the structure (comprised of both fused and individual bones) that supports the human body with the help of ligaments, tendons, muscles, and cartilage. The six main functions of the skeletal system include the following:
 Supporting the body and maintaining its shape
Supporting the body and maintaining its shape
 Permitting movement at the joints between the bones
Permitting movement at the joints between the bones
 Protecting vital organs
Protecting vital organs
 Serving as the site for hematopoiesis (blood cell formation)
Serving as the site for hematopoiesis (blood cell formation)
 Storing calcium and metabolizing calcium
Storing calcium and metabolizing calcium
 Regulating blood sugar by releasing a hormone called osteocalcin
Regulating blood sugar by releasing a hormone called osteocalcin
Bones within the skeletal system range in size from the tiny stapes bone in the middle ear to the femur, which is the largest bone in the body. In a normal adult, the skeleton comprises about 30 to 40 percent of total body weight, and half of the weight of the skeleton is water.
Putting some muscle on them bones
The muscular system enables humans to move and carry out a variety of other functions by way of the coordinated contraction of muscle tissue. For the most part, muscles don’t function on their own; their action is largely controlled and coordinated through the nervous system. The exception to this rule is certain autonomous muscles (such as the heart) that act independently of this control. (See the later section “Nervous? That’s your nervous system talking” for more on the nervous system.)
The muscular system consists of three types of muscles: skeletal muscles, cardiac muscles, and smooth (non-striated) muscles. In total, more than 600 different muscles make up the human body, ranging in size from the tiny inner-ear muscle that controls the movement of the stapes bone to the large gluteus maximus muscle in the buttock. Now coauthor Terry knows why he was so sore after walking for a year in Iraq.
The cardiovascular and respiratory systems
The cardiovascular system is made up of the heart, blood, and blood vessels, which serve to transport blood throughout the body. Blood is a liquid comprised of plasma (mostly water), oxygen-carrying red blood cells, disease-fighting white blood cells, and blood-clotting platelets. The typical adult body contains an average of five to six quarts of blood (approximately 4.7 to 5.7 liters).
The human cardiovascular system boasts two major kinds of circulation: pulmonary circulation, in which the heart’s pulmonary artery pumps oxygen-depleted blood through the lungs to be enriched with oxygen, and systemic circulation, in which the pulmonary vein moves oxygenated blood from the lungs back to the heart and then on to the rest of the body.
The respiratory system pulls air into the lungs, where the movement of the diaphragm and the contraction of respiration muscles facilitate gas exchange. Molecules of oxygen from the air are taken into the bloodstream, and carbon dioxide is expelled from the bloodstream in the lung alveoli, areas rich with very small blood vessels called capillaries.
Nervous? That’s your nervous system talking
Both the central and the peripheral nervous system play the primary role in the control of behavior and emotions. The central nervous system (CNS) processes the information it receives from the environment and then transmits impulses to the peripheral nervous system to coordinate movements and actions throughout the body.
The brain is the main organ of the nervous system; it’s divided into three parts. First is the brain stem, which is an extension of the spinal cord. It controls a lot of automatic responses and muscles. The second part is the forebrain (which consists mainly of the cerebrum), and the third is the cerebellum. Down the middle of the cerebrum and the cerebellum is a groove that divides the two hemispheres of the brain, which are further divided into lobes. These hemispheres are linked by a thick band of nerve fibers (the corpus callosum) that control electrical impulses back and forth. The brain is connected to various nerves throughout the body via the spinal column; these nerves provide a two-way communication system among the brain; the spinal cord; and parts of the arms, legs, neck, and trunk of the body.
The digestive system
Digestion is the breakdown of large food molecules by both chemical (for example, saliva and stomach acid) and mechanical (for example, chewing) means into smaller particles that can then be absorbed into the bloodstream to be utilized by the cells for energy production and other purposes.
The digestive breakdown starts when you chew the food you eat and is helped along by saliva. This initial process is called mastication. Food then moves down the esophagus into the stomach, where hydrochloric acid kills most contaminating microorganisms (though not all) and further breaks down and chemically alters the food. The hydrochloric acid has a low pH (a chemical scale that labels acidity), which enables digestive enzymes to work more efficiently. The result of the initial digestion is a thick liquid called chyme that goes through the small intestine, where 95 percent of nutrient absorption occurs. (The importance of small intestine absorption is why physicians become so concerned with bowel obstructions.) Finally, after the nutrient-depleted food passes into the large intestine, waste material is collected and water is removed to eventually become fecal matter purged from the body during defecation.
The endocrine system: It’s a hormone thing
The endocrine system is comprised of hormone-producing glands that — through the hormones they produce — are capable of regulating such things as metabolism, sexual development, growth, and much more. (Hormones are powerful chemical messengers that travel in the bloodstream and have an effect on cells, organs, and bodily functions.) Some of the major endocrine glands include the adrenal, pituitary, hypothalamus, thyroid, ovaries, and testes.
Making babies: The reproductive system
The reproductive system or genital system is the complex system of specialized sex organs that work together, along with fluids, hormones, and pheromones, for the purpose of reproduction, sustaining life, and evolution. Although males and females of a particular species often have similarities in major systems, their reproductive systems are typically quite different. For example, men and women have more or less the same circulatory system, but their reproductive systems vary greatly. The ultimate goal of the reproductive system is to produce living, viable offspring, and it accomplishes this goal by uniting one male sperm with one or more female eggs — combining genetic material and producing an individual who shares characteristics of both the father and the mother.
Traditional human reproduction begins through sexual intercourse and leads to internal fertilization. During this process, the male ejaculates semen, a liquid that contains sperm (the male reproductive cells); the sperm then makes its way through the vagina to the uterus and then on to the fallopian tubes where fertilization of the ovum — the female reproductive cells, or eggs — takes place. The successfully fertilized ovum implants itself on the inner wall of the uterus, where the resulting fetus matures for approximately nine months in a process called gestation. The natural process of childbirth is achieved through contractions of the uterus, dilation of the cervix, and the baby’s exit through the vagina. Unlike many other mammals, a human infant is nearly helpless and requires a high level of protection and parental support for years.
Flushing the system: The urinary system
The urinary system has the task of producing, storing, and excreting urine, the liquid waste product produced by your kidneys. In most people, the urinary system is composed of a pair of kidneys (though humans can survive with just a part of one kidney), ureters (which direct urine from the kidneys to the bladder, where urine is stored), and the urethra (which provides a pathway for the urine out of the body). Kidneys are complex organs that perform a variety of tasks, such as regulating electrolytes (such as sodium, potassium, and calcium) and blood pressure, concentrating urine, and maintaining equilibrium.
The lymphatic system
The lymphatic system is a network of vessels (separate from the blood vessels), nodes, and organs that transport lymph throughout the body. Lymph is a clear liquid comprised of interstitial fluid (the fluid surrounding cells in the body), white blood cells, proteins, and fats. The adenoids, spleen, tonsils, and thymus are all a part of the lymphatic system. The system is part of the immune system; it filters cancer cells and bacteria and produces white blood cells and other immune cells that fight disease.
Cracking Open the Chemistry Kit
Oh, chemistry — one of the subjects that tends to make people cringe. Chemistry is the science that deals with substance and matter, the distinct properties of certain types of matter, and how those kinds of matter interact with other types of matter. It also serves as a foundation or building block for other scientific studies, such as physics or biology.
Chemistry breaks down into two basic studies: inorganic and organic. Inorganic chemistry is the division of chemistry that deals with inorganic, or non-carbon compounds. It’s the basic branch of chemistry — the one you probably studied in your basic high-school or college chemistry course.
Organic chemistry is the division of chemistry involving carbon-based compounds, hydrocarbons, and their derivatives. Organic compounds form many everyday products, and organic reactions are chemical reactions involving those organic compounds. These organic reactions are the basis of virtually all biological mechanisms. (Don’t worry; you won’t encounter any complex organic chemistry equations on your flight aptitude test. However, you must be familiar with what organic chemistry is and some of its principal concepts.)
What stuff’s made of
Time to get down to the basic elements of what “stuff” is. The following sections go down to the smallest level so you can grasp the concept of how stuff is combined and how properties of certain elements function.
Matter
Matter is anything that has a definite mass and takes up volume. Three states of matter exist: solid, liquid, and gas. Solids have a defined shape, mass, and volume. Liquids have a defined mass and volume, but not a defined shape. Gases have a mass and no defined shape; they expand to fill any volume and take any shape. Usually, matter transforms in sequence from a solid to a liquid to a gas (for example, think of a solid ice cube that melts into liquid water and then evaporates into water vapor).
Elements
The 118 existing chemical elements make up the billions of different objects in the world. Most materials aren’t made up of just one type of element but rather of a combination of elements called compounds that are joined through chemical reactions (see the later section “The different types of chemical reactions”).
Atoms
The atom is a basic unit of matter. An atom has a compacted central nucleus surrounded by orbitals of negatively charged electrons. Inside the atomic nucleus, you find a mix of positively charged protons as well as neutrons with zero electronic charge. An atom’s electrons are attracted to the nucleus by electromagnetic force. (Head to the following section for details on these particles.) A group of atoms can remain bound to each other and form a molecule.
An atom with equal numbers of protons and electrons is electrically neutral. If the atom has fewer electrons than protons, it has a positive charge; more electrons than protons means a negative charge. An atom with a positive or negative charge is known as an ion. A positively charged ion is a cation, and a negatively charged ion is called an anion.
Subatomic particles
The atom is comprised of three major subatomic particles: protons, neutrons, and electrons. The protons and neutrons in an atom make up the small atomic nucleus (together, they’re called nucleons). All atoms of a particular element always have the same number of protons (referred to as the atomic number); however, atoms of a single element can have different numbers of neutrons. Such atoms are isotopes of that element; flip to the later section “The periodic table” for more details.
Here’s the lowdown on what you need to know about the subatomic particles:
 Electrons: The negatively charged electron has a mass of 9.11 × 10−31 kilograms; that’s the lowest mass of the three major subatomic particles. The electron’s size is too small to be measured; rather, its size has been determined through scientific experimentation and calculation.
Electrons: The negatively charged electron has a mass of 9.11 × 10−31 kilograms; that’s the lowest mass of the three major subatomic particles. The electron’s size is too small to be measured; rather, its size has been determined through scientific experimentation and calculation.
Electrons circle the nucleus in defined orbits. These electrons are transferred to and received from other elements during chemical reactions to form a bond (the strength varies). This exchange of electrons is the basis for millions of chemical reactions. Therefore, the number of electrons in an atom determines that atom’s chemical properties.
 Protons: The positively charged proton has a mass of 1.6726 × 10−27 kilograms (more than 1,800 times larger than the electron’s mass) and is about the same size as a neutron. As we note earlier in the section, the number of protons in an atom determines the element — period! Say you have an atom with six protons. Every atom that has six protons is a carbon atom, so you know your atom is carbon regardless of how many neutrons it has.
Protons: The positively charged proton has a mass of 1.6726 × 10−27 kilograms (more than 1,800 times larger than the electron’s mass) and is about the same size as a neutron. As we note earlier in the section, the number of protons in an atom determines the element — period! Say you have an atom with six protons. Every atom that has six protons is a carbon atom, so you know your atom is carbon regardless of how many neutrons it has.
 Neutrons: The neutrally charged neutron has a mass of 1.6929 × 10−27 kilograms (approximately the same as the proton). Elements are typically in their normal state when the number of protons and neutrons are equal (for your test purposes, you don’t need to worry about the exceptions).
Neutrons: The neutrally charged neutron has a mass of 1.6929 × 10−27 kilograms (approximately the same as the proton). Elements are typically in their normal state when the number of protons and neutrons are equal (for your test purposes, you don’t need to worry about the exceptions).
Orbital shells and valence electrons
An electron shell is basically an orbit or electronic cloud circling an atom’s nucleus. The fundamental principle of chemistry is the sharing of electrons from the outermost, or valence, shell between elements. The valence electrons are those electrons circling in the outermost shell of an atom that can give or capture valence electrons to or from another element. A single covalent bond occurs when both atoms contribute one valence electron to form a shared pair, thus making the molecule inherently more stable. For the main group elements (see the later section “The periodic table”), only the outer electrons are valence electrons. However, keep in mind that in the transition metals, some inner-shell electrons are also valence electrons capable of being shared.
Valence electrons are a crucial concept because they give you a clue for predicting chemical behavior. Atoms tend to have a set number of orbital shells. When an atom contains a complete (closed) outer shell, its bonding electrons are all accounted for. Such atoms are considered stable. Because stable atoms’ electrons don’t easily interact with one another, these atoms aren’t very reactive with other atoms. However, an atom with one or two more or fewer valence electrons in the outside orbital than what’s required for a closed shell can easily give those electrons or receive more electrons to equalize the shell and become stable. This kind of atom is highly reactive, or unstable.
The periodic table
The periodic table (or, more officially, the periodic table of elements) is a display of the 118 known chemical elements arranged according to particular properties of their atomic structures. Specifically, it orders elements by increasing atomic number in periods (rows) and groups (columns). Check out the periodic table online at www.dummies.com/how-to/content/periodic-table-of-elements0.navId-403202.html; as you can see, the rectangular table includes gaps in the periods to group together elements with similar properties. For example, the table contains groups for alkali metals and halogens.
This table may seem confusing at first, but it’s very useful after you understand how it works. When you read from left to right across the table, the elements are arranged in order of the number of protons in the nucleus. Hydrogen (H) is first because it has one proton; helium (He), the next element to the right (despite the gap), has two protons; and so on.
On some periodic tables, you also see an atomic mass number, which is the sum of the numbers of protons and neutrons, under the symbol; atomic masses correspondingly increase as you go across and down the table.
Basically, the elements within each group react similarly because they all have the same number of valence electrons in the outer shell or orbit (we discuss these concepts in the earlier section “Orbital shells and valence electrons”). An example is the column group 1A, which contains H, Li, Na, K, Rb, Cs, and Fr. These elements all have one electron in the outer shell. All elements in group 2A have two electrons in the outer shell. Group 6A’s elements contain six electrons in the outer orbit, and the elements in group 8A contain eight valence electrons. All the elements in a group either freely accept or give off an electron based on what will make the shell fuller and therefore more stable.
As we mention earlier in the chapter, an atom with a different number of neutrons than its element usually is an isotope of that element. For example, a copper atom has 29 protons; anything with 29 protons is a copper atom. But these 29-proton atoms don’t always have the same number of neutrons; some have 34 neutrons, and some have 36 neutrons. These different varieties are two different isotopes. Elemental isotopes, because they have a differing number of neutrons, have a different mass than the “regular” form of the element. The atomic mass listed on a periodic table is the average of the atomic masses for naturally occurring isotopes.
The different types of chemical reactions
A chemical reaction is the process that transforms one set of chemical substances called reactants or reagents into another substance or substances known as the product. For example, the reactants hydrogen and oxygen can chemically react to form the product water. The process of chemical reaction is responsible for life itself. Chemical reactions typically (but not always) require some form of energy input such as heat, light, or electricity to induce the reaction. Reactions can be exothermic, where energy is released (that is, the reaction gives off heat), or endothermic, where heat is absorbed.
How chemical reactions are written
Generally speaking, a chemical reaction is written to show the reactants’ transformation to the product. As an example, take one of the most basic fuels for equation purposes, propane. Propane (C3H8) is combined with oxygen (O2) to get carbon dioxide (CO2) plus water (H2O). You first write this reaction as (a) C3H8 + (b) O2 → (c) H2O, where a, b, and c are the numbers required to balance the equation to follow the law of conservation of mass. Your final equation is (1) C3H8 + (5) O2 → (3) CO2 + (4) H2O or C3H8 + 5O2 → 3CO2 + 4H2O.
It’s basic, or acidic
Acid-base reactions involve transferring protons from one molecule (an acid) to another (a base). Acids act as proton donors and bases as proton acceptors. The following equation represents an acid-base reaction, where HA is acid, B is base, A– is conjugated base, and HB+ is conjugated acid. (A conjugated acid or base is just one that loses or gains a proton.)

Picking up a Brief Course in Physics
The term physics can intimidate a lot of students; coauthor Terry recalls dreading the subject until his instructor told him one day that physics is simply a course on the application of math and science to everyday life. Hearing that put Terry more at ease; in fact, physics became his favorite subject. This section aims to follow that lead by reviewing how to apply math and science to everyday problems. Thank you, Mr. Schembeckler.
Mass (and weight)
Mass and weight are two different animals. Mass is the amount of matter contained within an object, while weight is the force exerted by gravity on the object’s mass. This force acts on an object whether the object is falling, resting, or being elevated and results in a downward acceleration of 9.81 meters per second squared. The formula for calculating weight is
W = mg
where W is weight, m is the mass of an object, and g is the acceleration that results from gravity.
Motion and Newton’s laws
Motion occurs when an object is moved from one point to another. Three types of motion exist: translational (linear or moving in a straight line), rotational (motion occurring about an axis), and vibrational (motion around a fixed point).
All motion is governed by Sir Isaac Newton’s three laws of motion. These laws are important to know for your aptitude test because every aviation achievement begins with these fundamental concepts; they’re the scientific building blocks enabling modern military aviation. Drumroll, please:
 Newton’s First Law of Motion: A body at rest tends to remain at rest, and a body in motion tends to remain in motion unless acted upon by an outside force.
Newton’s First Law of Motion: A body at rest tends to remain at rest, and a body in motion tends to remain in motion unless acted upon by an outside force.
 Newton’s Second Law of Motion: An object will change velocity if it is pushed or pulled on. When an object is acted upon by an outside force, the acceleration is directly proportional to the applied force and inversely proportional to the mass of the object. This law derives the formula of F = ma, where F is the force acting on an object, m stands for the object’s mass, and a is the object’s acceleration.
Newton’s Second Law of Motion: An object will change velocity if it is pushed or pulled on. When an object is acted upon by an outside force, the acceleration is directly proportional to the applied force and inversely proportional to the mass of the object. This law derives the formula of F = ma, where F is the force acting on an object, m stands for the object’s mass, and a is the object’s acceleration.
 Newton’s Third Law of Motion: For every action, there is an equal and opposite reaction.
Newton’s Third Law of Motion: For every action, there is an equal and opposite reaction.
The second law deals with acceleration. To understand acceleration, you must first look at velocity. Velocity is a descriptor of how fast an object is moving.
Velocity = Displacement ÷ time
(For comparison, the formula for speed is Speed = Distance traveled ÷ time.)
In general, the increase in velocity over time is called acceleration, and the decrease in velocity over time is called deceleration. You can calculate acceleration with the following formula:
Acceleration = Change in velocity ÷ time
Motion in a plane (not in an airplane)
The preceding section gives you some conceptual ideas on motion; here you can look at how motion is applied. Motion can occur in one dimension, such as a car moving along a road or a ball thrown upward into the air. The big difference between these examples is the effect of gravity on the objects.
Kinematics equations involve five variables and can be used to mathematically solve for velocity and acceleration. (Kinematics is the study of the motion of a body.) If you know any three of the variables, you can easily find the rest. The five variables are
 D = displacement
D = displacement
 a = acceleration
a = acceleration
 Vi = initial velocity
Vi = initial velocity
 Vf = final velocity
Vf = final velocity
 T = time
T = time
Given a constant acceleration, the equations that you use to find whichever variables you’re missing are
 Vf = Vi + aT
Vf = Vi + aT
 D = Vi T + 1/2aT2
D = Vi T + 1/2aT2
 D = 1/2(Vi + Vf) × T
D = 1/2(Vi + Vf) × T
 Vf2 = Vi2 + 2aD
Vf2 = Vi2 + 2aD
Force
Force is any kind of input that causes an object to experience a change in direction, speed, or shape. Force has both magnitude and direction, which makes it a vector quantity. The formula for force (measured in newtons) is as follows:
F = ma
where F is the force, m is the mass, and a is the acceleration.
Force can be frictional, electromagnetic, or gravitational. Static frictional force is the force that opposes any movement of an object when the object is at rest, and kinetic frictional force is created by the opposing forces between the surfaces of objects that are in relative motion. Gravitational force is the effect of gravity on an object. You don’t need to worry about electromagnetic force for the flight aptitude test. (Note: Other forces exist, but you don’t need those for the test either.)
Energy and work
Energy is defined as the potential to do work. The energy of an object can be divided into two types: potential and kinetic. Potential energy is the energy that an object has because of its position (for example, the bowling ball sitting in the upper shelf of your closet). Kinetic energy is energy that results from motion. Both potential and kinetic energy change when work is done by or on an object. Work is the transfer of energy to an object when the object, because of the application of a force, moves. You can calculate the work done on an object by using the following formula:
W = F × d
where W is work in joules, F is the force in newtons, and d is the distance in meters.
The work you do against gravity is gravitational potential energy, and you can calculate it by using the following formula:
PE = mgh
where PE is the potential energy in joules, m is the mass, g is the acceleration because of gravity (9.8 meters/second squared), and h is the height above ground in meters.
Pulleys
A pulley is a device used to exert rotational motion, alter an applied force’s direction, or gain mechanical advantage. Pulleys usually take the form of a belt, rope, cable, or chain that runs over a grooved wheel on a shaft. The mechanical advantage derived from the pulley or combination of pulleys is calculated by dividing the weight lifted by the lifting force.
Pulley systems come in three different types (see Figure 9-1):
 Fixed: As the name indicates, a fixed pulley is anchored in place. This kind of pulley is also known as a class 1 pulley.
Fixed: As the name indicates, a fixed pulley is anchored in place. This kind of pulley is also known as a class 1 pulley.
 Movable: A movable pulley is not anchored in place but rather is able to move. This kind of pulley is also known as a class 2 pulley.
Movable: A movable pulley is not anchored in place but rather is able to move. This kind of pulley is also known as a class 2 pulley.
 Compound: A compound pulley combines fixed and movable pulleys into a single system, gaining the advantages (and disadvantages) of each.
Compound: A compound pulley combines fixed and movable pulleys into a single system, gaining the advantages (and disadvantages) of each.
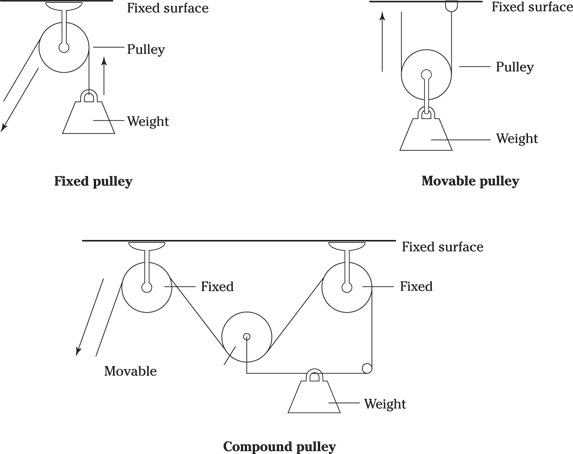
Illustration by Wiley, Composition Services Graphics
Figure 9-1: The three types of pulleys.
Levers
A lever is simply a mechanical device in which rotating around a central point (called the fulcrum) allows you to achieve your desired goal by either minimizing your input force or maximizing your output force. In short, a lever reduces the force required to move or lift a weight. Figure 9-2 shows you the two types of levers that you may encounter on the various flight aptitude tests.
 A first class lever (Figure 9-2a) rotates around a fulcrum to achieve a load, or effect, on the opposite side of the fulcrum from the applied force.
A first class lever (Figure 9-2a) rotates around a fulcrum to achieve a load, or effect, on the opposite side of the fulcrum from the applied force.
 A second class lever (Figure 9-2b) reduces the effort to achieve a lifting result with both forces on the same side of the fulcrum.
A second class lever (Figure 9-2b) reduces the effort to achieve a lifting result with both forces on the same side of the fulcrum.
Anyone who has ever used pliers or carried a wheelbarrow full of dirt has utilized a lever.
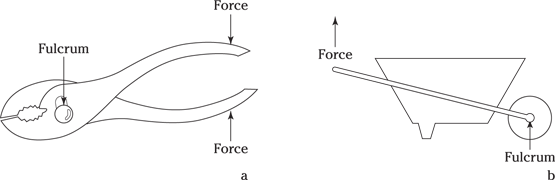
Illustration by Wiley, Composition Services Graphics
Figure 9-2: First (a) and second class (b) levers.
Fluids
A fluid is any substance that changes its shape under pressure; both gases and liquids are considered fluids. Pressure in a fluid is the force exerted on the area and is shown by the following equation:
P = F ÷ A
where P is the pressure (in pascals), F is the force (in newtons) and A is the area (in square meters).
Three principles govern fluids: Archimedes’s, Pascal’s, and Bernoulli’s.
 Archimedes’s principle: An object immersed in a fluid is buoyed up by a force equal to the weight of the fluid that the object displaces. The magnitude of the force is given by the equation
Archimedes’s principle: An object immersed in a fluid is buoyed up by a force equal to the weight of the fluid that the object displaces. The magnitude of the force is given by the equation
F = ρVg
where F is the force, ρ is the density of the fluid, V is the volume, and g is the acceleration because of gravity.
 Pascal’s principle: Any pressure applied to a confined fluid, at any point, is transmitted undiminished throughout the fluid. This principle is represented by the following equation:
Pascal’s principle: Any pressure applied to a confined fluid, at any point, is transmitted undiminished throughout the fluid. This principle is represented by the following equation:
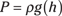
 Bernoulli’s principle: As the velocity of a fluid increases, the pressure exerted by that fluid decreases. This principle makes your future in aviation possible because the low area of pressure creates a lifting force that allows planes to fly.
Bernoulli’s principle: As the velocity of a fluid increases, the pressure exerted by that fluid decreases. This principle makes your future in aviation possible because the low area of pressure creates a lifting force that allows planes to fly.
The following equation shows Bernoulli’s principle:
P(static pressure) + P(dynamic pressure) = P(total pressure)
Dynamic pressure is the velocity pressure (or kinetic energy) for the fluid at a certain point. You can find dynamic pressure by using the following formula:
P(dynamic pressure) = (ρ × velocity2) ÷ 2
Electricity
Electricity involves the flow of an electrical current (energy) from a source (battery or electrical outlet) to a load (light or motor). A load is a device that transforms electrical energy into other forms of energy. The electrical energy transported via electrical current consists of a flow of electrons. For an electrical current to flow in a conductor (such as an extension cord), a potential difference or voltage must exist between the conductor’s ends. The greater the voltage is, the greater the current is. All substances, from a wire to a piece of wood, offer resistance to an electrical current; the amount resistance depends on the material’s length, area, temperature, and an intrinsic value of the property called resistivity.
According to Ohm’s law, when an electrical current passes through a conductor between two points, the current is proportional to the potential difference across the two points. Ohm’s law is represented by the following equation:
I = V ÷ R
where I is current in amperes, V is voltage in volts, and R is resistance in ohms.
The chart in Figure 9-3 helps you take any variables given and find the answer you seek.
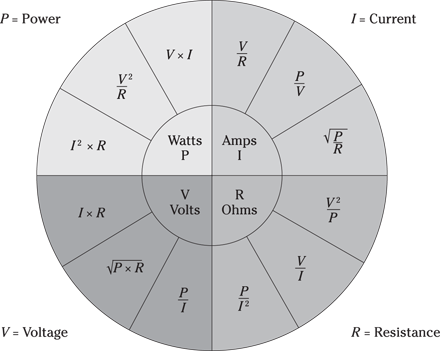
Illustration by Wiley, Composition Services Graphics
Figure 9-3: Various formulas dealing with electricity.
Sound and light waves
Two common physics principles are sound and light waves. Sound waves are pressure variations that are transmitted through matter. The speed at which sound travels depends on temperature and the medium (air) in which the sound waves travel. Here are a few sound wave highlights:
 When sound waves hit a hard surface, they reflect off it, causing an echo.
When sound waves hit a hard surface, they reflect off it, causing an echo.
 The number of compressions by sound waves that occur in one second is called the frequency or pitch of the sound.
The number of compressions by sound waves that occur in one second is called the frequency or pitch of the sound.
 If the source of the sound is in motion (such as a car coming down the street), you hear or perceive sound of higher or lower frequencies. This sensation is because of the Doppler effect.
If the source of the sound is in motion (such as a car coming down the street), you hear or perceive sound of higher or lower frequencies. This sensation is because of the Doppler effect.
Light waves can be visible or nonvisible. A light wave is measured in terms of its wavelength, or how far it travels before the wave’s shape repeats itself. If you know the distance between repeating corresponding points of the same phase of the wave, you can figure out wavelength.
The wavelength λ of a sinusoidal waveform (a smooth succession of curves) traveling at the speed of light v (about 3 × 108 meters per second) is given by
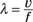
where f is the wave’s frequency.
Pendulums
For any pendulum problem, remember that given a small initial swing, the pendulum’s weight has no impact on the frequency (the number of periods — back-and-forth swings — the pendulum makes per second). Rather, the frequency (f) is affected by the length of the attachment string and the acceleration caused by gravity. Frequency is measured in hertz; you can calculate it by using the following formula, where g is the acceleration becasue of gravity and L is the length of the string:
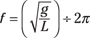
Figure 9-4 shows the movement of a pendulum.
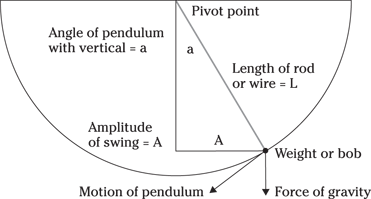
Illustration by Wiley, Composition Services Graphics
Figure 9-4: A pendulum.
Getting down to Earth Science
Earth science is the study of the world we live in and even other planets. Everything from the internal core of the earth to the upper atmosphere is covered in this branch of science. The following sections cover some important earth science topics.
Earth trivia
Here are some interesting facts about the earth to give you a better insight to the planet:
 Age: 4.5 to 4.6 billion years
Age: 4.5 to 4.6 billion years
 Atmospheric content: 77 percent nitrogen; 21 percent oxygen; and traces of argon, carbon dioxide, and water
Atmospheric content: 77 percent nitrogen; 21 percent oxygen; and traces of argon, carbon dioxide, and water
 Chemical composition: 34.6 percent iron, 29.5 percent oxygen, 15.2 percent silicon, 12.7 percent magnesium, 2.4 percent nickel, 1.9 percent sulfur, and 0.05 percent titanium
Chemical composition: 34.6 percent iron, 29.5 percent oxygen, 15.2 percent silicon, 12.7 percent magnesium, 2.4 percent nickel, 1.9 percent sulfur, and 0.05 percent titanium
 Ratio of water to land: 70.8 percent to 29.2 percent
Ratio of water to land: 70.8 percent to 29.2 percent
 Highest elevation: Mt. Everest, 29,035 feet (8,850 meters)
Highest elevation: Mt. Everest, 29,035 feet (8,850 meters)
 Tallest mountain from base to peak: Mauna Kea, Hawaii, 33,480 feet (10,204 meters)
Tallest mountain from base to peak: Mauna Kea, Hawaii, 33,480 feet (10,204 meters)
 Lowest elevation: Dead Sea, 1,369 feet (417.27 meters) below sea level
Lowest elevation: Dead Sea, 1,369 feet (417.27 meters) below sea level
 Deepest ocean depth: Challenger Deep, Mariana Trench (in the western Pacific Ocean), 35,840 feet (10,924 meters)
Deepest ocean depth: Challenger Deep, Mariana Trench (in the western Pacific Ocean), 35,840 feet (10,924 meters)
 Point at greatest distance from center of the earth: Peak of volcano Chimborazo, Ecuador, 20,561 feet (6,267 meters)
Point at greatest distance from center of the earth: Peak of volcano Chimborazo, Ecuador, 20,561 feet (6,267 meters)
 Highest recorded temperature: Al Aziziyah, Libya, September 13, 1922, 135.8 degrees Fahrenheit (57.7 degrees Celsius)
Highest recorded temperature: Al Aziziyah, Libya, September 13, 1922, 135.8 degrees Fahrenheit (57.7 degrees Celsius)
 Lowest recorded temperature: Vostok, Antarctica, July 21, 1983, –128.5 degrees Fahrenheit (–89.2 degrees Celsius)
Lowest recorded temperature: Vostok, Antarctica, July 21, 1983, –128.5 degrees Fahrenheit (–89.2 degrees Celsius)
 Diameter at the equator: 7,926.28 miles (12,756.1 kilometers)
Diameter at the equator: 7,926.28 miles (12,756.1 kilometers)
 Diameter at the North and South Poles: 7,899.80 miles (12,713.5 kilometers)
Diameter at the North and South Poles: 7,899.80 miles (12,713.5 kilometers)
 Circumference at the equator: 24,901.55 miles (40,075.16 kilometers)
Circumference at the equator: 24,901.55 miles (40,075.16 kilometers)
 Circumference between the North and South Poles: 24,859.82 miles (40,008 kilometers)
Circumference between the North and South Poles: 24,859.82 miles (40,008 kilometers)
 Rotation rate on axis: 23 hours, 56 minutes, and 4.09053 seconds
Rotation rate on axis: 23 hours, 56 minutes, and 4.09053 seconds
 Revolution rate around the sun: 365.2425 days
Revolution rate around the sun: 365.2425 days
 Distance from the sun: 93,020,000 miles (149,669,180 kilometers) on average
Distance from the sun: 93,020,000 miles (149,669,180 kilometers) on average
 Distance from the moon: 238,857 miles (384,403.1 km) on average
Distance from the moon: 238,857 miles (384,403.1 km) on average
Standard temperature and pressure use for aviation planning purposes is 59 degrees Fahrenheit (15 degrees Celsius) and 29.92 inches of mercury of pressure. These figures are a standard or average to base aircraft performance on. You calculate the actual performance based on flight performance charts and density altitude (the pressure altitude adjusted for non-standard temperature). Standard altitude temperature lapse rate is 2 degrees Celsius or 3 degrees Fahrenheit for every thousand feet of elevation climbed above the surface.
The earth in layers
Our planet is a fascinating example of planetary structure, evolution, and ecosystems. The following sections look at some of the structures that make this planet unique and capable of supporting life.
Layers of the planet
The interior structure of the earth is layered. You don’t need to get into too much depth here (no pun intended); just know that the earth has an outer crust, a mantle, a liquid outer core, and a solid inner core.
Atmospheric layers
Onward and upward. Most people who are reading this text have probably been fascinated with the sky and flying for as long as they can remember. The sky, or atmosphere, is really a sea of gas with varying levels of concentration and life-sustaining capabilities. Here’s a breakdown of your future office space:
 Troposphere: This layer begins at the earth’s surface and goes 4 to 12 miles (6 to 20 kilometers). You’ll do most of your flying in this layer. The transition boundary between the troposphere and the stratosphere is called the tropopause.
Troposphere: This layer begins at the earth’s surface and goes 4 to 12 miles (6 to 20 kilometers). You’ll do most of your flying in this layer. The transition boundary between the troposphere and the stratosphere is called the tropopause.
 In general, you have to start worrying about oxygen when you fly for a given time at 10,000 feet above sea level (well within the troposphere). Don’t worry; though; flight training will give you experience with oxygen depletion.
In general, you have to start worrying about oxygen when you fly for a given time at 10,000 feet above sea level (well within the troposphere). Don’t worry; though; flight training will give you experience with oxygen depletion.
 Stratosphere: The stratosphere extends from the top of the troposphere to around 31 miles (50 kilometers). This layer contains very little water vapor, but 19 percent of the atmosphere’s gases occur here. The boundary that separates the stratosphere from the mesosphere is called the stratopause. The troposphere and stratosphere together are known as the lower atmosphere.
Stratosphere: The stratosphere extends from the top of the troposphere to around 31 miles (50 kilometers). This layer contains very little water vapor, but 19 percent of the atmosphere’s gases occur here. The boundary that separates the stratosphere from the mesosphere is called the stratopause. The troposphere and stratosphere together are known as the lower atmosphere.
 Mesosphere: This atmospheric layer extends from the top of the stratosphere to about 56 miles (90 kilometers). Gases here continue to become thinner and thinner the higher you go. The boundary separating the mesosphere from the thermosphere is called the mesopause.
Mesosphere: This atmospheric layer extends from the top of the stratosphere to about 56 miles (90 kilometers). Gases here continue to become thinner and thinner the higher you go. The boundary separating the mesosphere from the thermosphere is called the mesopause.
 Thermosphere: Above the mesosphere, the thermosphere or upper atmosphere reaches to almost 375 miles (600 kilometers).
Thermosphere: Above the mesosphere, the thermosphere or upper atmosphere reaches to almost 375 miles (600 kilometers).
 Exosphere: The exosphere is the outermost layer of the atmosphere. It goes from the top of the thermosphere to 6,200 miles (10,000 kilometers) above the earth.
Exosphere: The exosphere is the outermost layer of the atmosphere. It goes from the top of the thermosphere to 6,200 miles (10,000 kilometers) above the earth.
Exploring Your Solar System
From Star Trek to Star Wars to Facebook pages demanding Pluto be reinstated as a planet, humans are fascinated with space and the distant planets. In the following sections, you can find some good information to know about our solar system for your aptitude test.
Sun facts and figures
The sun is a medium-sized, spherical star that just happens to be located at the center of our solar system. (Of course, medium is relative; the sun’s diameter is about 1.392 million kilometers, and its mass is approximately 2 × 1030 kilograms, nearly 99 percent of the total mass of the solar system.) It’s comprised of 71 percent hydrogen, 27.1 percent helium, and less than 2 percent of assorted other elements. The sun’s surface temperature is in the neighborhood of 5,780 degrees Kelvin. It’s a very hot place.
Closer to the sun: The inner planets
Astronomers decided somewhere along the line that dividing up the planets into two groups was a good idea. No, we’re not talking about lists of naughty and nice planets or anything like that. The divisions in question deal with the planets’ proximity to the sun. The inner planets include some of our personal favorites: Mercury, Venus, Earth, and Mars, which are also known as the terrestrial planets:
 Mercury: Mercury is the planet closest to the sun, and because it has just a smidgen of atmosphere, its surface is pocked with meteor craters. Mercury is the smallest of all the planets in the solar system, and it has no moons. Although it’s no sun, it can be a hot place, with high temperatures of up to about 800 degrees Fahrenheit. But it knows extreme cold, too; temperatures on the side away from the sun dip to about –280 degrees Fahrenheit.
Mercury: Mercury is the planet closest to the sun, and because it has just a smidgen of atmosphere, its surface is pocked with meteor craters. Mercury is the smallest of all the planets in the solar system, and it has no moons. Although it’s no sun, it can be a hot place, with high temperatures of up to about 800 degrees Fahrenheit. But it knows extreme cold, too; temperatures on the side away from the sun dip to about –280 degrees Fahrenheit.
 Venus: The second planet from the sun is Venus — a planet that shares much in common with Earth, including (approximately) size, mass, density, and elemental composition. However, the similarities with Earth pretty much end there. Venus’s thick, cloudy atmosphere (which makes it the brightest object in the night sky after Earth’s moon) would be toxic to humans — it’s comprised mostly of carbon dioxide gas with a fine mist of sulfuric acid droplets. (Sounds lovely, doesn’t it?) Surface temperatures on Venus run to about 880 degrees Fahrenheit, but scientists believe that the planet once had extensive oceans. Any oceans are long gone today, though, replaced by large fields of hardened basaltic lava.
Venus: The second planet from the sun is Venus — a planet that shares much in common with Earth, including (approximately) size, mass, density, and elemental composition. However, the similarities with Earth pretty much end there. Venus’s thick, cloudy atmosphere (which makes it the brightest object in the night sky after Earth’s moon) would be toxic to humans — it’s comprised mostly of carbon dioxide gas with a fine mist of sulfuric acid droplets. (Sounds lovely, doesn’t it?) Surface temperatures on Venus run to about 880 degrees Fahrenheit, but scientists believe that the planet once had extensive oceans. Any oceans are long gone today, though, replaced by large fields of hardened basaltic lava.
 Earth: The third planet from the sun is our home sweet home planet Earth. Check out the earlier section “Getting down to Earth Science” for the nitty-gritty on the earth.
Earth: The third planet from the sun is our home sweet home planet Earth. Check out the earlier section “Getting down to Earth Science” for the nitty-gritty on the earth.
 Mars: The fourth planet from the sun — and the last of the inner planets — is Mars. It’s also known as the Red Planet because of the iron oxide content of its surface, which gives it a red appearance when viewed from telescopes. Mars has a thin atmosphere with surface features that are similar to the craters of Earth’s moon, volcanoes, and ice caps. Mars has two moons, Phobos and Deimos, and surface temperatures that range from about –125 degrees Fahrenheit to about 23 degrees Fahrenheit.
Mars: The fourth planet from the sun — and the last of the inner planets — is Mars. It’s also known as the Red Planet because of the iron oxide content of its surface, which gives it a red appearance when viewed from telescopes. Mars has a thin atmosphere with surface features that are similar to the craters of Earth’s moon, volcanoes, and ice caps. Mars has two moons, Phobos and Deimos, and surface temperatures that range from about –125 degrees Fahrenheit to about 23 degrees Fahrenheit.
Planets on the fringe: The outer planets
The planets beyond Mars are known as the outer planets or Jovian gas giant planets. These planets are primarily made up of the same stuff as the sun: helium and hydrogen. Here are some other facts about these far-out neighbors:
 Jupiter: The fifth planet from the sun is Jupiter, by far the largest planet in our solar system. Hydrogen makes up about 75 percent of Jupiter’s total mass, and helium accounts for the other 25 percent. In fact, if Jupiter’s mass were just 80 times greater than it is, the planet would have ignited and become a second sun within our solar system. Jupiter’s upper atmosphere is quite turbulent, with strong winds that push the planet’s ammonia clouds into long stripes. Jupiter’s Great Red Spot is the result of a huge storm that has raged for centuries, and the planet’s magnetic field is 20,000 times stronger than the Earth’s. Jupiter’s average temperature is –234 degrees Fahrenheit.
Jupiter: The fifth planet from the sun is Jupiter, by far the largest planet in our solar system. Hydrogen makes up about 75 percent of Jupiter’s total mass, and helium accounts for the other 25 percent. In fact, if Jupiter’s mass were just 80 times greater than it is, the planet would have ignited and become a second sun within our solar system. Jupiter’s upper atmosphere is quite turbulent, with strong winds that push the planet’s ammonia clouds into long stripes. Jupiter’s Great Red Spot is the result of a huge storm that has raged for centuries, and the planet’s magnetic field is 20,000 times stronger than the Earth’s. Jupiter’s average temperature is –234 degrees Fahrenheit.
 Saturn: The sixth planet from the sun is Saturn. The planet has 52 known moons and is notable for its complex system of rings, comprised of nine continuous and three discontinuous main rings. The rings are primarily made up of ice crystals, dust, and rocky debris. Although Saturn’s mass is more than 95 times that of Earth’s, its average density is just one-eighth that of our own planet. The average temperature on Saturn is a frosty –288 degrees Fahrenheit.
Saturn: The sixth planet from the sun is Saturn. The planet has 52 known moons and is notable for its complex system of rings, comprised of nine continuous and three discontinuous main rings. The rings are primarily made up of ice crystals, dust, and rocky debris. Although Saturn’s mass is more than 95 times that of Earth’s, its average density is just one-eighth that of our own planet. The average temperature on Saturn is a frosty –288 degrees Fahrenheit.
 Uranus: The seventh planet from the Sun is Uranus, which has 11 known rings and 27 known moons. Uranus is larger than all the other planets except for Jupiter and Saturn, and its average temperature is –357 degrees Fahrenheit.
Uranus: The seventh planet from the Sun is Uranus, which has 11 known rings and 27 known moons. Uranus is larger than all the other planets except for Jupiter and Saturn, and its average temperature is –357 degrees Fahrenheit.
 Neptune: The planet farthest from the sun is Neptune, which is slightly smaller than Uranus; its mass is 17 times that of Earth. Neptune’s temperature averages –353 degrees Fahrenheit.
Neptune: The planet farthest from the sun is Neptune, which is slightly smaller than Uranus; its mass is 17 times that of Earth. Neptune’s temperature averages –353 degrees Fahrenheit.
Not quite a planet: Pluto
As you’ve probably heard by now, Pluto was categorized as a planet for decades. However, the International Astronomical Union stripped this designation from Pluto in 2006 and reclassified it as a dwarf planet. We’re including it in the discussion here anyway because it’s still an important entity. Pluto is the second-most-massive known dwarf planet and the tenth-most-massive body that directly orbits the sun. It has three known moons and is composed primarily of rock and ice, with a total mass approximately one-fifth the mass of Earth’s moon. It’s quite cold at a temperature of about –356 degrees F.
Putting Science to Good Use: Applied Sciences
The meaning of applied science is spelled out in its name; this branch applies concepts from other sciences toward your environment.
Digging agriculture: A major growth industry
Agriculture (also known as farming) is the backbone of most countries, and it deals with all the processes that get food on the table, including feed production, fertilizers, land management, and food growth. Subsistence agriculture, where a farmer produces enough food to meet his family’s needs, was the primary type of farming in the United States until the turn of the 20th century, and it’s still the principal type of farming in many undeveloped nations.
The change from subsistence to commercial agriculture, where growers produce crops in large quantities specifically to sell as a commercial product, was largely responsible for the migration of people in the rural United States to cities. Agriculture also produces consumption goods such as nursery plants, animal hides, industrial chemicals, fibers (such as cotton or wool), and now even fuel through the use of corn ethanol. A subsection of agriculture is animal husbandry, which concerns breeding and raising animals for meat and products (such as milk, eggs, or wool) on a continual basis.
One goal of farmers worldwide is to make farming more efficient in order to feed more people with the same amount of land and natural resources. In the United States, for example, improved science and farming practices, soil conservation, pesticides, genetic engineering, and modern machinery have greatly increased yields per acre. One of the key components of modern warfare is teaching unimproved nations how to implement subsistence farming and thereby win the hearts and minds of that country’s people. While serving in Iraq, coauthor Terry was fortunate enough to witness troops teaching the local populations drip irrigation, sugar refining, and catfish farming.
Planting your roots in forestry
Forestry is the practice of growing, managing, harvesting, and conserving forests and their associated resources for the use of mankind. The nature of forestry (no pun intended) is to continue a system that allows for a renewable resource that’s both very forward-thinking toward sustainability and able to meet the current needs of a growing economy. Forestry has come a long way from the past practices of clear-cutting entire forests and not worrying about future growth. The challenge of the forestry industry is to balance maintaining company profitability, meeting the resource needs of a growing population, and managing assets in a way that is acceptable to environmental groups.
Keeping others healthy with the health sciences
From medicine to dentistry, the health sciences are an all-encompassing field geared toward maintaining a healthy life, preventing of diseases, and repairing injuries. Two important fields in this category are medicine and nutrition, which you can read about in the following sections.
Medicine
Medicine is the science that deals with the health and well being of the population. Medicine includes current treatment, scientific research, and preventative practices, all in a variety of specialties. Many advances in the modern medical world have been developed by the different branches of the military services.
The medical field works as an interdisciplinary team and includes many highly trained health professionals; coauthor Terry is proud to now be a member of this community. In the lead are medical providers such as physicians, dentists, physician assistants, and nurse practitioners; these folks are assisted by nurses, emergency medical responders, pharmacists, X-ray and laboratory technicians, and many others, with behind-the-scenes research by scientists and research doctors. And of course, don’t forget the medical evacuation pilots and flight crews who transport the critically ill both from the front-line battlefield and to and from hospitals throughout the world.
Nutrition
Nutrition is providing, at a cellular level, the substances necessary to support life. A proper diet can prevent or alleviate many common health problems (such as cardiovascular disease, diabetes, and obesity). On the flip side, poor dietary practices can negatively impact health; they’ve been estimated to lead to approximately 30 to 50 percent of annual U.S. health care expenditures.
