An enormous number of ecological factors are associated with several hundred species of hummingbirds and their foraging adaptations, as well as related adaptations of the hundreds of species of plants that have coevolved with hummingbirds with respect to mechanisms favoring efficient pollination. The relationship between plants and hummingbirds is an old and close one; in western North America alone about 130 species of plants exhibit features apparently modified through evolution for foraging and pollination by hummingbirds (Grant and Grant, 1968). Another 20 species or more of eastern North American plants have been similarly affected (Austin, 1975), so that at least 150 species of North American flowering plants exhibit an “ornithophilous syndrome” (van der Pilj and Dodson, 1966).
Plants exhibiting these adaptations normally bear large flowers that are solitary or loosely clustered in a horizontal or pendant position, usually at the tip of flexible pedicels. The flowers are often red or red and yellow, holding large quantities of nectar at the base of a long, stout floral tube; and the corolla is often thickened or otherwise modified to protect it from accidental piercing by the bird’s beak or from nectar thievery by nonpollinators. The plants typically bloom during daylight hours, have little or no scent, and have projecting stamens and pistils that are likely to intercept the crown of the visiting pollinator. They also lack “landing platforms” suitable for nectar-drinking competitors such as bees, and may have other devices that tend to exclude visits by these and other nonpollinating nectar-drinkers, such as butterflies and moths (Grant and Grant 1968).
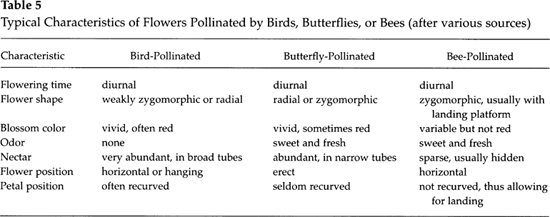
Van der Pilj and Dodson (1966) have listed some of the typical features of ornithophilous flowers as compared with those adapted for butterfly and bee pollination. As shown in Table 5, there are greater similarities between blossoms pollinated by hummingbirds and butterflies than between those adapted to birds and bees.
According to Grant and Grant (1968), most of the hummingbird-adapted plants of western North America are either perennial herbs or softwood subshrubs, with a few trees and very few annual herbs as well. Many of the plants are dicotyledons with fused corollas, and the vast majority are red or at least partially red. The Grants concluded that the most common ancestral condition for the hummingbird flowers of North America is a bee-pollinated system, with only a few genera from ancestral groups where lepidopteran (butterfly or moth) pollination typically occurs. Further, a few ornithophilous genera are centered in subtropical or tropical America and probably have been associated with birds for long periods. These presumably have followed the hummingbirds northward to occupy their current ranges in western North America.
In a review of the effect of the evolution of ornithophily on flowers, Stiles (1978) noted that, because this syndrome is energetically expensive for plants, it should occur only when birds provide the optimum vehicle for pollen flow. Pollinating adaptations exhibited by plants all revolve around nectar secretion and the specific manner of presenting it to birds or other pollinators. In dark habitats, plants may produce enough nectar to make bird pollination profitable; similarly, annual plants would benefit less from long-lived pollinators such as birds than would perennials. Likewise, trees benefit relatively little from hummingbirds, since such a rich nectar source would be divided up into small foraging territories, thus reducing pollen flow from tree to tree. On the other hand, many epiphytes are hummingbird pollinated; the small plant sizes associated with the epiphytic habit tend to ensure outcrossing even in territorial species of hummingbirds. Generally, hummingbird-pollinated flowers tend to bloom over a greater proportion of the year than do insect-pollinated flowers, a tendency which may help to stabilize the presence of the pollinator in the community. Many plant species have flowers that are specialized for hummingbird pollination, but usually not by specific bird species. This general rather than precise structural correspondence and codependence may help to buffer the ecosystem against sudden population fluctuations of any single bird or flower species (Stiles, 1978).
In a study area in the White Mountains of Arizona, nine species of hummingbird flowers coexist, all strongly convergent in flower color, size, and shape. Competition among them for pollination is evidently reduced by differences in orientation of anther and stigma location, so that different parts of the bird transport the pollen. Many of the flower species secrete nectar at similar rates, but the local population of cardinal flower produces no nectar at all, and instead attracts hummingbirds by mimicking the more abundant nectar-producing species (Brown and Kodric-Brown, 1979).
As Grant and Grant (1968) noted, the usual relationship between corolla length and bill length of the typical hummingbird visitor is only a general one; nevertheless, there is often a close relationship in both corolla length and corolla shape in these structures (Figures 12 to 14). Brown and Kodric-Brown (1979) noted that seven of the nine species of hummingbird-pollinated flowers they studied had very similar corolla length (20 to 25 millimeters), and the average culmen lengths of the three most common hummingbird visitors ranged from 15.7 to 20.2 millimeters.
A striking case of coevolution between bill length and corolla length in hummingbirds is that of the swordbill, an Andean species of hummingbird that has a culmen length substantially longer than any other known species. The culmen length in adults averages 83 millimeters, ranging to 105 millimeters in some individuals. In their study in Colombia, Snow and Snow (1980) observed that this measurement is apparently specifically dependent upon the blossoms of Passiflora mixta, a species of passion flower with a remarkably long corolla tube (about 114 millimeters). The long bill and extensile tongue of the swordbill species enable it to obtain the nectar from the long corolla tube, which is probably inaccessible to all other hummingbirds (Figure 12). The swordbill is evidently nonterritorial, and forages as a “trapliner” by visiting a large number of plants over a fairly wide area. Likewise, the giant hummingbird in Ecuador is heavily dependent upon the flowers of Agave americana, and its distribution seems to have spread with that of the plant (Ortiz-Crespo, 1974).
Many other species of plants, though perhaps not so specifically adjusted to bill length as Passiflora may be, nonetheless have remarkable adaptations that ensure cross-pollination. Pickens (1927) described the situation in Macranthera flammea, which has the typical hummingbird corolla shape (Figure 13) and color (bright orange), but bears an erect flower. As the corolla begins to open, the pistil quickly emerges and reaches its full length. A day or two later the pistil begins to wither, but by then the stamens have grown to the length of the drooping pistil, with the pollen-bearing anther surfaces tilted toward the center of the flower. By this device, a visiting hummingbird is bound to have its crown intercept either the anthers or the stigma, thus assuring cross-pollination when it visits another blossom at a slightly different stage of floral development, while at the same time avoiding self-pollination.

12. Andean swordbill and Passiflora mixta.
It is apparent that adaptations such as unusually long and curved stamens are common adaptations in hummingbird-adapted flowers. Many of these species have stamens that extend out well beyond the corolla, resulting in pollen deposition on the visiting hummingbird’s bill, forehead, or throat, depending on the particular plant species (Figure 14; see also Figure 21).
As hummingbirds are busily extracting nectar and pollen from such plants, they also are frequently receiving uninvited guests in the form of nasal mites (Rhinoseius and Proctolaelaps species) (Figure 14G). These are almost microscopically small mites that spend most of their lives in the blossoms of flowering plants, and compete significantly with hummingbirds for the plant’s nectar and pollen supplies. Adult mites often move from blossom to blossom in a single flower cluster, but in order to move from plant to plant they need the assistance of a visiting hummingbird as it pokes its bill into the flower’s corolla. During the few seconds of such a visit, the mites are able nimbly to climb aboard the hummingbird’s bill and quickly run to its nasal opening. There the mites remain, causing no apparent damage to the host, but instead simply waiting until they are provided with a “deplaning” opportunity when the hummingbird again visits the host plant. Experiments by Robert Colwell (1985, 1995) have shown that the mites are attracted to the nectar from their own host plant species in preference to others, and apparently they can identify their specific host species by its odor. They reproduce in the host plant’s blossoms, taking about a week to pass from egg to adulthood, then the mites begin their patient wait for another free ride.

13. Comparisons of corolla shapes and hummingbird bills, showing coevolved characteristics. Heliconia bihai, associated with hairy hermit (A) and green hermit (B); Centropogon valerii, with green violet-ear (C); Macranthera flammea, with ruby-throated hummingbird (D); Salvia cardinalis, with blue-throated hummingbird (E); Salvia mexicana, with white-eared hummingbird (F); Penstemon eatoni, with black-chinned hummingbird (G); and Ipomopsis aggregata, with calliope hummingbird (H). (After various sources)

14. Some comparative flower shapes and pollination adaptations with straight-billed North American hummingbirds, including white-eared and Loesellia mexicana (A), berylline and Cinna barina (B), ruby-throated and Monarda didyma (C), Costa and Fouqueirria splendens (D), black-chinned and Trichostemia lanatum (E), and rufous and Penstemon labrosus (F). Also shown is a hummingbird flower mite (Rhinoseius) within a hummingbird’s nostril (G). (After various sources)
Wagner (1946a) described an interesting pollination adaptation in the Mexican plant Centropogon cordifolius. In the early stages of floral development, the stamens mature first and tilt downward. After the stamens have withered, the stigmas occupy essentially the same position in the blossom, where they are likely to strike the top of the head of a foraging hummingbird (Figure 15). In a similar case, the stigma of Lamourouxia exserta develops earlier than the anthers, so only cross-pollination is possible. Even more remarkable is the highly specialized structure of Marcgravia, which has a group of nectaries directly below a series of developing flowers located horizontally above on long, thick stalks around the upper part of the main flower axis. The blossoms face downward toward the nectary below and exhibit “protandry,” with the pollen-bearing anthers developing first and the stigmas later. As the whummingbird finishes drinking at the nectary and rises backward and upward, its head comes into contact first with the clustered stamens and later with the pistil (Figure 15), again assuring cross-pollination (Wagner, 1946a). Similarly, in the southern Andes, the Andean hillstar pollinates a species of oranged-blossomed composite (Chuquiraga spinosa) that exhibits protandrous development of the stamens. As the growing style passes through the corolla tube, it pulls the mature pollen upward with it, coating the inside of the corolla tube and the style itself with pollen. The lobes of the stigma remain closed until the style’s elongation has lifted the stigma well above the level of the corolla tube, thus avoiding self-pollination (Carpenter, 1976).

15. Pollination adaptations of Maregravia pieta (top), Centropogon (middle), and Lamourouxia exserta (bottom), after drawings by H. Wagner (1946a). See text for explanation. In top drawing, b = bud, N = nectary, n = nectar, p = pistil, rf = rudimentary flower, and s = stamens.
A number of investigators have reviewed the usual association of bird-pollinated flowers and red coloration. Grant and Grant (1968) suggested that a single coloration used by a group of hummingbird-adapted species serves as a common advertisement for food sources; thus each plant species benefits from becoming a part of a pool of similar nectar-producing species. Moreover, many hummingbird-pollinated forms probably evolved from bee-pollinated ancestors that were usually blue. The shift from blue to red, which is not attractive to bees, may have augmented the development of a bird-adapted flower form. Thus, in the genus Penstemon, which is commonly bee-pollinated, the species P. centranthifolius is red, with flowers that are trumpet-shaped and attractive to hummingbirds. The closely related form P. grinnelli, which has pale blue, widely bilabiate flowers, is bee-pollinated; and a third species, P. spectabilis, has smaller blue, somewhat bilabiate flowers and is wasp-pollinated (Straw, 1956). Grant (1952) described a similar case of floral isolation by flower color, shape, and position in two closely related species of Aquilegia found in the Sierra Nevada mountains; these differences reproductively isolate a hummingbird-adapted species from a hawk-moth-adapted species.
Hummingbirds do not exhibit any innate preference for red coloration, but they certainly can learn to associate particular colors with nectar sources. Thus the development of red coloration in bird-adapted flowers provides a convenient, uniformly recognized “flag” for the birds, which is conspicuous against a green background. It is also unlikely to attract bees, which have color vision ranges that barely reach the red portion of the spectrum.
Studies by Stiles (1976) indicated that hummingbirds respond more strongly to energetic aspects of fluid solutions (concentration of sugar, rate of nectar flow) than they do to taste considerations (composition of sugar), and to taste in turn more strongly than to the color of feeder or flower. Among sugars, they select sucrose over glucose, and glucose over fructose. The preferred colors tend to be near the long-wavelength or red end of the spectrum for both tropical and temperate-zone flowers; thus the presence or absence of hummingbird migratory behavior is probably insignificant in fixing the colors of plants.
Besides individualized specialization between birds and plants, specialist foragers such as hummingbirds tend to exhibit a considerable degree of ecological segregation into habitats where interspecific competition from related species are minimal. Of the few quantitative investigations in this area on North American species, studies such as those of Des Granges (1979) have indicated a high degree of interspecific organization of hummingbird guilds in tropical environments. Table 6 presents an approximation of the tendencies for ecological segregation in the species of hummingbirds that breed predominantly in western North America, and Table 7 comprises a similar ecological and geographical tabulation of the primarily Mexican species discussed individually in this text. Neither table allows for a precise estimate of ecological overlaps or interspecific competition between any two species, but both tables provide some indication of the most probable cases of ecological contact between species while on their breeding grounds. Further segregation of habitat between the sexes exists for at least six of the seven species of North American hummingbirds presented in Table 6 (Pitelka, 1951b; Stiles, 1972b), as well as in Mexican species such as the white-eared hummingbird (Des Granges, 1979) and the blue-throated hummingbird (Wagner, 1952). At least one species of hermit (the saw-billed) is sexually dimorphic in bill shape (Selander, 1966).


In a study area in Mexico on the border of Colima and Jalisco, Des Granges (1979) found a foraging guild of some 21 species of hummingbirds, comprising three different groups: (a) the resident group of tropical species inhabiting particular habitats throughout the year; (b) wandering species that visit several habitats during the year and follow seasonal blooms of flowering plants; and (c) migrants present only during the winter. Most of the resident species are territorial, feeding preferentially on tubular flowers. The wanderers are typically “trapliners”—nonterritorial birds that move about, foraging on a variety of blossom types. The migrant species are territorial, defending flowers that provide nectar in excess of the resident and wanderer’s requirements, and supplementing their diets with insects. There were several types of ecological segregation, including special segregation of species (dominant and territorial birds defending the tops of trees and shrubs, or areas of tightly packed flowers; and subordinate or nonterritorial birds defending lower areas and often more scattered flowers), seasonal segregation of some species, and a limited degree of sexual segregation in two species. There was no definite indication of diurnal segregation.
At least in the more tropical areas, the species in hummingbird communities tend to fall into one of four foraging modes. “High-reward trapliners” have relatively specialized bills, which have coevolved with particular blossom types. Such species effectively exploit the nectar sources by repeated visits but do not defend specific foraging territories. “Low-reward trapliners” are similar but usually have smaller, straighter bills and are more generalized foragers, visiting more dispersed and less specialized flower types. Typical “territorialists” defend foraging territories, visiting all the suitable flower types within them. Finally, the “territory parasites” are either large species that can feed with impunity in the territories of smaller and less dominant species, or relatively small and fugitive forms that can effectively infiltrate the territories of other species (Feinsinger and Colwell, 1978). Territorial species often have greater flight acceleration and maneuverability than do traplining species, but they hover less effectively and usually have a higher wing–disc loading (the ratio of body weight to the area covered by the outstretched wings) than do trapliners.
In a review, Pyke (1980) noted that honeyeaters of Australia show convergences with hummingbirds in that both groups feed on a combination of nectar and insects; both have long, curved bills and tongues adapted to nectar foraging; and both feed at long red flowers. He summarized studies which indicate that 7 nonhermit species of hummingbirds spent about 84 percent of their time gathering nectar and the remainder of their time catching insects. Studies of 15 species indicated that 86 percent of the observations of foraging were associated with nectar-gathering; 14 percent with insect-catching. Hummingbirds catch insects in many ways, including hawking in flight, gleaning from recesses, variants of gleaning, and sometimes even running or walking (Mobbs, 1979).