CHAPTER 5
Comparative Behavior
The behavior of complex animals such as hummingbirds generally can be organized into three very broad categories: (a) those concerned with the survival and maintenance of the individual (egocentric activities), (b) those essentially self-directed but tending to bring about aggregations of individuals in common habitats or areas of common activity (quasi-social actions), and (c) those directed toward and dependent upon the presence of other organisms for their expression (social behaviors).
Egocentric behaviors of hummingbirds include fundamental features of individual survival such as respiration, ingestion, defecation, and the like, as well as more complex activities such as preening, oiling, shaking, and stretching—all of which might fall under a collective heading of “comfort activities.” Unlike most birds, hummingbirds sleep with the neck retracted, head directed forward, bill pointed upward at a distinct angle, and body feathers variably fluffed. Essentially the same posture is typical of fully torpid individuals (Figure 16F). Upon awakening, the bird arches its neck and raises its partly closed wings. It then opens one or both wings fully and stretches them down alongside the body, but not to the rear as in most birds (Figure 16A and C). The tail may be fanned simultaneously. Although hummingbird feet are very small, perching on a single foot has been observed in several genera (Mobbs, 1971).
Apparently hummingbirds never engage in mutual preening, but instead spend a good deal of time in self-preening. For most of this, in common with other birds, they use the bill (Figure 17B), but hummingbirds are remarkably adept at preening themselves with their claws (scratch-preening) in areas of the head and neck that cannot be reached by the bill. Sometimes the claws are used to preen the wing coverts, but not the primaries themselves. Most hummingbirds scratch by raising the foot up and over the wing, as do typical perching birds (Figure 17C), but some long-billed species scratch by bringing the foot forward under the wing (Mobbs, 1973).

16. Hummingbird behavior patterns, including (A) unilateral wing-stretching, (B) “courtship feeding” in Andean emerald, (C) bilateral wing-stretching, (D) feeding of young by female, (E) fighting by sparkling violet-ear, and (F) nocturnal torpor of Andean hillstar. (After various sources)
Hummingbirds seem to enjoy bathing very much, and will often bathe in the water film that has accumulated on a large, flattened leaf. At other times they may fly into the flowing water of a small waterfall or other spray and bathe while hovering in the air. When flying around in the jet of a water spray coming from below, the birds will allow themselves to be drenched from beneath and will sometimes catch individual drops of water in the bill with great skill. When bathing on a leafy surface, they will rub their abdomens against the leaves and move backwards and forwards over the wet leaf surface (Figure 17A), occasionally sliding off into the air only to fly back and begin the activity again (Scheithauer, 1967).
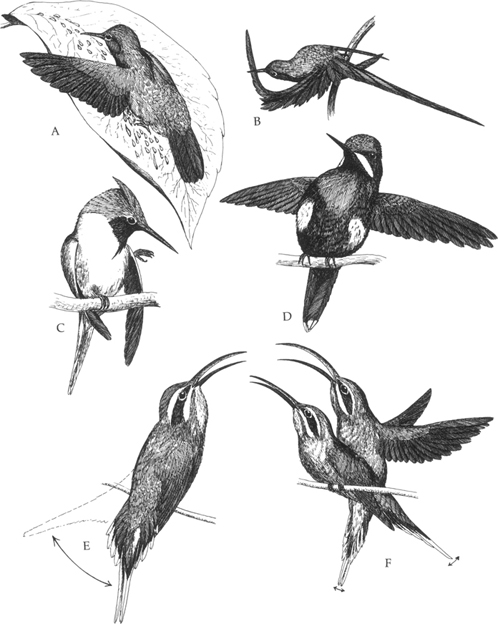
17. Hummingbird behavior patterns, including (A) leaf-bathing by rufous-tailed hummingbird, (B) preening by blue-tailed sylph, (C), over-the-wing preening by horned sungem, (D) threat display by green thorntail, (E) singing with aggressive tail-wagging by long-tailed hermit, and (F) copulation by long-tailed hermit. (After various sources)
Quasi-social behaviors include investigative, shelter-seeking, and similar activities that bring individuals into social contact, even though this is not the chief purpose of the activity. Thus, aggregations may develop around limited foraging areas, in localized bathing or roosting sites, and even in favorable nesting sites (albeit rarely). Examples of nesting aggregations include a clustering of five Andean hillstar nests in a small cave in Ecuador (Smith, 1949) and the presence of six Costa hummingbird nests within a 30-meter radius in a cocklebur thicket (Bent, 1940).
The remarkable tendency of hummingbirds to investigate unusual features of their environment probably is related to their constant need to find new and rich sources of food. Thus, they are likely to examine almost anything that is brightly colored, from a red tin can on a camp table to a bright-colored cap. I have seen rufous hummingbirds closely investigate the red stripes of canvas that support the poles of my tent, and of course they will visit red hummingbird feeders almost immediately after one installs them in areas the birds frequent. Such curiosity occasionally can be disastrous, as, for example, when a bird gets caught in the sticky head of a purple thistle and is unable to escape, or is otherwise trapped in an unfamiliar situation.
Closely related to a hummingbird’s curiosity is its apparently excellent memory, which enables it to locate food sources perhaps remembered from previous years. Hummingbirds have a remarkable capability of associating food sources with location and color (Miller and Miller, 1971; Scheithauer, 1967), which fosters foraging success. Fitzpatrick (1966) recounted an amazing example of a hummingbird’s memory and capabilities for detailed human recognition: He placed a hummingbird feeder outside his bedroom window while he was recuperating from tuberculosis in a California sanitarium. Soon a rufous hummingbird took possession of the feeder, and thereafter Fitzpatrick watched it closely for several months. When he was finally able to go outside in a wheelchair, Fitzpatrick was immediately “greeted” by the hummingbird, which careened around his head and hovered in front of his eyes. After almost a year, when Fitzpatrick returned to his home some 13 kilometers away, the rufous somehow managed to follow him and took up residence near his house. Later, the bird usually accompanied him on his daily walks. It sometimes called his attention to the presence of other animals that he might have otherwise overlooked—once noting a half-hidden rattlesnake—and eventually rode on the rawhide lace that served as a rifle sling. When Fitzpatrick had fully recovered from his illness, he left his house for a month. Yet only moments after he returned and got out of his car, the hummingbird was there, zooming about his head and hovering in front of his eyes!
This remarkable story introduces the area of social behavior, comprising all aspects concerned with individual interactions within and between species. Social behavior includes such altruistic responses as care-giving and care-soliciting behavior, which in hummingbirds is essentially limited to relationships between parents and offspring, although there are a few cases of adult hummingbirds feeding youngsters other than their own. Thus, parental nurturing (Figure 16D) is probably the only type of altruistic behavior among hummingbirds; there is no evidence of succoring behavior between adults, although Weydemeyer (1971) reported seeing a possible example of this. Adults rarely even touch each other, although Poley (1976) photographed contact behavior between two adult female hummingbirds and also photographed what seemed to be typical courtship feeding behavior (Figure 16B). However, the existence of true courtship feeding in hummingbirds is still unproved and unlikely.
The other major aspects of social behavior are agonistic interactions (attack–escape behavior) and sexual activities. In hummingbirds the two components are extremely difficult to separate, for a good deal of what passes for sexual behavior is probably little different from agonistic responses (Figures 16E and 17D). For example, male territoriality in most hummingbirds centers on a supply of food for itself, rather than encompassing a nesting site or available food resources for the female and any offspring. Thus, except in lek-forming species, only secondarily does the territory serve as a mating station, and the display flights of male hummingbirds are probably essentially an intimidation device (Pitelka, 1942). Thus, the bright coloration exhibited by males during territorial advertisement and defense may be essentially agonistic rather than sexual in function. Yet, to the degree that females can discriminate among individual males, and possibly tend to mate with those that are relatively more dominant or conspicuous, the role of sexual selection in the evolution of male plumages and displays cannot be overlooked.
Perhaps the most complete attempt to understand the diversity and significance of hummingbird sexual behavior is that by Wagner (1954), based on his long experience with numerous species of Mexican hummingbirds. A brief summary of his observations can serve as a basis for further discussion. According to Wagner, the female hummingbird searches for a mate only after nest completion. She is likely to mate with the first conspecific male that she meets, and their period of union lasts for only a few hours.
The courtship of the male has two phases: “luring” the attention of females that are ready to mate by species-specific plumage display and associated behavior, followed by the nuptial flight, performed by both sexes immediately before copulation. The luring phase is generally associated with not only posturing but also sound production effected either mechanically or by feather vibration during display flights, and vocalizations. Vocalizations in turn include short-note “songs” of a single bird, group singing in “song-assemblies,” and sounds produced during display flights.
For the courtship phase, or nuptial flight, the male must take the initiative, although the female’s actions determine the locality of the activity. In different species there are widely varying degrees of sexual dimorphism and coloration associated with precopulatory behavior. In Wagner’s (1954) experience, the degree of sexual dimorphism in plumage is closely correlated with the degree of differentiation of the nuptial flight; the more complicated the latter, the greater the degree of sexual dimorphism. In some species this phase of courtship consists only of a high intensity of activities typical of the luring phase; in others it is made up entirely of different instinctive movements.
However, Pitelka’s (1942) study regarded male display flights, as well as singing assemblies, essentially as devices for territorial proclamation and intimidation of conspecifics, including females, and courtship or invitation roles were not apparent in their performance. In his opinion, displays in which both the male and the female participated did not indicate the height of courtship, but the contrary, with the female’s displays perhaps only an effort to resist the male. He believed that most descriptions of hummingbird copulation were actually simply examples of the usual aggressive clashes between sexes, and that very few credible descriptions of copulation actually exist.
Observations by Scheithauer (1967) tend to confirm the idea that males probably attain copulation primarily by intimidation rather than by courtship. For example, a male blue-tailed emerald displayed for weeks in front of a female, hovering in front of her only 12 millimeters from the tip of her bill as she perched on a branch, following his rapid movements with her head, so that their bills pointed at each other like needles. Invariably, before he was able to take the decisive step toward mating she would dart away and elude him, so that the two birds never paired. Yet, in another case when a female brown inca completed a nest but had not yet secured a mate, she courted a male fawn-breasted brilliant by “dancing” up and down in front of him with a piece of cottonwool in her bill.
Similarly, Stiles (1982) reported that the dive displays (Figure 18) of North American hummingbirds are essentially aggressive displays associated, in most cases, with the defense of the breeding territory. However, they may also play some role in the initial phases of courtship. The most important displays in courtship per se are close-range, back-and-forth flights by the male, above or in front of the perched female. These “shuttle-flights” are accompanied by species-specific sounds (song in Calypte species, wing strokes in other genera), and they are highly species specific in terms of rate, direction, and amplitude of the “shuttles.” The displays immediately precede copulation in nearly all cases, but have never been explicitly described, probably because they usually occur in dense vegetation (as does copulation itself) (Figure 19).
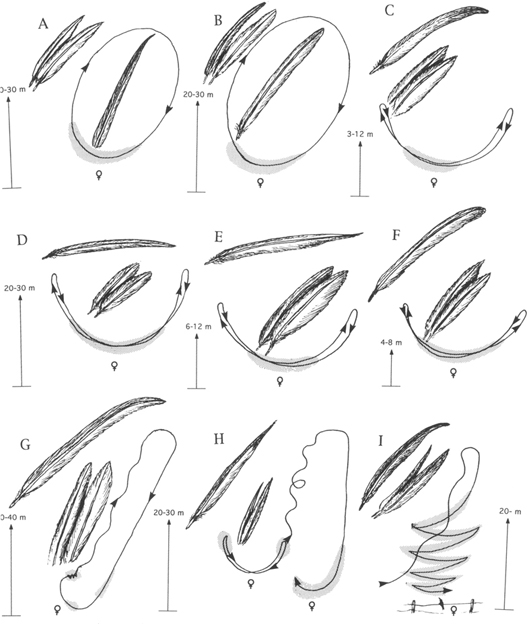
18. Male dive and shuttle-flight displays of nine hummingbirds, together with sketches of outermost male primaries and two outermost rectrices. Species shown are rufous (A), Costa (B), ruby-throated (C), calliope (D), broad-tailed (E), black-chinned (F), Anna (G), Allen (H), and lucifer (I). Stippled areas indicate points of associated vocal or mechanical sounds. (After various sources)
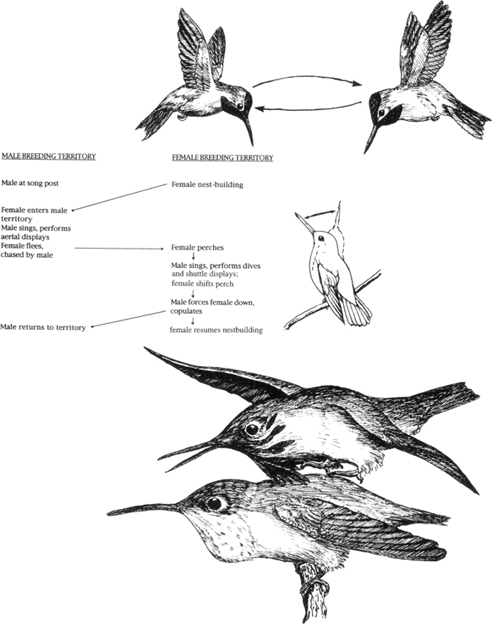
19. Shuttle-flight display of Anna hummingbird (above), with a generalized sequence of sexual behavior in this species. (Adapted from sketch and diagram of Stiles, 1982) Copulation by the calliope hummingbird (below). (After a photo by C. W. Schwartz)
Among the most interesting of the social behaviors of hummingbirds are the song assemblages of territorial males, especially the hermits. Such singing assemblies are present in Trinidad little hermits from about November until the post-breeding molt in July. During this eight-month period each male is at its singing perch for a high proportion of the daylight hours—in one case, 70 percent of the entire time. While on its perch, the male sings about once every 2 seconds (or 12,000 times a day). The songs of individual males vary considerably, but males with neighboring perches tend to have similar song-types. The singing assemblies apparently function as leks, which the females visit for the sole purpose of mating. However, observation of actual copulation in the species has not yet been reported according to Snow (1968). A study of song-types in the little hermit has tended to confirm Snow’s finding that similarities exist among the song patterns of different singing assemblages, perhaps around a founding individual that performed an imperfect imitation of a previously existing song pattern. The surprisingly elaborate songs of the species may be related to the fact that the birds display in relatively dark locations, unsuited for visual displays, and also may facilitate the differentiation of song dialects (Wiley, 1971).
Stiles and Wolf (1979) completed a more thorough study of lek behavior for the long-tailed hermit. Of the approximately 30 species of hermits, at least 3 form leks throughout their ranges (long-tailed, green, and little), and a fourth (reddish) forms leks in part of its range. Others, such as the planalto hermit, evidently do not form leks at all. Lek behavior has been observed in the genera Threnetes and Eutoxeres, but seems to be especially typical of the genus Phaethornis. In the long-tailed hermit, leks have been active on the same site for as long as 12 years, with as many as 25 males occurring in a single lek. Territories in the leks evidently serve as mating stations only and are never sufficiently rich in flowers to affect the energy budgets of the resident males.
Song display (Figure 17E) was the major type of territorial advertisement noted by Stiles and Wolf, and visual displays were observed only between birds close to each other. The sexes are identical in this species, and the same displays were given to females as to male intruders. Females apparently signal their sex simply by sitting still long enough for the male to mount, so there are both heterosexual and homosexual mating copulations (Figure 17F). Mating sequences apparently always begin within the male’s territory, although actual copulation may take place elsewhere.
Most of the displays of the long-tailed hermit seem to serve both as aggressive signals (toward other males) and as sexual signals, and include aerial activities such as the “float” display (Figure 20A), the “gape and bill-pop” display (Figure 20B), “perch-exchange” behavior (Figure 20C), and “side-by-side” display (Figure 20D).
In the leks of this species, at least, the most dominant males occupy central territories, and subordinate individuals are restricted to more peripheral areas. These central territories were the most stable over time, and the most strongly contested. Resident males returned to the same territories in subsequent years or moved into vacant territories closer to the center of the lek. Most movements toward the lek center occurred after the deaths of central residents, but the dominance status of individual males seemed to change little with age, even over several years. The rate of turnover of lek residents was about 50 percent annually—a high mortality rate which surpasses that of the green hermit in Trinidad, where nearly all resident males survived more than one year (Snow, 1974). In this species mating takes place on the male’s territorial perch. Moreover, “false matings” by males with leaves or other objects occur frequently in this and other hermit hummingbirds. Male green hermits have visited active nests as well, but nest defense by males has not been recorded.

20. Display behavior of long-tailed hermit, including float display (A), aerial gaping and bill-popping (B), perch-exchange sequence (C), and side-by-side with tail-wagging (D). (Based on sketches of Stiles and Wolf, 1979.) Also shown is gorget display by a male black-capped coquette (E), and sketches of a female calliope hummingbird (F) and a male calliope in normal non-display posture (G), versus during male gorget display (H). Corresponding postures of the Costa hummingbird are also shown (I–K). (Original, by author)
As is the case with leks of grouse and other lek-forming species such as the ruff (Philomachus pugnax), females may cue on activity centers in lek-forming hummingbirds; unlike these species, however, there is no sexual dimorphism in plumage and very little weight dimorphism. In the hermits, the peripheral males apparently have at least some chance of mating, and as a result there is seemingly a less steep dominance/fitness gradient in these birds than in grouse or other species with highly structured leks and extremely localized mating opportunities. This is perhaps partly a result of the rapid turnover rate in resident males, the dense vegetation of the lek that restricts effective widespread dominance by a single male, and the fact that even dominant males must frequently leave the lek to forage, thus increasing mating opportunities for all the remaining individuals. Since nearly all hummingbirds possess the essential prerequisites for lek behavior—male emancipation for breeding participation and extensive available nonmaintenance time and energy for territorial advertisement and defense as well as courtship—it is perhaps surprising that so few species have adopted this breeding strategy. Lek behavior seems to have evolved more frequently in the hermit group than in the more widespread trochiline hummingbirds, because the former have concurrently evolved a high degree of morphological specialization for exploiting, but not defending flowers that have coevolved with these species. However, feeding-resource-centered, territorial, defensive behavior seems to be the most efficient use of male activities for most of the trochiline species, including the North American forms (Stiles and Wolf, 1979).
Among the trochiline hummingbirds, substantial sexual dimorphism in adult plumage is the general rule, and during sexual or aggressive displays males exaggerate their already marked differences from females by erection of usually iridescent crown and throat feathers to produce spectacular visual effects (Figure 20E–K). Clearly the displaying bird is aware of the importance of visual angle in its orientation toward the recipient of the visual signaling, and even seems aware of the importance of direct sunlight in achieving the maximum visual effects. It is impossible for a human to judge how these colors are perceived by another hummingbird, but if ultraviolet perception is within the visual range of birds (for which there is now increasingly good evidence), then the overall effect may be quite different from our own color perception (Goldsmith, 1980; Bowmaker, 1988).




