

I sent for Ratcliffe; was so ill,
That other doctors gave me over:
He felt my pulse, prescribed his pill,
And I was likely to recover.
But, when the wit began to wheeze,
And wine had warmed the politician,
Cured yesterday of my disease,
I died last night of my physician.
Matthew Prior, The Remedy Worse Than the Disease, c. 1715
Poison has traditionally been part of the physician’s armory, and the best poisons are surely, for most of us, those that have benefited humanity. The Squibb Pannier was a medical chest designed to be carried into the field by Union medics in the Civil War, and it reflects medical thinking in the 1860s, a time when medical knowledge was changing fast. Nonetheless, practitioners still relied on strong poisons for a strong enemy, because in war at that time, more would die of disease than would die of bullets.
More than half of the drugs in the Squibb Pannier are immediately identifiable as poisons: Spanish fly, silver nitrate, iodine, tartar emetic, calomel (mercury chloride), strong alcohol, chloroform, ammonia, laudanum, quinine, camphorated tincture of opium, iron sulfate, compound cathartic pills, colocynth and ipecac pills, powder of ipecac and opium, potassium chlorate, morphine sulfate solution, camphor and opium pills, blue mass (mercury) pills, creosote, fluid extract of aconite root, fluid extract of colchicum seed, fluid extract of ipecac, ferric chloride, lead acetate, and zinc sulfate were all there, sometimes in more than one form.
v a cure. Sometimes, of course, the evil-tasting substances can in fact do some good but still taste seriously evil. That is when honey and sugar come into their own, as a means of
If poisons were powerful, they usually, had the advantage of tasting bad. The rule has long been that the more evil-tasting a preparation and the nastier its effects, the more efficacious it must be as getting vile- tasting material past the taste buds. (Tea, coffee, and sugar were also in the pannier.)

Cephaëlis ipecacuanha
A common poison that wasn’t in the pannier but that is still used today is digitalis. Some believe Vincent van Gogh to have been poisoned by digitalis, Digitalis purpurea or the purple foxglove. Not long before his death, he painted portraits of his homeopath, Dr. Gachet, and his daughter. He later painted a second portrait of the doctor. In both, Gachet was represented with a foxglove, the significance of which is not clear.
There is no evidence that Gachet ever prescribed digitalis for his companion, but some claim van Gogh’s penchant for yellow and his instability both stemmed from digitalis poisoning. According to all accounts, however, van Gogh was also fond of eating his oil paints and drinking turpentine and had long been highly unstable and self- destructive. Gachet himself believed van Gogh’s unpredictable behavior could be attributed to turpentine poisoning and over-exposure to the intense Provençal sun.
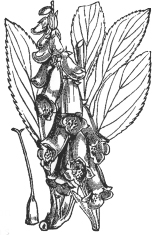
Digitalis purpurea
Then again, there is another faction who would rather blame van Gogh’s whole situation on absinthe. Absinthe is alcohol adulterated by oil of wormwood and an outlawed beverage in many countries since just before World War I. It can cause forgetfulness, delirium, convulsions, and brain damage, or so we are told. It is emerald green and a favorite with intellectuals and artists, but the thujone it contains was supposedly a serious problem. In fact, it seems more likely the problem was one of bad publicity, given that renowned wicked men such as Charles Baudelaire, Arthur Rimbaud, Aleister Crowley, Paul Verlaine, and Oscar Wilde all drank it! (The modern version, sold as “absinth,” is said to lack the hallucinogenic components.)
Absinthe starts with ordinary wormwood, Artemisia absinthium, which is soaked in ethanol to extract the thujone and also some horridly bitter substances called absinthins. A distillation of the steep liquor got rid of those, then other herbs (including the Roman wormwood, Artemisia pontica) were added, along with the vital green coloring, usually chlorophyll.
The drink’s evil reputation was not aided by the infamous van Gogh ear- slicing incident, also blamed, possibly unfairly, on absinthe, or by the alleged but erroneous chemical structure of alpha-thujone, said to be similar to THC, the active ingredient in cannabis. Some now doubt the toxicity of alpha-thujone, because it is only a monoterpene, similar to eucalyptol and menthol. It is a harmless enough substance found in herbs like sage, tansy, and tarragon and even in Vicks VapoRub, a popular chest ointment sold around the world to ease the breathing of young babies. Wormwood itself has long been known to nursing mothers: here is Juliet’s (wet) nurse, describing how she weaned her charge.
But, as I said,
When it did taste the wormwood on the nipple
Of my dug and felt it bitter, pretty fool,
To see it tetchy and fall out with the dug!
William Shakespeare, Romeo and Juliet, Act I, scene iii
On the other hand, there can be no doubt that oil of wormwood in large doses was far from benign. Alfred Taylor describes a case where a druggist’s shopman was found early one morning by his master, lying on the floor of the shop, completely unconscious, convulsed, and foaming at the mouth. In a short time, he was no longer violently convulsed but remained insensible, with clenched jaws and dilated pupils.
There was a bottle of oil of wormwood nearby, and it was clear he had consumed some of it, perhaps half an ounce (15 milliliters). His pulse was weak, compressible, and slow. On recovering, he had totally forgotten all the circumstances connected with the case and maintained he knew no reason why he should have taken the oil. Taylor suggests he may have imagined himself suffering from worms and sought relief in an unusual dose of this oil.
Assorted species of Artemisia, the mugworts, make powerful antimalarials, not for our benefit but to control the roundworms that would otherwise infest their roots. In China, artemisia, in the form of qinghao, has been used on malaria for more than a century, and in 1972 Chinese scientists isolated the active ingredient (called artemisinin) from Artemisia annua, or sweet wormwood. Since then, quinine resistance problems in Southeast Asia have grown worse, and medical workers have been using artemisinin on a large scale to treat infected humans.
Nobody really knows how it works, but researchers think the artemisinin may kill the Plasmodium parasite by producing free radicals when it binds to heme. These highly active chemical fragments may act as a poison and kill the parasite by attacking the proteins and lipids in its cell membrane. Work on new anti-malarials is well-timed, as quinine and its derivatives are losing the fight, and that other powerful antimalarial poison, DDT, is under fire from well-meaning reformers.
Until recently, its ready availability made quinine the preferred suicide agent in Greece, and when it was sold over the counter in Britain, it was ostensibly intended for the treatment of colds and fevers but was in fact mainly used to terminate pregnancies. To have any effect on the uterus, however, it had to be delivered at close to toxic levels, and so it was banned in Britain.
Quinine was included in many antimalarial tonics because it tasted so bad, but it actually poisons the malarial parasites only a little more than it poisons us. It is toxic in medium doses, and repeated dosing can lead to cinchonism, which presents as tinnitus, headaches, nausea, abdominal pain, and skin rashes. In a severe malarial case, quinine used to be given at close to a lethal dose and could cause blackwater fever.
Quinine had one unexpectedly deadly effect on humans, in that it allowed Europeans to survive in their equatorial colonies, to the detriment of the locals. Its absence could be equally deadly, as could religious intolerance. In Oliver Cromwell’s time, quinine was known as “the Jesuits’ bark.” For this reason, Cromwell, when struck down with a dose of malaria (common in Britain in those days), declined the Jesuitical treatment and died of ague, ignorance, and bigotry in roughly equal proportions.

Cinchona succirubra
Ignorance also seems to have played a role in most or all cases of poisoning with diethylene glycol, or DEG. In 1937, something like a hundred people died in the United States when DEG was used to dilute sulfanilamide. This was before the first of the antibiotics, and sulfanilamide had only just become available as a weapon against previously deadly bacterial infections. The snag was that it was served up as a mixture containing 10 percent sulfanilamide and 72 percent DEG.
We know now that DEG, most commonly used as an antifreeze and as an industrial solvent, is metabolized by alcohol dehydrogenase (ADH), the substance that protects us from alcohol, into deadly oxalic acid. The acid is fairly mild as acids go, but it forms crystals in the brain, causing irreversible damage, and in the kidney tubules, so victims die of acute kidney failure. Around 2,000 plants contain oxalic acid, including many of the lilies and the oxalis that gives it its name.
The involvement of ADH is an oddity. Because of ADH’s role in turning DEG into a poison, this form of intoxication is sometimes treated with alcohol, either orally or intravenously. The ADH attacks the ethanol preferentially, leaving the DEG intact and thus giving the body more time to eliminate it, still as unconverted DEG. DEG tastes sweet, and about a cupful is probably a lethal dose, but reports indicated that some survivors had consumed ten ounces, while some died from as little as an ounce.
Nobody knew any of this in 1937, but reports of people dying after taking the sulfa drug began almost immediately. Before long, animal studies confirmed that DEG was the culprit, and it was banned, but some 105 people had already died. The U.S. Congress passed the Federal Food, Drug, and Cosmetic Act in 1938. This required new drugs to be tested for toxicity before they went on the market, but while this might prevent the deliberate and ignorant use of DEG, it was not enough to stop further instances of DEG poisoning through carelessness or worse.
In 1986, 14 people died in Mumbai (then Bombay) when they received medications made up with glycerine contaminated with diethylene glycol. In 1992, there was a similar case in Nigeria, and, as recently as 1995, a terrible case in Haiti saw 60 to 80 children killed when glycerine mixed with DEG was used in an acetaminophen syrup. The adulterated glycerine originated in China and was shipped to Haiti from the Netherlands via a German company. It was labeled GLYCERINE 98 PCT USP, a claim that implies the glycerine is suitable for medicinal use, even though the shippers apparently were in a position to know it was only 53.9 percent glycerine.
As the events of 1937 showed, one of the problems in solving these cases is that the lethal dose varies widely from person to person, and the dosages taken may vary because many patients stop taking medicine as soon as there is an improvement in their condition. On top of that, there is always the possibility of genetic variation in the risk involved—as we have seen, Native Americans have less efficient alcohol dehydrogenase, and so may well have a better chance of eliminating DEG before it is converted to oxalic acid.
Another problem specific to DEG is that the victims of kidney failure do not present all at one time. Any number of diseases and contaminants can cause kidney failure, and the poison cases will be seen alongside disease-driven kidney failure, so it takes a subtle mind to identify the cases that are “wrong.” Jagvir Singh and his colleagues outlined their investigation of a 1998 mass poisoning in India, when at least 33 children died. Researchers visited the houses of many of the dead children—all of whom had died of kidney failure—looking for any common factor. Naturally enough, they concentrated on obvious causes and effects. Blood and stool samples were taken from sick children, and water samples from tanks and tube wells.
The outbreak was centered in the Gurgaon district of Haryana state. Of the 25 children for whom treatment histories were available, 15 had been to the same health clinic, and while there were no records kept at the clinic, the qualified pediatrician said he had not changed any procedures recently. He had treated them with antibiotics, acetaminophen syrup, cough syrup, or rehydration salts. He treated about 100 children each day, and commented that it was odd so few had become ill.
The art of epidemiology is the art of finding the smoking gun, but here it seemed that maybe the firing pin had missed—there was something not right. The breakthrough came, say the researchers, when a paper in a medical journal described the outbreak of DEG poisoning in the Haiti case and also referred to the 1986 case in Mumbai. Investigators then realized that while most of the children would have been given the more commonly prescribed acetaminophen syrup, only a few would have been given the cough syrup.
Chromatography revealed that a locally manufactured cough syrup was 17.5 percent diethylene glycol. It also confirmed that none of the other prescribed medications contained any diethylene glycol. Some of the children who had been prescribed cough syrup had also been given injections of antibiotics. These can harm kidneys previously damaged or under attack, so in this case it required a particular, terrible combination to kill a patient.
Unlike in Haiti, where activists have been enthusiastic in blaming large and heartless pharmaceutical companies for the contamination, or in Spain, no blame seems to have been allocated for either the Indian or Nigerian outbreaks.
Other poisons that can cause disease are under investigation. In research conducted at Cornell University in 1998, pregnant rats were given drinking water contaminated with lead. The lead level was comparable with that in drinking water from some areas of the United States, and the rats produced offspring with damaged immune systems. If the same effects occurred in humans, fetal exposure to lead could account for the upsurge in asthma and other allergies, as well as cancers.
Those who have asthma and rely on “traditional” or complementary medicines face an extra hurdle. In 1975, 74 patients in Singapore were found to have arsenic poisoning. The source was a variety of anti-asthmatic herbal preparations, some containing up to 107,000 ppm of arsenic, or almost 11 percent! More recently, potentially toxic levels of arsenic have been found in traditional Chinese herbal balls sold in the United States.

Historical analysis of famous deaths is a popular parlor game for medical practitioners, but it is hampered by the fact that our ancestors’ lack of knowledge about microbes and infection led them to attribute any rapid death to poison. Extra confusion arises from the regular medical use and abuse of poisons in “cures,” and the option for the cleverer doctors to keep quiet about some of their worst excesses. Not that they always did hide the facts—in some cases the doctor was sufficiently convinced that his treatment was fine; it was just that his patient had been uncooperative and died. In those cases the full report is available for us to examine in hindsight.
That alone is why we know that Philip II of Spain died in 1598 after enduring two months of hideous purging, and that Louis XIV had a close shave with death in 1658. In what follows, keep in mind that purging and bleeding were standard treatments until well into the nineteenth century, and taught in all the best medical schools. By themselves, they were terrible; in combination, they could be deadly. In some ways, it would seem, the poor were better off than the rich because they could not afford to be treated by doctors. Louis XIV was rich enough to afford the best and lucky enough to survive their ministrations.
At the age of 20, Louis was diagnosed with typhoid while on campaign in Flanders. First, his doctors tried bleeding him, but he developed a fever and convulsions and was in considerable pain. His doctors then debated whether or not to dose him with antimony, in the form of antimony wine. This was prepared by leaving wine to stand in a vessel made of antimony. The acidic wine slowly dissolved the metal, but the final concentration depended on the type of wine and the purity of the antimony. This was a remarkably risky preparation, and if luck had not been running Louis’s way, history could easily have been rather different. As it was, he survived, and his kingdom flourished.
According to Dr. Johnson, our first lexicographer, antimony gained its name after a German monk named Basil Valentine gave an antimony supplement to pigs in their feed, and they thrived on it. Inspired by this, he fed the same mix to his brother monks, says the worthy doctor. The ungrateful recipients promptly sickened and died, causing the mix to be called antimonachus (or anti-moine—monks’-bane), meaning “bad for monks.” A nice tale, even if untrue, but a fair indication that antimony was already recognized as unpleasant stuff in the mid-eighteenth century. Mostly, it was used in tartar emetic, a poison used to make the patient vomit and cast up whatever else might be troubling him or her.
Kings and queens were not the only victims of officious and official poisoners. In the United States, Benjamin Rush laid about him with bloodletting and calomel (mercury chloride), greeting the resultant mercurial fever as a sign the patient was recovering, and reassuring his students that it was very hard to bleed a patient to death. George Washington was forced to submit to the tender mercies of Rush disciples in December 1799, when he came down with a fever and sore throat.
First, local bleeders took three pints of blood, and the hapless patient was given two doses of calomel and a purging enema. Then another pint and a half of blood was taken, and the former president was dosed with 10 grains of calomel—enough to stop a fit man in his tracks. This was followed by several doses of tartar emetic, and a blistering compound was applied to the throat (blisters being supposed to draw off the harmful elements). Then, for good measure, he was prescribed a vinegar poultice, and the soles of his feet were blistered. When Washington managed to make himself heard, he, not surprisingly, asked to be left to die in peace. He died less than 24 hours after being taken ill, almost certainly a victim of his doctors’ enthusiasm for poisons.
Abraham Lincoln, on the other hand, may have poisoned himself over a number of years. In 1858, Lincoln became so excitable during a debate that he grabbed a former fellow congressman, O. B. Ficklin, by the coat collar and lifted him from his seat, roaring, “Fellow-citizens, here is Ficklin, who was at that time in Congress with me, and he knows it is a lie.” Legend has it he shook Ficklin until the man’s teeth chattered. Fearing Lincoln would shake Ficklin’s head off, Ward Hill Lamon grasped his hand and broke his grip. After the debate, Ficklin, who remained a lifelong friend, said, “Lincoln, you nearly shook all the Democracy out of me today.”
Modern opinion is that Lincoln’s volatility was caused by mercury poisoning. Lincoln’s famous composure during his presidency could be explained by the fact that the behavioral effects of mercury poisoning can be reversed. Apparently Lincoln had taken a mercury pill called “blue mass” for some years to treat his persistent “melancholia,” but in 1861, a few months after the inauguration, he stopped using it, saying it made him “cross.”
These blue mass pills were compounded from licorice root, rose water, honey and sugar, rose petals, and 375 micrograms of mercury. Today, the safe maximum level for an average adult is 21 micrograms, and Lincoln would have taken two of his tablets at a time—40 times the safe dose.
Mercury was widely used not only by physicans but also by quacks, simply because, as a poison, it killed many diseases (as it did quite a few patients). Daniel Defoe is our witness:
‘Tis sufficient from these to apprise any one of the humour of those times, and how a set of thieves and pickpockets not only robbed and cheated the poor people of their money, but poisoned their bodies with odious and fatal preparations; some with mercury, and some with other things as bad, perfectly remote from the thing pretended to, and rather hurtful than serviceable to the body in case an infection followed.
Daniel Defoe, Journal of the Plague Year, 1722
Strychnine could be found in at least one quack medicine, Fellow’s Compound Syrup of Hypophosphites, which was sold as a “tonic alkaloid.” Each fluid dram was said to contain 1/64 grain of strychnine. Easton’s Syrup, on the other hand, contained quinine and strychnine, in addition to iron: it was supposedly an all-purpose tonic—though it sounds as though it might have had a secondary function as an abortifacient.
Nature abounds in poisons, more of which we shall meet later. The traditional medicines we extract from animals, plants, and minerals often have a long history. Hippocrates recognized that willow bark—aspirin to you and me—eased aches and pains. Many of the older drugs in the pharmacopoeia are truly ancient. More importantly, many of them are poisons, but they have to be to kill the germs that infect the patient. In an ideal situation they are more poisonous to the germ than the patient.
From a modern perspective, some of the more traditional remedies seem just plain odd. John Hall, physician and son-in-law to William Shakespeare, quotes one cure that was probably as harmless as it was free of any effect. Then again, a hot onion in the crotch might just be unusual enough to shock people into urinating:
To the region of the bladder and between the Yard and the Anus was applied hot the next: Take a good big Onion: and head of Garlick, fry them with butter and vinegar. These thus used, procured Urine within an hour, with some stones and gravel . . .
John Hall, Select Observations on English Bodies, 1657
Onions, even when applied to one’s private parts, are not poisonous to humans. They are poisonous to dogs, however, causing hemolytic anemia if ingested in sufficient quantities.
A poison could be defined as any substance that destroys the health or life of a living organism, but 100 cups of coffee, 250 grams of salt, and 200 kilograms of potatoes will all kill an adult human. Vitamin D is essential in small doses, but large doses will kill. As Alfred Taylor said, “A poison in a small dose is a medicine, and a medicine in a large dose is a poison.”
A poison is a substance that interferes in some way with some process of biochemistry: it is an atomic- or molecular-level monkey wrench in the works. The toxicity of a substance will be demonstrated in the way it attacks the liver or the kidney, or blocks the delivery of oxygen to where it is needed, or the way an enzyme is either formed or operates. In the strict sense, a poison is a substance that can cause illness or death when taken in small amounts. In the legal sense, it is usually taken as a chemical with an LD50 of less than 50 milligrams per kilogram, but that leaves us in need of a definition of this term.
The LD50 is what we use in most cases to measure toxic effects. Shorthand for “the lethal dose required to kill 50 percent of a sample,” it is defined more formally as the dosage, measured in milligrams per kilogram of body weight (the same as parts per million or ppm), required to kill 50 percent of a sample population within 14 days. Any substance, even something that is not legally a poison, can have an LD50—table salt, sodium chloride, has a human LD50 of about 3 grams for each kilogram of body weight, while the LD50 of solanine, a poison found in potatoes, would be consumed by an adult eating 200 kilograms of potatoes in one hit.
It might be worth noting here that when we talk about LD50 values in humans, nobody has taken human populations and fed them varying doses of poison to see how many die. Instead, the values are inferred from case histories, one of the reasons why human LD50 values are generally stated as approximations. On top of this, some chemicals irritate without poisoning, but a sufficient irritation can lead to fluid in the lungs and so kill indirectly. Corrosives can also kill when they are swallowed, and, if the LD50 fits, they may be regarded as poisons.
Gases are a little different, and here we speak instead of the concentration–time combination needed to kill 50 percent, the LCt50, which is going to be given as something like 20 minutes at 100 milligrams per cubic meter, or 10 minutes at 200 mg/m3. In each case, the product (and so the LCt50) is 2,000 mg min/m3. We can extrapolate and say that, if the concentration is 10 mg/m3, we would expect half of a sample to survive for 200 minutes, but some would die much sooner, and at some point the dilution will be so great that the body’s defenses may be able to destroy all the poison as it is breathed in. Such is the cold mathematical precision of toxicology.
Modern medicines are generally designed to poison a microbe that is harming or poisoning us with some waste product. The value of the poison/medicine lies in it being more poisonous to the microbe than it is to us. Before there was a serious germ theory of disease, the assumption was that all disease was caused by some poison or another, and so the best treatment was to expel the poison by applying some form of emetic or laxative. (This was justified by doctors who could show that many illnesses and many poisons caused violent expulsion at one or more ends of the alimentary canal. In any case, people felt so wretched when being purged that they felt much better afterward—so purging just had to be good for them.)
At least as far back as Dioscorides, emetics were seen as appropriate, and indeed they still can be for some cases of poisoning, where there is no risk of the expelled poison getting sidetracked into the lungs and doing even more harm. All the same, we need to be a little careful in reading old recipes. John Hall mentions using agaric. He was, I hope, talking about a tree fungus from larches in the Levant, a common purgative. The same name was also used for fly agaric, which was mixed with water and put out as a fly poison, and also eaten to cause a form of intoxication. The Viking berserkers are thought to have eaten fly agaric to prepare themselves for battles, but Hall’s agaric had no such interesting properties.
We will come back to Hall’s remedies later, but doctors were not the only ones with poisons in reach. Gardens typically had a number of herbs with either known or reputed properties. A number of these poisonous substances were also believed to be useful if somebody wanted to expel an unwanted fetus. In short, they were abortifacients.
Abortion is, and perhaps always will be, an issue that arouses strong emotions. The Hippocratic oath included a promise not to administer abortifacients, but it is fairly clear that prostitutes in ancient Greece relied on abortion, and it must have been fairly common, since temple inscriptions indicate that a woman was regarded as being impure for ten days after being aborted. According to the Stoics, a fetus was more plant-like than animallike, and only became an animal at birth, so abortion posed no ethical problem for them.
In the age of the Roman republic, it appears to have been both acceptable and normal for women to rely on abortion, and both Galen and Dioscorides list many plants that could produce an abortion, taken either orally or as a vaginal suppository. Even the emperor Domitian, who died in AD 96, is said to have poisoned his niece, Julia, in an attempt to abort their incestuous child.
Then somewhere around the time of Severus and Caracalla, not too far from AD 211, abortion became a crime against the rights of parents, an offense punishable by temporary exile. The early Christians regarded abortion after the fetus was formed at 40 days as killing a living being, an attitude that was probably reflected in the later emphasis on emmenagogues, treatments taken not to abort a fetus but to restore a temporarily interrupted menstrual flow—the same thing by a different name, in many cases, since one way of restoring the flow was to abort the fetus.
The range of possible treatments was wide. Cantharides, which we will meet elsewhere as a fabled means of provoking unbridled lust, was used also to make good the damage that lust might wreak, but so too were colocynth, aloes, hemlock, saffron, pennyroyal oil, and juniper. There are at least 25, and perhaps as many as 50, species of juniper, but the abortifacient comes from just one of these, Juniperus sabina. This is the source of savin, or oil of savin, an effective killer of worms, and also, it appears, of fetuses. Traditionally, gin is flavored with juniper berries, and so it was thought to be an effective agent when a miscarriage was desired.
The name gin is derived from the French name for juniper, genièvre, and the juniper in gin is Juniperus communis. This yields oil of juniper, a powerful diuretic that is still in the British and U.S. pharmacopoeias. Unlike oil of savin, this appears to have no effect on the fetus. Yet another juniper, Juniperus oxycedrus, provides the cedar oil still found in most of the European pharmacopoeias but not in that of Britain.

Juniperus communis
This oil was a key ingredient in the discovery of bacteria in the nineteenth and early twentieth centuries. Much of what was discovered arose from the extra magnification seen when the object under scrutiny was attached to the cover slip of a slide by a drop of the strong-smelling oil. This arrangement became known as the oil-immersion lens, and it delivered decent resolution at a magnification of 1,000. In that way, one oil of juniper has saved many, many lives.
Alfred Taylor was not convinced oil of savin actually caused a miscarriage, but speculated that it may have caused a shock to the system and so indirectly have procured an abortion. He noted that as long as the oil was used with this intention, it was likely to poison young women from time to time. Rough treatments like oil of savin aside, women traditionally grew a number of herbs that could, when applied the right way, relieve them of an unwanted burden. But how safe were these old remedies? Used correctly, they were still reasonably safe—and effective.
The aconite described by Dioscorides was leopard’s bane, Doronicum pardalianches, a member of the daisy family, which he said was used in eye medicines. Listing the common names, Dioscorides identified monkshood, the modern aconite, as “the other aconite,” and said it was the one used against wild animals. Dioscorides reported no medical uses for “the other aconite,” which modern botanists call Aconitum napellus, and which is a member of the buttercup family. Later on, though, Galen would assert that both had the same medical properties. As we will see later with hemlock, common names can bring their own special problems.
In 1987, John Riddle listed 257 drugs used in ancient Greece and found that 230 of them still appeared in at least one pharmacopoeia, somewhere in the modern world. Of course, new discoveries have been made since classical times, like camphor, discovered in the sixth century on the Malay peninsula (or thereabouts). Camphor reached Europe by the ninth century, courtesy of Arab traders, and has been there ever since. Riddle also notes that, by 1979, 20 of the 25 most commonly prescribed drugs had been discovered since 1950, and this process has been accelerating. We still have our poison drugs, less familiar ones now, but no less toxic for that.
But if the medicine chest or the herb garden did not yield enough poisons, the boudoir had a few unpleasant items to offer. Fowler’s Solution, a 1 percent solution of potassium arsenite, was a market leader in its time and so worth considering in a little detail. During the latter part of the eighteenth century, in the reign of King George III, this patent medicine was advertised as an “infallible remedy for agues and intermitting fevers” and able to work its miracles even where “the bark” (quinine) had failed. It was said to be derived from “cobalt,” probably an arsenical mineral. The drops had the advantage that, unlike the incredibly bitter quinine, they were tasteless, and the remedy was sometimes used in hospitals, where it seems Dr. Fowler first encountered it.
He must have been impressed with what he saw, because in 1783 he asked an apothecary named Hughes to duplicate the preparation. The apothecary dutifully made up an alkaline solution of white arsenic that actually possessed therapeutic properties. In time, this became known, a little unfairly, as Fowler’s Solution. It was first listed officially in the London pharmacopoeia of 1809, and it then became popular with women, who drank the solution for their complexions. Some used it as a cosmetic wash, and others even rubbed it into their hair and scalp to destroy vermin.
We will look at this in more detail in the next chapter, but while prostitutes commonly used arsenic for their complexions, women regarded as respectable did so as well. Elizabeth Siddal was first the model, and later the wife, of Dante Gabriel Rossetti. She became a great arsenic user, because she wanted to retain her youthful looks, bright eyes, and clear complexion. Unlike some of her poorer and less-educated sisters, she apparently knew that once she started she would not be able to stop. This may have something to do with her ultimate suicide from an overdose of medicinal laudanum in 1862, but even then she was denied eternal rest.
In 1869, Rossetti obtained permission to have his wife exhumed from her grave in Highgate Cemetery, as she had been buried with the only copy of some of his poems, verse that he now regretted having placed in such security. The Home Secretary gave the necessary permission, and the evil-smelling book was retrieved, disinfected, and later dried and copied. The poems were published in 1870, giving literary Victorians a subtle twinge of vicarious necrophilia.
When we look at some of the fashionable beauty treatments of today, we might be excused for wondering just how far, if at all, we have traveled from the nineteenth century. Let’s take Botox, botulinum toxin, as an example. On the one hand, the media warn us that this is a fearful substance that terrorists might well use to murder us in our beds, but women’s magazines and wrinkle-free models and celebrities sing its praises—to such an extent that fashion-conscious teenagers as young as fourteen and fifteen are reportedly receiving Botox injections in cities like New York and Miami.
In simple terms, the toxin makes muscles relax. It eliminates frown lines by immobilizing the muscles that yield them. Botulinum toxin is a by-product of Clostridium botulinum, an anaerobic bacterium. In real life (or death, perhaps), the effect of the toxin is to kill nearby flesh, providing more oxygen-free living space for the bacteria.
The toxin blocks the release of acetylcholine from the endings of motor nerves. As the nerves cannot deliver signals, the muscles they would usually operate are effectively paralyzed. This paralysis lasts for some months, even up to a year, before the patients have to come back for another dose, or have a suddenly wrinkly face. It is a license for its purveyors to print money.
Botox was first used for strictly medicinal purposes, to treat lazy eye and uncontrolled blinking, but researchers soon noticed that wrinkles were also affected. A few side effects have been seen in some cases—minor allergic reactions, bruising, or a temporary drooping of the eye, but nobody has died of the tiny dose used.
Botox also has some rather more important cosmetic effects for those unfortunates who suffer from a form of excessive sweating, called hyperhidrosis. Sufferers become saturated, even when they use pads and antiperspirants, causing them intense emotional upset and social stigma. The quick fix is a Botox injection into the underarm skin, the palms of the hands, or the soles of the feet, where it paralyzes the sweat glands for six months to a year.
Botoxing is not really the thing to have done at parties by people who think Ab Fab is a documentary—after all, how many people would change their hair color at a “hair color party”? Nonetheless, there would seem to be at least a few valid reasons why some people may need small amounts of Botox from time to time, but it’s not the only poison in the makeup bag.