

Mr. Pritchard, failed bookmaker, who maddened by besoming, swabbing and scrubbing, the voice of the vacuum-cleaner and the fume of polish, ironically swallowed disinfectant.
Dylan Thomas, Under Milk Wood, 1953
The popularity of arsenic for cosmetic purposes seems to have come either from the fabled “fair Circassians” or from the Albanians, but it was by no means a craze limited to women’s cosmetics. Florie Maybrick soaked flypapers to remove the arsenic they contained. She used the resulting solution as a facial toner, and, as we saw in chapter 5, many patent solutions and creams—for tightening the chin, removing freckles, or brightening the complexion—contained copious quantities of arsenic.
Arsenic even had a role to play in death, whether or not it was the cause. The embalmer’s arsenic bleached skin to an acceptable white and left the cadaver more supple, so it could be posed more naturally. Embalming became popular in the Civil War, when families wanted their war heroes brought home from the battlefield for burial. The amount of arsenic used on a corpse reportedly varied from “four ounces to 12 pounds,” but even the lowest dose would have made it easier for poison murderers to escape discovery, so, over time, arsenic was replaced by formaldehyde.
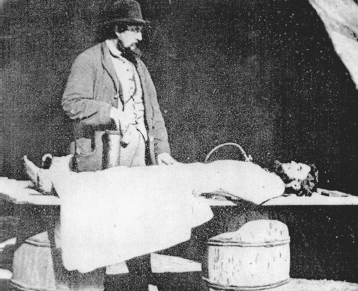
Dr. Richard Burr performing the embalming process on a body recovered from the battlefield.
Women and war dead were not the only users of arsenic: in chapter 2 we met two men whose partners were accused of their murders but who shared the curious habit of arsenic eating (arsenophagy). This is not quite as bizarre as it sounds, since we consume between 12 and 50 milligrams of arsenic each day in our normal diet. We can handle this because the amount of arsenic excreted in the urine each day is generally up to 50 milligrams. The Japanese, whose diet is high in fish and shellfish, have been found to have an arsenic content in their daily urine as high as 148 milligrams.
The men of the mining district of Styria, southwest of Vienna and centered on today’s Graz, were renowned for their enthusiastic arsenophagy. They ate arsenic to improve both their skin and their breathing and to give them longer wind—most desirable in a mountainous area where a great deal of climbing was required. News of the practice appeared first in a Viennese medical journal in 1851 and soon after was being discussed in England, both among medical men and in the popular press. Arsenic became a core ingredient in preparations for everything from venereal disease to tapeworms.
While there can be no doubt that some Styrian arsenic eaters did indeed swallow large amounts of arsenic, quite large enough to poison a normal human, most of their intake probably passed out again without having been absorbed, because the grains of the mineral were too coarse to allow rapid uptake. So a lot of the arsenic eating may have been for show.
Arsenic was not the only poison found in cosmetic preparations. Belladonna, an extract of the deadly nightshade, could be applied to the eyes, where it would dilate the pupils. Despite the desirability of this “doe-eyed” look, it has become apparent only recently that women’s pupils dilate when they are sexually interested in somebody, so the gentlemen were being led on, even when the women were completely unaware of what they were apparently “responding” to, or their “response.”
Doctors also believed lead had some definite curative and cosmetic properties. John Hall noted that he used cerussa, otherwise white lead or lead carbonate, as a treatment for “pustles,” or pustules, as we would say today:
[Alice] Austin, a Maid, had her Face full of red spots, with red Pustles, very ill favoured, although otherwise very comely, and of an excellent wit.... The Body thus purged, her Face was anointed with the following Liquor: . . . Litharge of Gold powdered, one ounce. Alum one dram. Borax three drams. Cerussa half an ounce. Vinegar two ounces. Rose-water and plantain water, each three ounces. Boyl them to the wasting of the third part, after strain them, and add the juyce of lemons, half an ounce.... I advised her morning and night (the Pustles opened, broken and crushed) she should wash the Pustles daily with the said Water.
John Hall, Select Observations on English Bodies, 1657
This would have been extremely painful with broken flesh, but the pain probably made any cure seem even more effective. Hall also used “litharge of gold,” a yellow crystalline lead oxide, as a face powder. In France, lead compounds were known as poudre de la succession (“succession powder”), owing to their effectiveness in removing annoying barriers between an individual and an inheritance, but even that did not sound any alarms at first.
In Tudor times, those desirous of appearing in the forefront of fashion might whiten their faces with white lead. Kohl, for millennia a common cosmetic, is an antimony compound, but one of the most bizarre uses of a poison must have been when thallium was found to cause hair loss.
Thallium acetate was first given therapeutically to terminal tuberculosis cases, in order to suppress night sweats. It may or may not have worked, but the side effects of the treatment were quite obvious—the patients’ hair fell out. Accordingly, in 1898 the chief dermatologist at the St. Louis Hospital in Paris introduced thallium as a pretreatment for ringworm of the scalp. After World War I, it was extremely popular for this purpose, and Hamilton and Hardy record its use:
Thallium is absorbed through the skin, and so was used as a depilatory to get rid of parasitic diseases of the hair follicles in children. If the dose is right, children will completely lose their hair on the 16th to 18th day.
Asher Finkel (ed.), Hamilton and Hardy’s Industrial Toxicology, 1983
Thallium acetate was sold in the United States as a depilatory under the name Koremlu Cream, until the mounting flood of damages claims for ailments ranging from neuritis to myalgia, arthralgia, and permanent loss of scalp hair drove the makers into bankruptcy. Even if you decided to keep your hair and dye it, you could be in trouble, as Mrs. Anna White was at pains to point out to her lady readers:
Dyeing the hair is a very dangerous business, as most of the hair-dyes have for their base sugar of lead, caustic alkalis, limes, litharge and arsenic, all of which burn the hair. We have known of cases of paralysis of the brain occasioned by the inordinate use of hair-dyes which their makers asserted were ‘perfectly harmless.’
Shampooing is a great detriment to the beauty of the hair. Soap fades the hair, often turning it a yellow. Brushing is the only safe method of removing the dust from the head, with the occasional use of the whites of eggs. Perfect rinsing and drying should follow all washing of the head.
Mrs. Anna R. White, Youth’s Educator for Home and Society, 1896
The lead salts in hair dyes attach to disulfide bridges in the protein of hair to form black lead sulfide, destroying the bridge in the process. Lead in your system interferes with heme and por-phyrin synthesis, which can lead to lower hemoglobin levels. Other symptoms of lead poisoning can include colic and abdominal pain, tiredness, and constipation, and even very low levels seem to affect intellect and learning.
Other salts used in hair dyes include copper, iron, nickel, cobalt, and bismuth, but women were not the only casualties of poisonous cosmetics. One American whisker dye was found to be as dangerous as the dyes offered to women because it contained excessive amounts of silver, and men also used whisker removers containing lead acetate, which could cause permanent baldness.
Mrs. White was full of good ideas for improving one’s appearance. Lily-white hands had to be matched with lily-white complexions. She offered harmless remedies, such as bran mittens for keeping the hands white in spite of the disfiguring effects of housework. These were large mittens, filled with wet bran or oatmeal, that were to be worn overnight. This replaced a toxic seventeenth-century method of applying sorrel juice (containing oxalic acids or oxalates) to the hands. Freckles annoyed many ladies, she said, recommending Unction de Maintenon, which contained, among other things, oil of bitter almonds:
Venice soap, 1 ounce
Lemon juice, 1/2 ounce
Oil of bitter almonds, 1/4 ounce
Deliquidated oil of tartar, 1/4 ounce
Oil of rhodium, 3 drops
Dissolve the soap in the lemon juice, add the two oils, and put the whole in the sun till it becomes of ointment-like consistency, and then add the rhodium. Bathe the freckled face at night with this lotion, and wash it in the morning with clear, cold water, or if convenient, with a wash of elder flower and rose water.
Mrs. Anna R. White, Youth’s Educator for Home and Society, 1896
Mrs. White possibly wasn’t up to speed on her history when she named her preparation. Madame de Maintenon was the last mistress (and secret wife) of Louis XIV, whom we met in chapter 5. Louis and Madame had the dubious honor of presiding over one of the most poison-riddled courts in history. Mrs. White’s freckle soap contained poison, cyanide to be specific, in the quarter ounce of oil of bitter almonds.
In murder fiction, the odor of bitter almonds is always associated with sodium, potassium, and hydrogen cyanide, but only 40 to 60 percent of people can detect this, and the real number is perhaps less, as few are willing to undertake the experiment. While cyanide’s effects were known to the Egyptians 5,000 years ago, the first description of poisoning by almond extract was in 1679, and of cherry laurel water in 1731, both of them forms of hydrogen cyanide. The Swedish chemist Carl Wilhelm Scheele (we will meet him later as the discoverer of Scheele’s Green) first isolated hydrogen cyanide (HCN), or prussic acid, from the dye Prussian blue in 1782. Four years later, he died of his own discovery when he broke a vial of prussic acid. The way of the chemist was never easy.
Prussic acid can be extracted from a variety of fruit seeds, and it seems likely its presence evolved because it made life unpleasant for any animals crunching the seed, while it did no harm to those merely swallowing the seed whole and carrying it away. In this way, the cyanide worked like the capsaicin in peppers—selecting those animals that would spread the seed most effectively, and with the least harm.
Unfortunately for almonds and a few other types of seed, cooks found oil of bitter almonds to be a most attractive addition to various meals. According to Taylor, the essential oil of bitter almonds contains a variable amount of HCN, rising as high as 12 percent, while almond flavor or essence of peach kernels contains one dram of the essential oil to seven drams of rectified spirit. In either case, the stuff is fairly toxic. Fifty milligrams of HCN—a bit less than a grain—is enough to kill, and a bit of quick figuring reveals that a one-ounce bottle of the essential oil would contain enough to kill about 60 people. Strong stuff to keep in a kitchen if the cook has a temper, but an even bigger threat in the Victorian kitchen came from nitrobenzene, which has a similar smell and taste. It gives confectionery the smell, but not the pleasant taste, of bitter almonds, and packs a far bigger kick.
Cyanide acts on the blood. It has its effect, in simple terms, by starving the body of oxygen, which cuts off the formation of adenosine triphosphate (ATP), an essential part of getting energy out of food in a form that cells can use. Cyanide victims have cherry-red blood, because the hemoglobin is saturated with oxygen that can go no further, and in the midst of plenty, the victim dies. Just 50 mg of hydrogen cyanide or 375 mg of a cyanide salt can kill a human.
Technically, cyanide is a rapid-acting poison that inactivates cytochrome c oxidase when the cyanide ion reacts with ferric (trivalent) iron in cytochrome c oxidase. This enzyme is involved in the final stage of the electron transfer chain, which delivers energy. Cyanide inhibits the electron transfer chain and stops ATP from forming. Because of this, there is a buildup of lactate, leading to acidosis, especially in the brain.
Chemistry in the body is mainly a matter of checks and balances, with chemicals formed in one process being broken down in another, so as to reset the state of something. At some synapses—links between nerves—a chemical called acetyl-choline is used to carry the message from one nerve to the next. The synapse is then reset as an enzyme called cholines-terase wipes the acetylcholine out again. Some nerve gases and organophosphate insecticides block cholinesterase, so the acetylcholine keeps on triggering the second nerve.
Strychnine attacks another sort of synapse transmission, one relying on glycine. Glycine works to inhibit signals, but strychnine locks the glycine out, with the result that the nerves keep firing, reflex arcs fire over and over again, and a form of spastic paralysis sets in, followed by convulsions and paralysis of the respiratory muscles, so the victim dies. The muscular contractions of strychnine produce characteristic contortions of the body, arched backward so only the heels and the top of the head touch the ground, and on the face, a risus sardonicus, or, if you recall your Tom Lehrer, a hideous grin.
But while Lehrer’s victim was said to have died with the spoon in her hand, there are other ways of taking in poison than swallowing it. We have about two square yards of skin, and some 90 square yards of lung surface, so it is possible to inhale some poisons and to absorb others through the skin. Nitroglycerin, for example, can be absorbed through the mucous membranes of the mouth, which is why it is placed under the tongue when it is used as a treatment for angina pectoris.
Taylor notes that the symptoms of nitrobenzene poisoning are remarkably similar to those of cyanide. In at least one case he examines, essential oil of almonds was at first believed to be the cause. A woman who was making pastry tasted the bottle of what she had assumed was oil of almonds. Finding it acrid, she spat it out. She was unwell for a long time but survived her encounter with nitrobenzene. In another case, a 13-year-old boy tested a bottle to his lips and died some 12 hours later. The household’s cook also applied the bottle to her lips and became ill, but later recovered. The boy may have swallowed some of the liquid, as his stomach contents had the characteristic smell of the liquid when an autopsy was carried out. The bottle had been wrongly labeled as oil of bitter almonds.
Serious chemical discovery got under way in about 1780 and, after a slow start, took off in the nineteenth century, as people worked out ways to harness the new knowledge. Much of it related to colorful (that is, generally reactive and potentially poisonous) new compounds. One famous, although putative, victim of this knowledge was Napoleon Bonaparte, eventually released from his miserable exile by a lingering and painful death.
A later check of the deposed emperor’s hair revealed that there was indeed arsenic in Napoleon’s remains, but this does not mean he was given a lethal dose. On the other hand, a large number of people would have been willing to see him dead. Alive, he was a threat to peace, in case he escaped, just as he had on Elba, but somehow, people could not bring themselves to kill him. Perhaps nobody wanted to carry on the tradition of executing kings and emperors, not even despised ex-emperors.
Ambroise Paré claimed after the event that the sudden and fatal illness in 1534 of Pope Clement VII, one of the Medicis, was caused by him inhaling arsenical fumes from a torch carried by one of his attendants in a procession. Others claim that both the pope and Leopold I of Austria were killed by the fumes from arsenic-laden candles.
These claims are impossible to prove, but there is a sound basis for them. Researchers have known for some years that lead wicks in candles can release significant amounts of lead into a room. These candles tend to be long-burning scented and ceremonial candles. In one set of tests, carried out in 1999, the lead levels emitted reached as high as 65 micrograms per cubic meter (μg m-3) against a U.S. safety standard of 1.5 (μg m-3. In the same year, lead wick candles were banned in Australia, but they were not banned in the United States until 2003.
Diehard Napoleonists are still convinced that some person or persons unknown actually murdered the emperor. The issue was raised in Paris at a meeting of the International Napoleonic Society on the eve of the 179th anniversary of Napoleon’s death, May 5, 2000. In the 1960s arsenic had been found in hair purported to belong to the emperor, and now it was claimed that the FBI had run further tests in 1995, finding levels of 20 to 50 parts per million (ppm) of arsenic, where 1 ppm is more normal.
Contemporary reports of Napoleon’s terminal illness mention that he complained of light sensitization, loss of hair, sleep problems, and neurological disturbances. All these are consistent with arsenical poisoning, and postmortem reports suggest he was still fat when he died, which is inconsistent with the official diagnosis of gastric cancer. The problem with the claims is that there is no proof that the hair specimens actually came from Napoleon, and important (and hard to miss) signs of arsenical poisoning, such as leathery texture of palms and soles of feet, were not present in Napoleon’s corpse.
There were no less than eight doctors at the postmortem, and they agreed that extensive stomach cancer was present at death. More importantly, one of them was one Francesco Antommarchi, who attended Napoleon through his last illness. Antommarchi was a Corsican, who had been sent to Elba by Napoleon’s mother. It is unlikely he was part of a British plot to poison his patient, and he surely would have noticed and reported symptoms of arsenical poisoning. Let us assume, however, for the sake of argument, that the various samples of Napoleonic hair are genuine and do indeed contain excessive amounts of arsenic: there are quite a few ways for the arsenic to have gotten there.
Beginning about March 1821, Napoleon was given tartar emetic to induce vomiting. This may have produced the opposite result—an inability to vomit—if the mucous lining of the stomach was already corroded. In April 1821, he was given an almond drink that would have contained mandelonitrile, a substance that can decompose to produce prussic acid. Finally, on May 3, 1821, Napoleon was given a relatively large dose of 10 grains of calomel, ostensibly to relieve constipation. Reaction of calomel and mandelonitrile, or the prussic acid it formed, may have produced mercury cyanide. As Napoleon was unable to vomit, this toxic cyanide could have been retained in his system until it killed him on May 6.
On the other hand, there are two perfectly feasible ways for Napoleon to have ingested a certain amount of arsenic, possibly enough to kill him, in complete innocence and with no plot intended. In 1856, a Dr. Boner alleged that Napoleon had been in the habit of taking arsenic as a precaution against being poisoned, and this was confirmed by others. A second, and more likely, possibility is that it came from the wallpaper.
In 1778, Carl Scheele announced the discovery of a new copper arsenate dye, and over the next few years a number of slight variations on the theme were discovered. This was at a time when most dyes were vegetable based and given to fading or washing out. The new mineral pigments held their hue far better—they were brilliant and colorful, and they caught on in a big way. By 1814, the Wilhelm Sattler Dye and White Lead Company in Schweinfurt began making a mixed copper acetate–arsenate salt that gave a beautiful green in paper, textiles, and confectioneries.
In no time at all, Schweinfurter green was the new green all across the civilized wallpaper-conscious world. Its big plus was that it did not turn gray when it was exposed to sulfides—important at a time when coal was a common source of heat. A couple of minuses were that wallpapers were attached with starch paste or, rarely, animal glue, and damp-proofing at the start of the nineteenth century was far from ideal. As a result, many rooms ended up lined with soggy paper smothered in poisonous Scheele’s green, Paris green, or Schweinfurter green and with a good supply of adhesive to provide fungus food.
In chapter 7 we will encounter biomethylation as a defense against toxic metals. Many fungi have the ability to drive this reaction, which makes metals more accessible, and in the soggy rooms of the civilized and tastefully wallpapered world small biochemical reactions were started, as the fungi struggled to survive the arsenical realm they found themselves in. (The dyes were also in artificial flowers, carpets, furs, dress fabrics, and black stockings, but the wallpaper was the main problem.)
People realized there was a problem with the new colors fairly quickly, because in northern Europe people soon noticed how bedbugs died in rooms papered with the new designs. At first even more of this useful paper was sold, but then people began to notice a garlic odor and that those sleeping in such rooms got sick; some died.
Somewhere around 1865, according to legend, somebody dumped some Paris green on a potato patch in America and noted that it killed insects while leaving the plants alone. This started a tradition of arsenical pesticides.
By 1838, the Prussian government had banned the use of poisonous substances in wallpapers. Regrettably, other parts of what we now call Germany—and the rest of Europe, for that matter—were not so strict. It was, in any case, too late for Napoleon.
We have known some parts of the story since 1897, when Bartolomeo Gosio (1863–1944) showed how a fungus then known as Penicillium brevicaulum, now known as Scopulariopsis brevicaula, attacked the starch paste and sizing, excreting an arsenic compound he could not identify. It became known as Gosio gas, but while his name is recalled by specialists, and while there is a via Bartolomeo Gosio in Rome, he is little remembered today, except by some who suspect crib death or SIDS may be caused by Gosio gas.
Gosio was investigating a number of deaths that appeared to be caused by a volatile, garlic-smelling arsenic compound emanating from damp, moldy rooms. The wallpapers in these rooms were colored with arsenic-containing pigments, and Gosio isolated a number of microorganisms associated with the gas. His assistant, Biginelli, trapped some of the gas as a complex with mercury(II) chloride. After chemical analysis, he suggested it might be diethylarsine, (C2H5)2AsH.
When Clare Boothe Luce was U.S. ambassador to Italy, she was poisoned by arsenic, though it turned out to be an accident that should have been kept under wraps. Luce was aware that her behavior was irrational, and reported to President Eisenhower that she felt as though she was drunk or drugged on several social occasions. This report got out when Eisenhower’s press secretary added the snippet to one of Ike’s news briefings. At no time did Luce say she had been poisoned, only that her behavior was being oddly affected by something.
Richard Helms of the CIA later established the presence of arsenic in the ceiling of the ambassador’s bedroom—as was common with many Italian ceilings—and it was claimed her bed was uncanopied (actually, they said she was sleeping on a sofa). Apparently there was a laundromat on the floor above, which caused the ceiling to vibrate, making one wonder: if the ambassador was living in primitive conditions like this, stuck on a sofa under the laundromat, where had they put the hired help?
The American press was delighted, because the lady and her husband (Henry Luce, owner of Life and Time magazines) had long been labeled Arsenic and Old Luce. The idea of Arsenic being poisoned with arsenic was too attractive to be allowed to rest, but on this occasion, even given the Italian setting, there was no state-sponsored poisoning going on. Arsenic is merely a common feature of room surfaces in old Italian buildings.
The Gosio gas was finally identified by Frederick Challenger as trimethylarsine in 1945, completing research he had begun in 1931. So when the arsenic was found in Napoleon’s alleged hair, the basic information was in place. Around 1980, David Jones gave a radio talk on the issue and wondered what color Napoleon’s wallpaper might have been.
There is a certain sort of delightful person who reads books or listens to radio talks and then writes to the author, providing an interesting and key snippet, fact, date, or name. If you are one of those, know that you are valued, for your snippets are the thread that ties the pieces together, launching your chosen recipient in an entirely new direction. Blessed are the snippet-passers of this world, though these days we often find them on the Internet.
In those days before the Internet took off, Jones received a letter from one Shirley Bradley, who had inherited a fragment of Napoleon’s wallpaper in a scrapbook. The wallpaper showed a green and brown star, but it may once have been green and gold, the imperial colors, and have faded. The fragment was found to contain arsenic, so if Napoleon was poisoned with arsenic, it is quite likely it was by mischance. If this is what happened, it may be a poetic form of justice, as it is widely believed that Napoleon was not above trying to poison others, especially King Louis XVIII.
In 1804, Louis XVIII of France was living outside Warsaw, in what was then Prussian territory. One day, one of the king-without-a-throne’s servants, one Coulon, was offered 400 louis d’or to add some hollow, poison-filled carrots to the soup that was to be served to the exiled royal and his family. Coulon was loyal: he accepted the carrots but denounced the emissaries. The Prussian police, conveniently, were careless enough to allow them to escape, arousing suspicions then and later that Napoleon himself may have been behind the plot, as he had a fair amount of clout at the time in the locality.
The carrots were later found to be filled with a paste of white, yellow, and red arsenic. Napoleon’s involvement was never proved, but during the Franco-Prussian War his nephew, Napoleon III of France, had no qualms about proposing that French bayonets be tipped with cyanide. Perhaps he was just joking, given that cyanide is also known as prussic acid, but in 1870 such ideas were treated with some horror. As we will see in chapter 9, this horror at the prospect of using poison in battle would not last.

It is interesting to consider whether we have more or less poison in our households today than we did a few generations back. In the nineteenth century, homes needed lye (caustic soda) to make soap, arsenic flypapers, arsenic powder for treating termite infestations, iodine, and copperas (iron sulfate) and bluestone (copper sulfate) for cleaning and scouring. Matches contained phosphorus, which was also found in cockroach poison. In the mid-nineteenth century, arsenical rat poison was replaced by phosphorus-based rat poisons, but these were equally effective in doing away with both human and rodent. There was lead in the paint and pipes, and arsenic in the wallpaper, while at least some of the dyed clothing was quite likely to be poisonous.
Today, we would expect to see antifreeze in cold climates, assorted caustic drain and toilet cleaners, oven cleaners, dishwasher detergent, bleach, insecticides, rat and mouse poisons, nail polish removers, paint thinners, disinfectants, mothballs, alcoholic drinks, tobacco products, and, in a few places, flaking toxic paint. For the most part, today’s household poisons are clearly labeled and generally have distinctive additives—color, taste, and smell—that make them unsuitable for a stealthy attack.
Disposing of lead-based paints continues to be a problem where old homes are being renovated, but at least people are now aware of the problems. Equally, the disposal of poisons from the workplace is gradually coming under better control. Our past record, however, is not good, so far as toxic working and living conditions are concerned.