- Charge is determined by the number of electrons present. Atomic number is determined by the number of protons. Isotope is determined by the number of neutrons (while protons make up part of the mass number, it is the number of neutrons that explains the variability between isotopes).
- 18O: 8 p+, 10 n0, 8 e–. 18F: 9 p+, 9 n0, 9 e–.
- Atomic mass is (just slightly less than) the sum of the masses of protons and neutrons in a given atom of an element. Atoms of the same element with different mass numbers are isotopes of each other. The atomic weight is the weighted average of the naturally occurring isotopes of an element.
-
This ratio is an equivalent concept. It is therefore acceptable, as long as units can be cancelled in dimensional analysis.
-
Isotope Protons Neutrons Electrons 19O 8 11 8 16O 8 8 8 17O 8 9 8 19F 9 10 9 16F 9 7 9 238U 92 146 92 240U 92 148 92
-

In both cases, the value is negative, indicating that energy is absorbed. This is consistent with an electron moving from a lower to a higher shell.
-
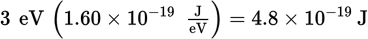
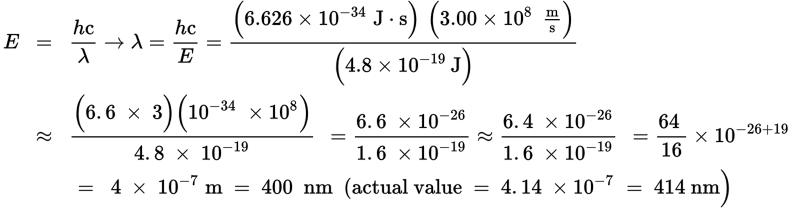
-

-
n l Possible Elements 2 1 2p: B, C, N, O, F, Ne 3 0 3s: Na, Mg 5 3 5f: Actinide series 4 2 4d: Y, Zr, Nb, Mo, Tc, Ru, Rh, Pd, Ag, Cd - Both O and O2– have fully filled 1s- and 2s-orbitals. O has four electrons in the 2p subshell; two are paired, and the other two each have their own orbital. O2– has six electrons in the 2p subshell, all of which are paired in the three p-orbitals.
-
Both these molecules have unfilled valence electron shells with relatively few paired electrons; therefore, they are paramagnetic.
-
Atom s-electrons p-electrons d-electrons f-electrons Total Valence Electrons P in PO43− 2 6 2 0 10 O in PO43− 2 6 0 0 8 Ir 2 0 7 0 9 Cf 2 0 0 10 12