Discrete Practice Answers
- A
This process may be adiabatic. Given that the gas was cooled, it did not maintain constant temperature, eliminating (C). Isobaric and isovolumetric processes appear as horizontal and vertical lines in pressure–volume graphs, respectively, eliminating (B) and (D). Adiabatic processes appear hyperbolic on pressure–volume graphs, as illustrated here. - D
There is not enough information in the problem to determine whether or not the reaction is spontaneous. If the signs of enthalpy and entropy are the same, the reaction is temperature dependent according to ΔG = ΔH – TΔS. Without the temperature, we cannot determine if this reaction is spontaneous, nonspontaneous, or at equilibrium. - D
Solve this question using the equation ΔG°rxn = −RT ln Keq. ΔG°rxn is negative (as it must be for a spontaneous reaction), and R and T are always positive. Therefore, lnKeq must also be positive for the sign convention to work out correctly. Since ln(1) = 0, the natural logarithm of any number greater than 1 will be positive, and the natural logarithm of any number less than 1 will be negative. In order for lnKeq to be a positive number, Keq must be greater than 1. - D
Combustion often involves the reaction of a hydrocarbon with oxygen to produce carbon dioxide and water. Longer hydrocarbon chains yield greater amounts of combustion products and release more heat in the process—that is, the reaction is more exothermic. Of the hydrocarbons listed here, n-pentane is the longest chain. - A
At first glance, this might seem like a math-heavy problem, but it really doesn’t require any calculations at all. We just have to keep track of which bonds are broken and which bonds are formed. Remember, breaking bonds requires energy, while forming bonds releases energy. Two bonds are broken: a C–O bond between the carbonyl carbon and oxygen of acetic acid 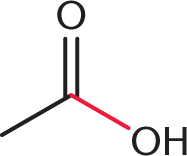 , and an O–H bond between the hydroxyl oxygen and hydrogen of methanol
, and an O–H bond between the hydroxyl oxygen and hydrogen of methanol 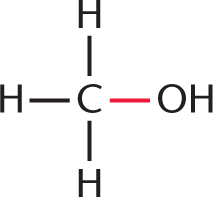 . Two bonds are also formed: a C–O bond between the carbonyl carbon and the oxygen of methyl acetate
. Two bonds are also formed: a C–O bond between the carbonyl carbon and the oxygen of methyl acetate 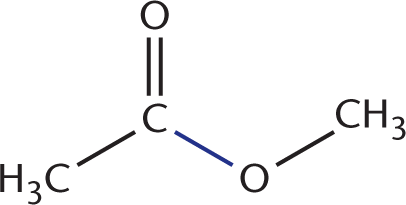 , and an O–H bond between a hydroxyl group and a hydrogen to form water
, and an O–H bond between a hydroxyl group and a hydrogen to form water 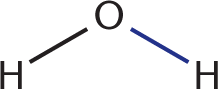 . Given that the same two bonds are broken and formed in this reaction, the energy change must be
. Given that the same two bonds are broken and formed in this reaction, the energy change must be

- C
Standard temperature and pressure indicate 0°C and 1 atm. Gibbs free energy is temperature dependent, but if a reaction is at equilibrium, ΔG = 0. - B
Solve this question using the equation ΔG°rxn = −RT ln Keq. ΔG°rxn° is
 R is
R is
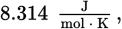 and T = 298 K because the reaction is occurring under standard conditions. Because ΔG°rxn uses kilojoules in its units and R uses joules, one will have to be converted. Plugging into the equation, we get:
and T = 298 K because the reaction is occurring under standard conditions. Because ΔG°rxn uses kilojoules in its units and R uses joules, one will have to be converted. Plugging into the equation, we get: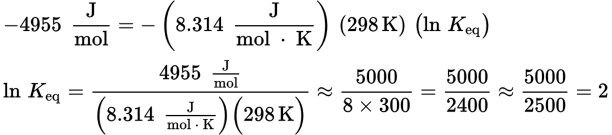
If ln Keq = 2, then Keq = e2. The value of e is approximately 2.7, so e2 = 2.72 will be a number between 22 = 4 and 32= 9. Both (B) and (C) fit these criteria; however, 8.9 is very close to 9, so we can assume that its square root is very, very close to 3. The answer choice should be a bit smaller, so (B), 7.4, is correct.
- D
There is not enough information available to determine the free energy of this reaction. While the entropy is clearly increasing (there are more particles in the system), it is unclear what the enthalpy change is. Because bonds are breaking, the reaction should be endothermic, meaning that both ΔS and ΔH are positive. In this case, it is a temperature-dependent process, and—without a temperature given—we cannot determine the sign on ΔG. - A
This problem asks for the free energy of a reaction at nonstandard conditions, which can be determined with the equation ΔG = ΔG° + RT ln Q. - A
A reaction with a negative enthalpy is, by definition, exothermic. Because both enthalpy and entropy are negative, this is a temperature-dependent process, and the reaction will be both endergonic and exergonic—but only at particular temperatures, eliminating (C) and (D). - C
For a process to progress forward spontaneously, Q must be less than Keq and will therefore have a tendency to move in the direction toward equilibrium. A spontaneous reaction’s free energy is negative by convention. - B
A calorimeter measures heat transfer. Although calorimeters often incorporate thermometers, the thermometer itself only tracks temperature, not the heat transfer itself, so (A) is incorrect. (C) is irrelevant; barometers measure changes in pressure. (D) is also incorrect because volumetric flasks measure quantities of liquid, not the heat capacity of the liquid. - A
Eliminate (C) and (D), which describe the free energy of reaction and cannot be determined from this graph. While most reaction coordinate graphs we’ve explored in this book use free energy for the y-axis, this one uses potential energy (enthalpy). If the heat of formation of the products is greater than that of the reactants, the reaction is endothermic. We can determine this information from their relative positions on the graph: because the products are higher than the reactants, this is an endothermic reaction. - C
Gases have the highest entropy, and solids have the lowest. Therefore, a phase change from a gas to a solid—deposition—would have the largest decrease in entropy of any phase change process. - A
In an explosion, a significant amount of heat energy is released, meaning that the reaction is exothermic (ΔH < 0), eliminating (B). The entropy change associated with an explosion is positive because energy is dispersed over a much larger area, eliminating (C). If this is true, the expression ΔH – TΔS must be negative, indicating that this is an exergonic process (ΔG < 0). Absolute temperature can never be negative, eliminating (D).