
(9.1) Percent composition by mass:

(9.2) Mole fraction:

(9.3) Molarity:
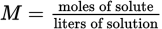
(9.4) Molality:
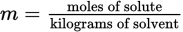
(9.5) Dilution formula: MiVi = MfVf
(9.6) Solubility product constant: Ksp = [An+]m[Bm−]n
(9.7) Ion product: IP = [An+]m[Bm−]n
(9.8) Raoult’s law (vapor pressure depression): PA = XAPA°
(9.9) Boiling point elevation: ΔTb = iKbm
(9.10) Freezing point depression: ΔTf = iKfm
(9.11) Osmotic pressure: ∏ = iMRT