3
INSOLUBLE: THE POWER OF PRECIPITATION
The processes of chemistry have wonderfully evocative names that seem to hint at an ancient, almost ritualistic character to the craft: evaporation, distillation, precipitation. This aura hangs over the routine tasks of the laboratory: chemical work can be repetitive to the point of boredom, but there is an almost devotional dimension to its demands of patience, care, and awareness. Primo Levi described this attitude with his characteristic lyricism:
Distilling is beautiful. First of all, because it is a slow, philosophic, and silent occupation, which keeps you busy but gives you time to think of other things, somewhat like riding a bike. Then, because it involves a metamorphosis from liquid to vapor (invisible), and from this once again to liquid; but in this double journey, up and down, purity is attained, an ambiguous and fascinating condition, which starts with chemistry and goes very far. And finally, when you set about distilling, you acquire the consciousness of repeating a ritual consecrated by the centuries, almost a religious act, in which from imperfect material you obtain the essence . . . the spirit.
That final word tells you that the religious analogies are neither casual nor superficial. The chemical laboratory used to be full of what its practitioners called “spirits”: spirit of salts (hydrochloric acid), spirit of wine (alcohol), spirit of hartshorn (ammonia). These are typically liquids distilled from some other substance: heat liberates the more volatile, easily evaporated compounds into the air, from which they are recaptured in pure form by cooling and condensation. In earlier times there was thought to be a genuine analogy between spirit as a volatile yet tangible substance and spirit as a kind of soul or essence.
When the art and science of what today we call chemistry was being refined in the sixteenth and seventeenth centuries, the processes of transformation that took place in the crucibles and retorts of the laboratory—evaporating, precipitating, purifying—were truly thought by some practitioners to be equivalent to those that happened in the wider world. You could conjure up a cosmos in a flask. The idea underpinning what became known as the chemical philosophy was that the “macrocosm” of the world was reflected in the “microcosm” of the human body and the glass vessels of the chemical workshop, so that you could understand one by studying the other. The body's health was thought to be governed by vapors or spirits called humors that could rise and fall, wax and wane. For the chemical philosophers, the processes of nature were mimicked in chemical (most at that time would say alchemical or chymical) transformations. Water rises into the sky and condenses again in clouds and rain just as liquids circulate during distillation—a process then already important for making alcoholic drinks. Even the origin of the world as described in the biblical book of Genesis, where the waters were separated to expose dry land, was a story about (al)chemical transformation.
“You could conjure up a cosmos in a flask.”
So it's no coincidence that the word “precipitation” is used even today to refer both to the fall of rain and snow and to the way solid substances may coalesce out of a solution in chemistry.

We learn from a young age that some things (like salt) dissolve in water and some (like sand) do not. But in fact, that's not quite right. The question is really how much of things dissolve in water. Every substance dissolves at least a little, but some dissolve a lot. You can keep adding sugar to water until you have syrup.
It's hard to figure out what the rules are. Take the classic precipitation experiment: mixing a solution of barium chloride with a solution of a soluble sulfate salt such as potassium sulfate. Both solutions are colorless—they look just like water, although they are loaded with ions of barium, potassium, chloride, and sulfate. But when mixed, out billows a white discharge of fine, insoluble particles of barium sulfate, like cumulus clouds blooming in the sky.
This is odd. Evidently each of those ions can be soluble individually—so why won't barium and sulfate stay dissolved when they share the same solution? Most chemistry textbooks are maddeningly evasive about this. They talk about something called a “solubility product,” which is (loosely speaking) a number calculated by multiplying together the maximum possible concentrations of the different ions in a solution of a salt with limited solubility, like barium sulfate. From the solubility product you can predict when the compound will precipitate as the concentrations of the ions increases.
That might be handy—but it's a description, not an explanation. (Beware: science is full of descriptions masquerading as explanations.) It doesn't offer any clue about why ions that are soluble in isolation are insoluble—and will precipitate—in combination. The real answer, as with any chemical reaction, is that the outcome depends on the overall changes in energy (strictly, in free energy—see chapter 8). Ions of opposite electrical charge, like those of barium and sulfate, will tend to attract one another and stick together, creating a solid. They will remain in solution only if this tendency can be suppressed. It can be if the ions are rendered more energetically stable by holding onto their shell of surrounding water molecules than by shedding them and gathering together in a crystal lattice. Which is the most stable situation depends on the ions concerned. For barium + chloride, retaining the shell of water molecules in solution is best. For barium + sulfate, it's not. But until you develop a chemist's intuition about such things, you can't expect to guess in advance which way the balance will tip.


As a general principle, many ions are readily dissolved (chemists would say solvated, which means “surrounded with solvent”) in water. Because water molecules are polar—they have a slightly negatively charged part (the oxygen atoms) and slightly positive parts (the hydrogens)—it is generally possible to arrange water molecules around an ion so that the parts of the molecules with opposite charge to the ion sit closest to it: that's an energetically comfortable arrangement. Water's polar nature makes it a good solvent for ions—so salts of all sorts, and also many minerals, will dissolve quite readily. Water is also a good solvent for other molecules that are polar, which is to say, ones possessing imbalanced distributions of charge. Alcohols such as ethanol are like this, and will stay dissolved at concentrations ranging all the way from, as one might express it, ales to wines to whiskey.
For the same reason, water is good at solvating sugars. But alcohols and sugars are among the molecules that also gain solubility from a rather more special property of water: its ability to form weak chemical bonds called hydrogen bonds. These arise from an electrical (at least, it is largely electrical) attraction between hydrogen atoms and atoms that have pairs of electrons “dangling” from them, taking no part in the regular chemical bonds that the atom forms with others. Oxygen and nitrogen are the most significant representatives among the atoms that tend to sport these “lone pairs” of electrons. The hydrogens in these partnerships have acquired a slight positive charge by being attached to some other atom that has a tendency to pull electrons toward it—both oxygen and nitrogen do this too, whereas a hydrogen atom attached to carbon, which has none of that electron-hogging character, won't form hydrogen bonds. We'll see shortly how important this distinction is.
What exactly do we mean by “dangling” pairs of electrons? If electrons in the outermost part of an atom's electron cloud aren't involved in chemical bonding to other atoms, they will typically pair up in lobe-shaped clouds: these are the lone pairs. Oxygen atoms have a total of six electrons in their outermost “shell.” In a water molecule (H2O), two of these electrons are paired up with those from the hydrogen atoms, while the other four are sequestered into two lone pairs, which can be pictured (beware of taking such pictures too literally, a caveat that applies in any description of atoms) as lobelike electron clouds protruding from the oxygen atoms rather like rabbit ears.
Because electrons are negatively charged, these lone pairs can attract the slightly positive hydrogen atoms on other water molecules. They will stick together, binding the hydrogen of one molecule to the oxygen of another. It's a loose and floppy bond, about ten times weaker than that which joins hydrogen and oxygen atoms within a given water molecule. But this hydrogen bond is nonetheless one of the most important molecular glues in nature.
For one thing, it makes water what it is: a liquid at the temperatures and pressures typically found at the surface of our planet. Without hydrogen bonds, water would be a gas under these conditions—like methane, which is similar in some ways to water (it is a small molecule having a central atom—of carbon—with hydrogens attached) but lacks the capacity to form hydrogen bonds. The hydrogen bonds between water molecules give them some extra cohesion that stops them from flying apart at everyday temperatures. Sure, a puddle will evaporate into water vapor eventually—but as the vapor rises into the sky, carried on convection currents of warm air, eventually the air will cool enough for the water to condense again into tiny droplets of liquid. And there's your cumulus cloud (or cirrus, stratus, nimbus, as the case may be).
Each water molecule can form four hydrogen bonds: one from each of the two lone pairs on the oxygen, and one from each of the hydrogen atoms. These are arranged around each molecule in just the right way to join water molecules into a three-dimensional network of hydrogen bonds, with each molecule making these fragile handshakes to four neighbors. It's a loose, imperfect network, because hydrogen bonds are too weak to hold for long in the face of the random jittering of a water molecule in the liquid. Typically, each hydrogen bond lasts for only about one trillionth of a second before breaking and then reforming with another partner. This means that the water molecules are constantly executing an elaborate dance, and at any moment many will lack all four partners. That's why water is a liquid at all. If the hydrogen bonds held them rigidly, the network would freeze—literally. This is what ice looks like at the molecular scale: a hydrogen-bonded array of water molecules locked into place as the molecular jitters are cooled away. One way to think of water's structure is as a sort of flawed and dynamic ice, shot through with disorder and motion.
“Water molecules are constantly executing an elaborate dance of hydrogen bonds.”
Actually, it's been a matter of long debate (some of it rather forceful) whether that is indeed the best way to describe water's structure. You could alternatively view it from the other direction as a kind of sticky vapor, the randomness of the molecular gyrations given a degree of order by the formation of these hydrogen-bond handclasps. Even today, deciding on the best description of water's delicately poised balance between order and disorder is not a settled matter.
Ammonia can form hydrogen bonds bridging its molecules too. But it doesn't have the right shape for these to generate a three-dimensional network, and so ammonia doesn't gain as much cohesion. It's fairly easy to liquefy by cooling or pressurizing, but it's still a gas under everyday conditions.

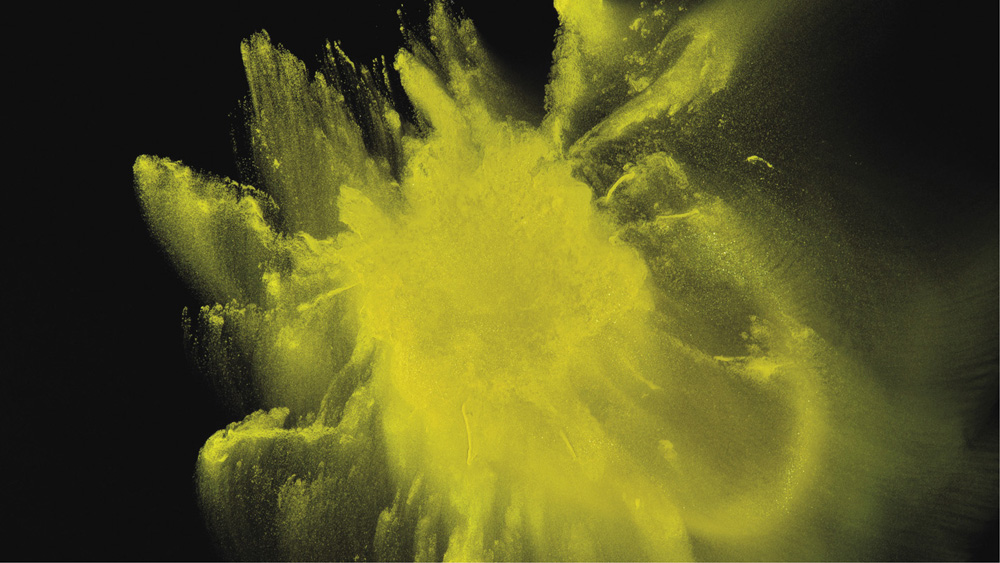

The importance of hydrogen bonding goes way beyond its role in pure water. It's because alcohols and sugars have oxygen and hydrogen atoms capable of hydrogen bonding that they are so good at dissolving in water. Some of the hydrogen bonds between water molecules that must be broken to open up a space for these larger molecules in solution can be recovered as links between the water molecules (the solvent) and the dissolved substance (the solute)—so the energetic price for admitting the molecules into solution is not too great.
The same is true for protein molecules, which are ubiquitous and essential ingredients of living cells. Many of these proteins act as enzymes: catalysts for biochemical reactions, such as those involved in metabolism (the processes in which food and other ingested compounds are concerted to energy and building blocks of biomolecules themselves). They float about in the watery fluid of the cell, called the cytoplasm. As we saw, protein molecules are chainlike molecules, typically folded into compact shapes. Their molecular building blocks are called amino acids, which contain hydrogen, oxygen, and nitrogen atoms all capable of forming hydrogen bonds with water (or with each other) when exposed on the surface of a crumpled-up protein chain.
Hydrogen bonds between the components of protein chains can help to glue them into the shapes they need to do their job. One of the most common shapes adopted by parts of protein chains is a corkscrew called an alpha helix, in which the spiraling form is generally held in place by hydrogen bonds linking adjacent coils. Perhaps the most renowned role of hydrogen bonds in biology, however, is as the glue that binds the double helix of DNA, the molecule that bears the genes. We saw in the previous chapter that when DNA is replicated, the twin strands are unzipped and each acts as a template for a new strand to be assembled. This unzipping would be impossible if the strands were linked by regular chemical bonds—they are too strong, and breaking them would require too much energy. But hydrogen bonds are weak enough to be easily broken one by one, even if collectively they supply a secure adhesive force between strands.
These attributes make hydrogen bonds the perfect glue for the chemistry of life. This chemistry relies on molecular dialogue: on molecules coming together, forming unions, exchanging information, and arranging themselves and each other with order and precision. Too strong a binding force here would seal the molecules into inanimate rigidity: they would become like rocks and minerals, locked into place for eons. Life persists at the boundary of order and chaos, and it needs the ability to make and break unions: now united, now free. It depends on the finely tuned chemistry of weak bonds.
“The chemistry of life relies on molecular dialogue: on molecules coming together, forming unions, exchanging information, and arranging themselves with order and precision.”
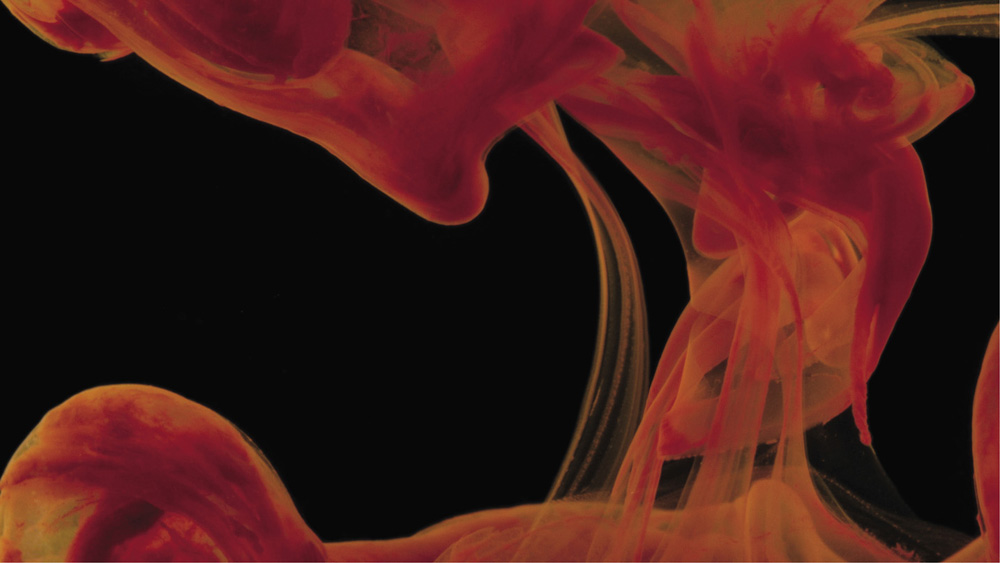
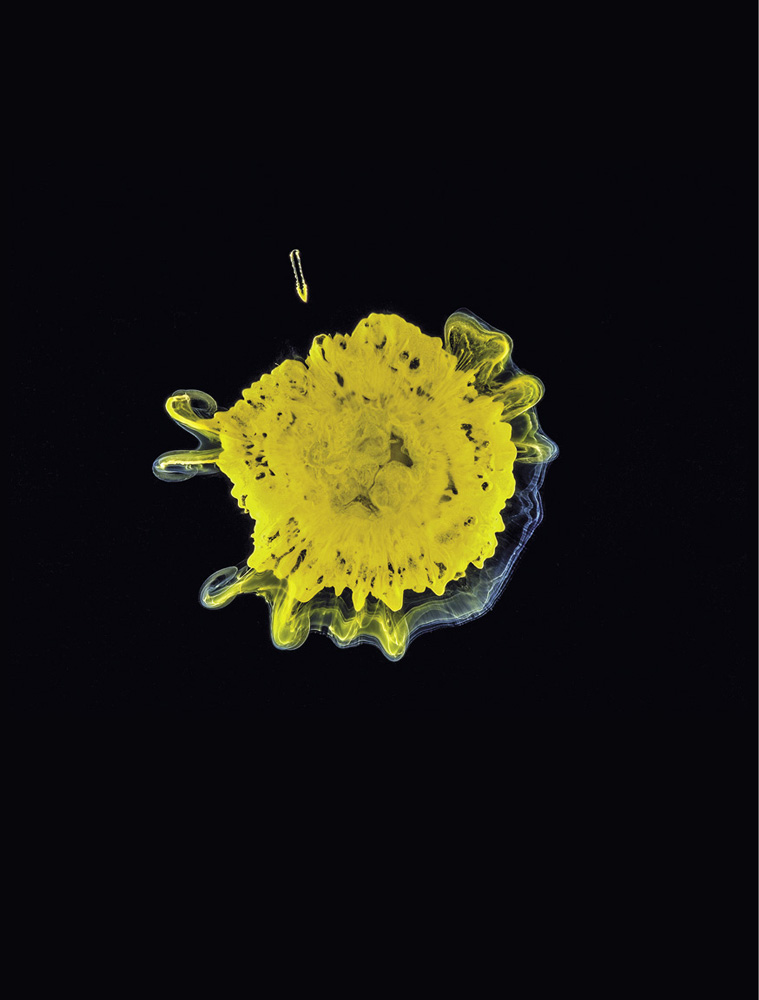
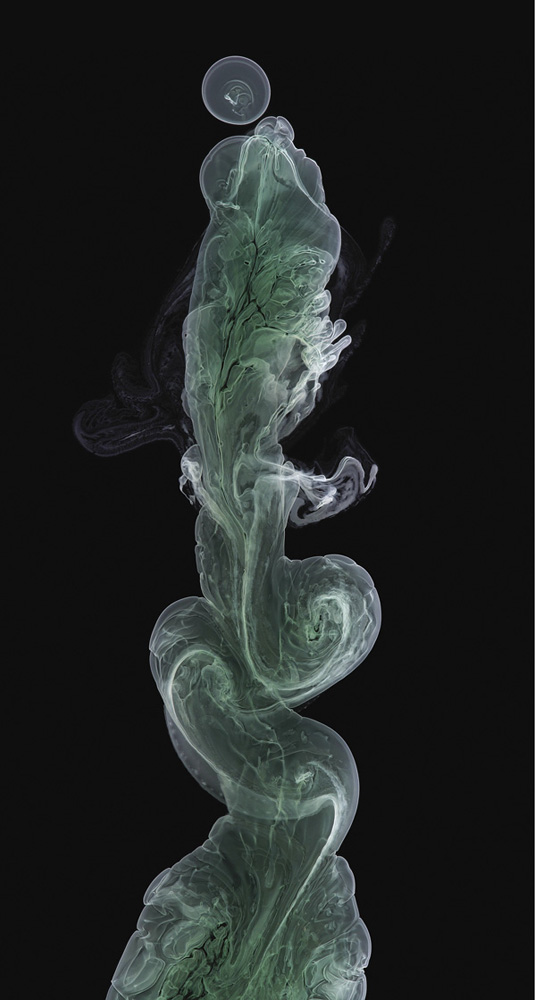
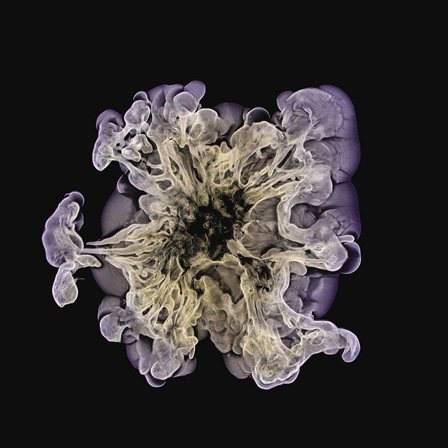
The precipitation of a mixture of cobalt(II), copper(II), and iron(III) hydroxides


Hydrogen bonds are not the only option for molecules such as proteins that depend for their biological function on a versatile ability to shape-shift, to engage and separate. Some of the “stickiness” that holds protein chains in the proper configuration comes simply from their being immersed in the watery solvent of the cell.
Proteins typically possess a finely tuned mixture of solubility and insolubility. Parts of their molecular chain are chemically similar to oils and fats (made up of nonpolar hydrocarbon units): they repel water and won't dissolve well in it.
Other parts resemble sugars or alcohols in being imbued with polar chemical groups capable of forming hydrogen bonds to water molecules: they are perfectly soluble. Roughly speaking, the protein chains fold up so that the soluble parts are exposed on the outside of the compact globule and the insoluble parts are buried inside—that's the most stable folded configuration. How a collapsing molecular chain finds this configuration from among the countless possible ways it could fold has long been a source of puzzlement, even wonder, among biochemists. But proteins seem to have evolved to be efficient at folding to the required shape, and sometimes this process is assisted by other proteins, aptly named molecular chaperones.

One might reasonably say that the protein chain is programmed to fold in the way needed to adopt the shape that gives it its function. The programming is inherent in the sequence with which its components—20 varieties of amino acid—are linked along the chain. That in turn is specified by a gene in DNA that encodes the protein. In this sense, biological molecules are examples of what chemistry Nobel laureate Jean-Marie Lehn has called “informed matter”: physical substance that contains within its atomic arrangements the information that tells it “what it should do.”
“Biological molecules are examples of informed matter: physical substance that contains within its atomic arrangements the information that tells it ‘what it should do.’”
If so, that information is specific to a watery existence. Proteins only fold properly when immersed in water. In other liquids they may lose their shape, and consequently their biological function too. At the same time, the water solvent mustn't lock the folded chain too rigidly in place, because proteins need some flexibility. When an enzyme binds to the target molecule that it will transform in a catalytic chemical reaction, it needs to adjust its shape in small (or sometimes in substantial) ways. The folded protein achieves an exquisite balance between a well-defined conformation and a panoply of minor variations on that theme.

In their apparent wish to stay hidden from water, the insoluble parts of a protein chain act as if they are attracted to one another. In fact, to all intents and purposes the tendency of these regions to clump together, shielding each other from water, does resemble a real, physical force: it is called the hydrophobic (“water-fearing”) attraction. The parts of the protein that dissolve well in water are, conversely, said to be hydrophilic: “water-loving.” Proteins are mixtures of hydrophobic and hydrophilic regions within a single molecule, and we can regard the sticking-together of hydrophobic parts as a kind of local precipitation on the scale of molecules themselves.
It's too simplistic to say that proteins are entirely hydrophilic on the outside and hydrophobic inside; they are really a mixture of both, inside and out. In fact, it may be an important aspect of a protein's design to have a partly hydrophobic exterior, since this can make proteins stick to one another, congregating into groups that accomplish their goal via teamwork. Some of the more complex molecular machines of the cell, such as the ribosome where proteins are made according to the plan encoded in nucleic acid molecules, are aggregates of several proteins, perhaps with other molecules included too.
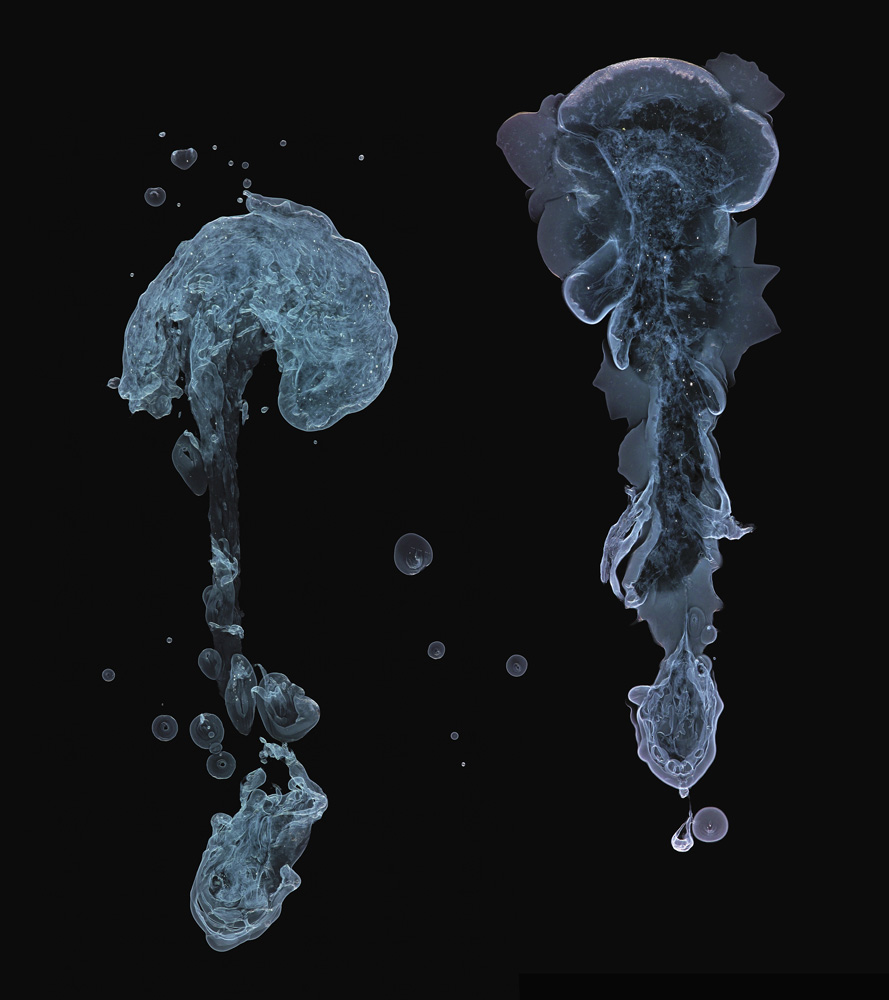
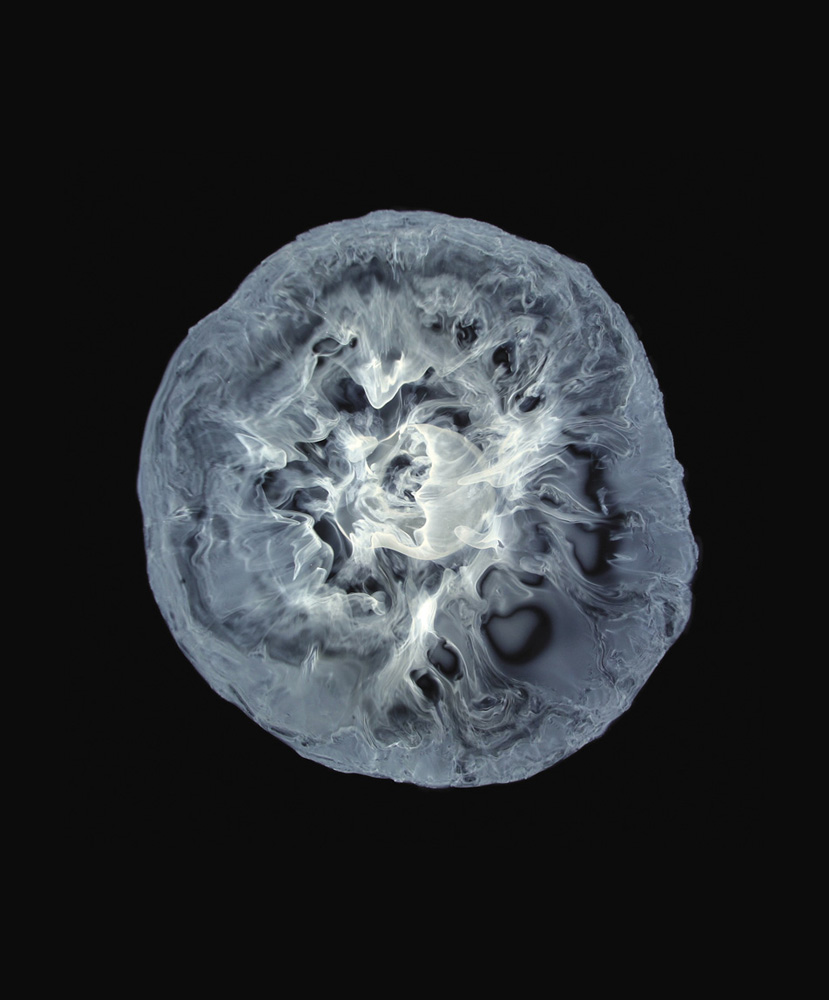
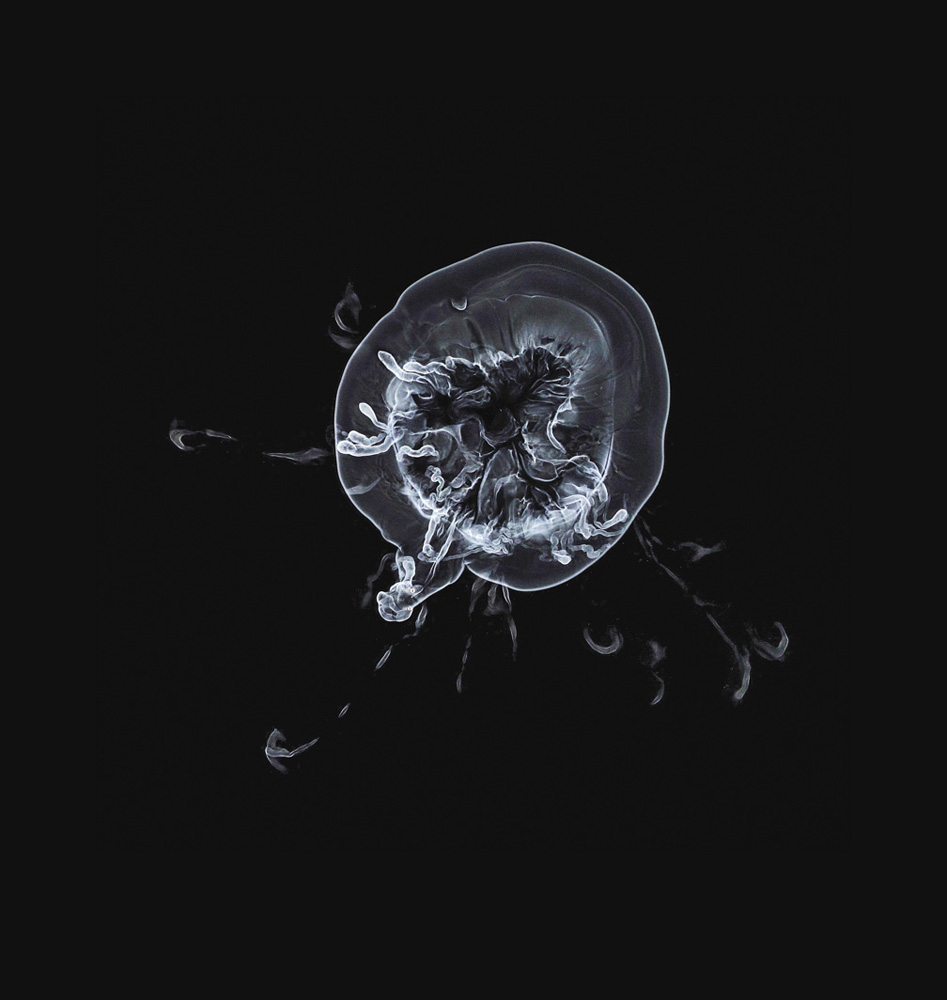
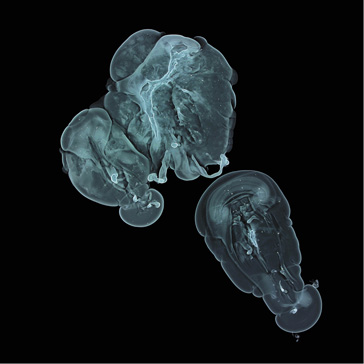
A precipitating mixture of copper(II) and nickel(II) hydroxides

Some proteins, meanwhile, are equipped with hydrophobic regions on their surface so that, rather than remaining soluble in the cell fluid, they will become embedded within its membranes. That's another way for such regions to stay shielded from water. Membrane proteins are a tremendously important class of biological agents. They can act as relay systems that carry messages received at the outside of a cell membrane—a hormone molecule, say—into the cell interior. They can be conduits that admit small molecules and ions into and out of individual cells—for example, proteins called ion channels are rather like tubes that puncture a membrane and let ions through. These channels are selective: they will only admit specific ions (sodium but not potassium, say), and in one direction but not the other. And they may be “gated,” opening and closing in response to signals they receive from their surroundings. Ion channels in the membranes of nerve cells (neurons) conduct sodium and potassium ions in and out of the cells, and the resulting change in the electrical charge on either side of the membrane creates an electrical pulse that travels along the neuron: the electrical activity that underpins nerve action and thought itself.
Cell membranes are also examples of how a mixture of solubility and insolubility is useful to biology. They are mostly made up of molecules called lipids, which have a long fatty “tail” attached to a small water-soluble “head.”
Molecules like this, with a simple division of hydrophilic and hydrophobic parts, are called amphiphiles (“both-loving”); soaps have a similar character, which is why they can surround globules of fat and make them soluble in water. In cell membranes the lipid molecules cluster together in a structure that shields the tails from water while exposing the heads. They line up side by side with all the heads pointing in the same direction, creating a sheet, and then two such sheets adhere back to back so that both sides present an array of water-soluble heads. This arrangement is called a lipid bilayer.
In cells these membranes may curl up on themselves to form closed compartments, the largest example being the cell membrane that encloses the entire cell. Some of these compartments inside cells, called organelles, contain proteins and other molecules that conduct specialized tasks. Other membrane structures crinkle into complicated, labyrinthine folds, studded with membrane proteins. One especially important membrane-bound organelle in our own cells (and those of other animals, as well as plants) is the cell nucleus, which contains our DNA, packaged into chromosomes. In this way, a great deal of the organization on which life depends is maintained via molecules that combine solubility and insolubility in water.
“A great deal of the organization on which life depends is maintained via molecules that combine solubility and insolubility in water.”

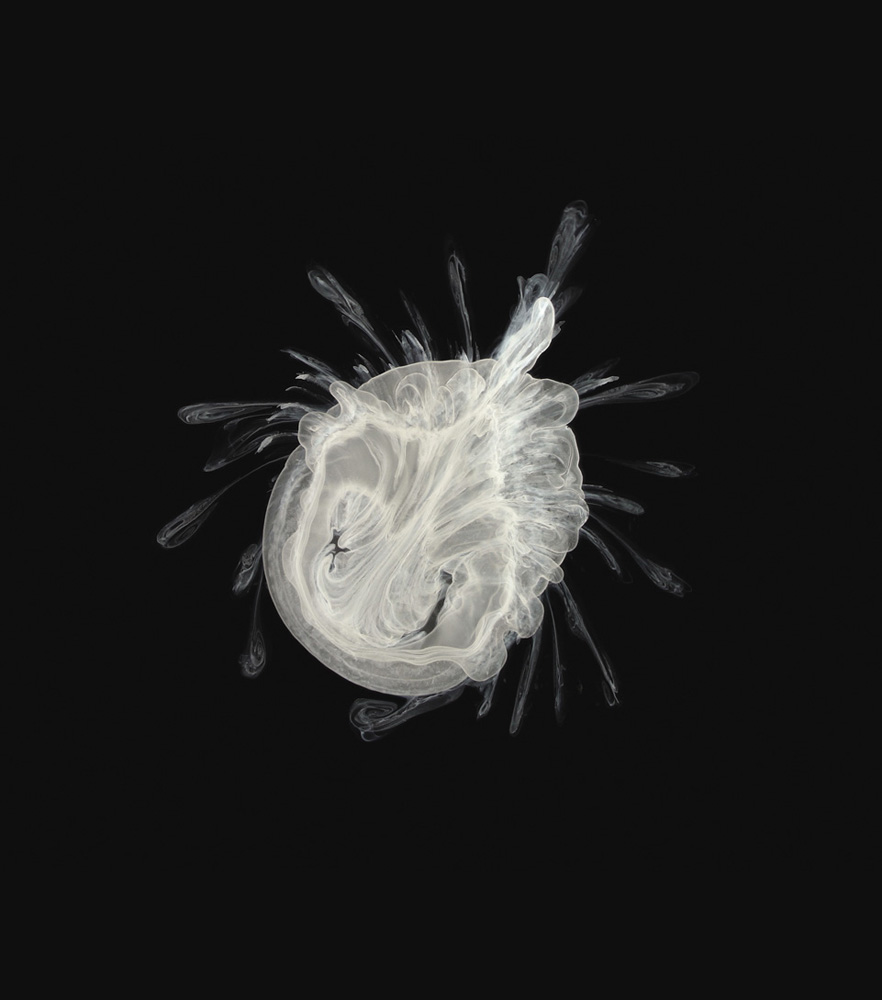
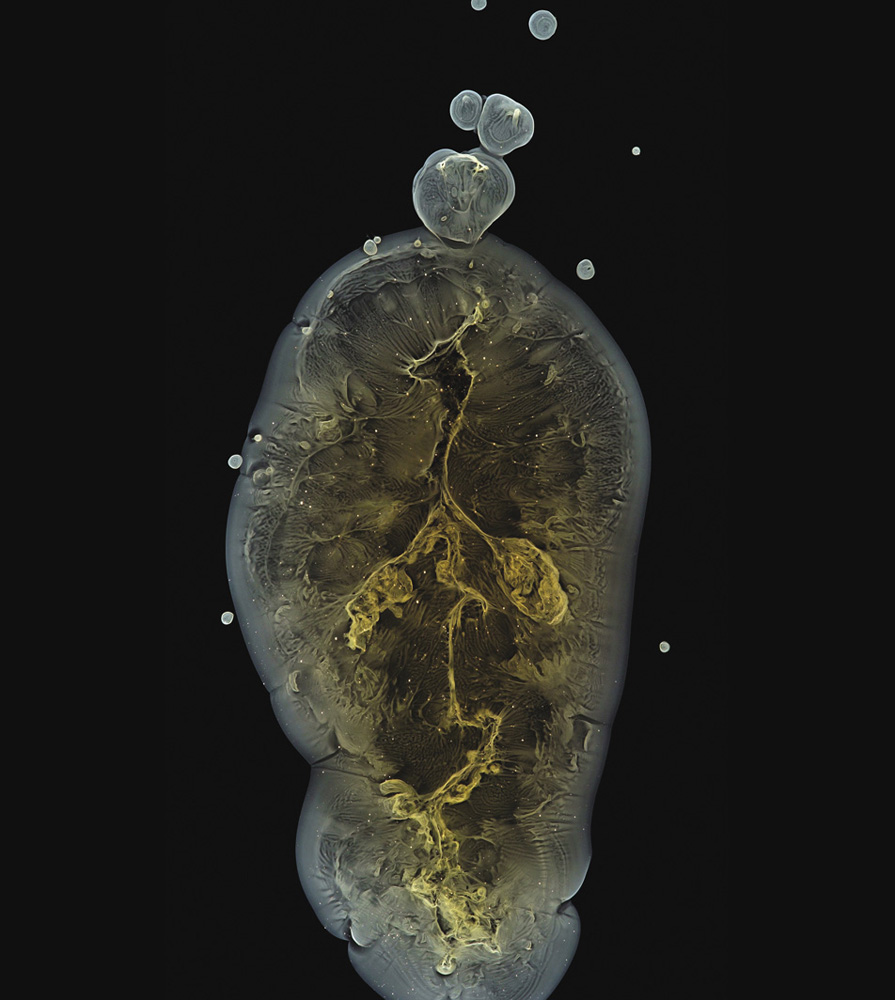
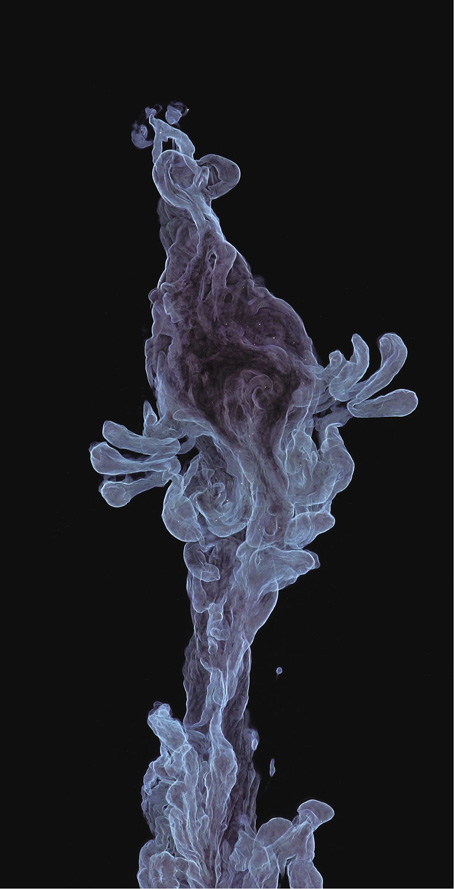
Precipitation of a mixture of iron(III) and nickel(II) hydroxides

The balance between a protein's tendency to fold and the looseness that allows it to adjust its shape is finely tuned. It usually holds only under the conditions found inside cells—that's to say, at body temperature and ordinary pressures, in a solution with the right degree of saltiness. Make the temperature too high or too low, the pressure too intense, or the liquid too salty, and the balance is lost.
Then the protein “denatures”—which is to say, it unfolds. Exposed to water, the hydrophobic regions on different chains might then stick to one another, and the protein coagulates into insoluble goo. This is what happens to the protein called albumin in egg white when it is heated, turning the substance from clear and water-soluble to opaque and insoluble—and creating the rubbery white skirt of a fried egg.
It's because proteins (and other biomolecules) need the right degree of saltiness to keep their shape and solubility that cells have evolved ways of controlling their salt content. If a membrane through which water can pass has different concentrations of salt on either side, water will be drawn from the less concentrated to the more concentrated side until the concentrations on each side are equal. This is called osmosis. Living cells in salty environments, such as the Dead Sea, have to actively oppose that process so that they don't lose their water and become desiccated. They can't simply admit high levels of salt inside, because that would risk denaturing the proteins and inflicting other kinds of salt-induced damage. They might store away salt in organelles called vacuoles, or they might produce other kinds of soluble molecules, such as amino acids or sugars, that can “balance” the high concentrations of salt outside the membrane without incurring the damaging effects of salt itself. Organisms that can withstand very salty conditions are examples of so-called extremophiles, which survive in extreme conditions. They might offer clues about whether life could survive in environments on other planets that seem by our standards to be less clement than those on Earth.

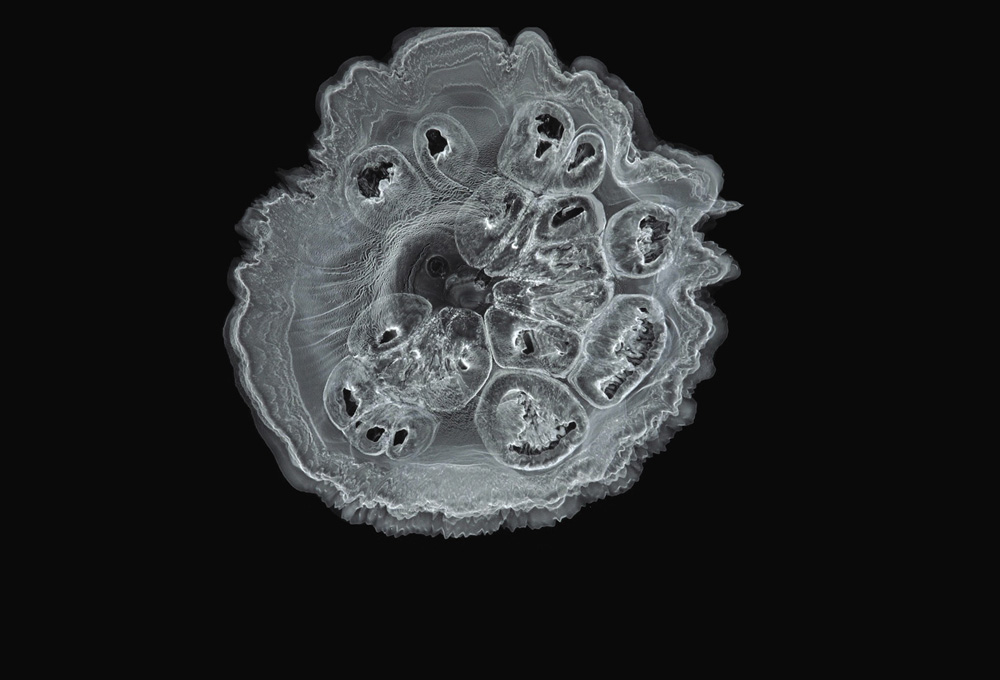
Precipitation of a mixture of copper(II) and nickel(II) hydroxides

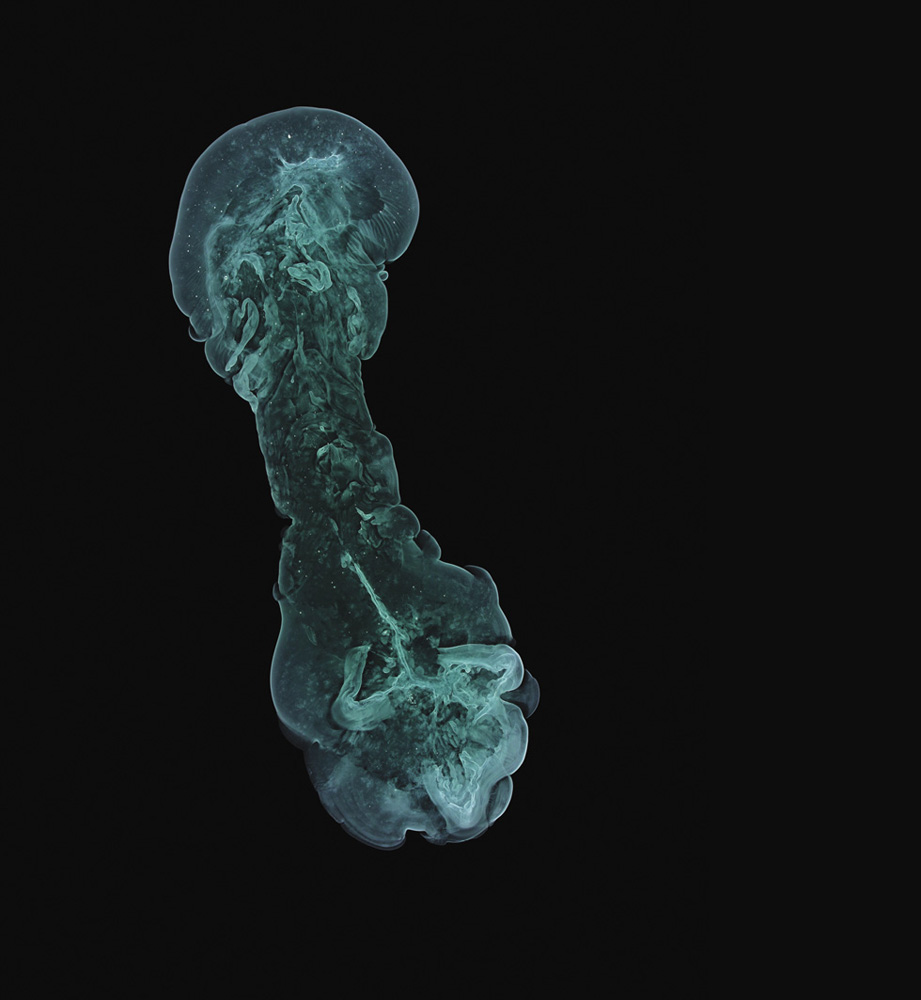
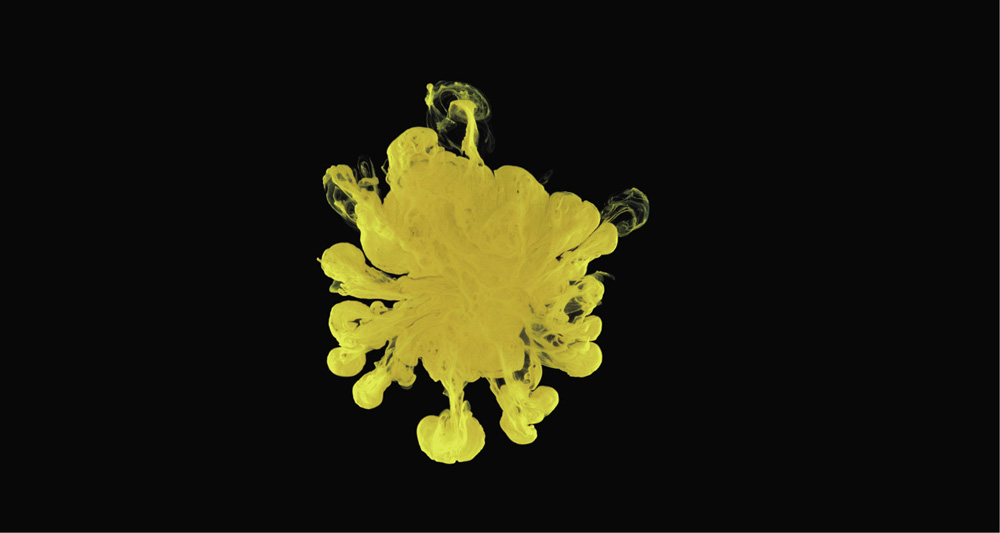
A precipitating mixture of nickel(II), magnesium, iron(III), copper(II), and cobalt(II) hydroxides
Proteins aren't the only substances that can coagulate and precipitate when there's a lot of salt in the water. It can happen to many kinds of organic matter dissolved or suspended as tiny particles in water, such as sediments in rivers. That's why, as a river flows into its estuary and mixes with sea water, the water can become murky: the sediment particles stick together in larger clumps, and what further upstream was clear water now resembles a muddy effluent. The formation of large flakes of material from much smaller, dissolved or suspended particles is called flocculation.
How it happens in estuaries is a complex affair that is still not fully understood—it depends, among other things, on how turbulent the flow is. But one way in which dissolved salt can cause coagulation is by neutralizing electrical charges on the surface of the particles. Many small sediment particles, for example those made from clays, have ions on their surface that give them a charge and make them repel one another. But if there are salt ions of the opposite charge in the water, they may be attracted toward the particles where they neutralize the surface charge, allowing the particles to come together and aggregate.
Looked at from high above, the outflow of turbid, sediment-laden water from an estuary traces out baroque patterns in coastal waters. Seen one way, it might appear messy—as if the land is disgorging its detritus into the bountiful blue of the ocean. But for marine coastal organisms this influx of matter is often a welcome injection of nutrients, helping estuarine ecosystems to thrive. And here too, we see played out in the natural world a macrocosmic reflection of the processes of precipitation that come swirling into the laboratory flask: a coalescence of substance from absence, the invisible made visible. Chemistry in action, transforming the world.
