6
GALVANIZING: THE ENCHANTMENT OF ELECTROCHEMISTRY

Patterns formed when droplets of potassium permanganate solution were added to a solution of sucrose and sodium hydroxide

Gold crystals grown by depositing the metal from a gold-rich vapor

“We Spaniards know a sickness of the heart that only gold can cure.” When he wrote these words, the conquistador Hernán Cortés was by no means the first to fall under the spell of gold—for the love of which he ransacked the Aztec empire of central Mexico, just as his compatriot Francisco Pizarro extorted tons of the yellow metal from the Incas.
But do we value gold because of its gleaming allure, or do we revel in that sight because of its value? Which is chicken and which is egg?
There surely is a magical quality in the reflective sheen of metals—the play of light that distorts the world into strange geometries, here mirror-bright, there streaked with darkness. The silvered mirror invites us into a looking-glass world where everything is reversed, left for right: the same but not the same. It's no surprise that mirrors in fantastical stories become portals into other realms or through which supernatural agencies speak and emerge—think of Snow White's queen, or Dracula's lack of a reflection. Mirrors seem to multiply space, and thereby to defy its laws.
Among the gallery of bright metals, gold is special: it is one of the few that does not tarnish. Silverware needs a rub from time to time to remove the dark discoloration, but gold never dims. Platinum has that incorruptibility too, and is similarly prized for it (although it was not recognized as a metallic element in its own right until the eighteenth century; the name means “little silver”). It is no wonder that, to the alchemists, gold seemed to hold a promise of eternal youth. The quest of the alchemists of China became more about discovering an elixir of immortality than about making gold itself from less precious metals. Aurum potabile—a ruby-red suspension of gold that one could drink—was regarded as a cure-all for diseases.
Most of us, however, can't afford gold in quantities exceeding what it takes to make a wedding ring. An alternative is to give cheaper metals the appearance of gold by applying a thin coating of the yellow metal: by gold-plating. It's the same for silver, once commonly coated onto the cutlery and kitchenware of the aspiring household to dazzle guests and to make the items less susceptible to tarnishing than if they were forged from steel.
The gleam of metals is enjoyed in its own right, not merely as a superficial symbol of opulence. Think, for example, of the chrome-plated fenders of a Cadillac, or the jaunty sparkle of a brass band. Glittering surfaces like these are often produced by a process called electroplating, which is a form of electrodeposition: the depositing of a layer of material using electricity. It's a classic and sometimes spectacular example of how electricity can be used to do chemistry.
“Electro-” here can stand for electricity, but equally it might connote the electron itself: a fundamental subatomic particle, from rearrangements of which all of chemistry arises. Every chemical process involves some movements of electrons. They are the glue that holds atoms together in molecules. When chemical bonds are made or broken, the electrons are redistributed: more glue here, less there.
In some of these reactions, an atom or a molecule might entirely lose or gain an electron, or several of them. Because an electron has a negative electrical charge, transactions of this sort result in a change in the overall charge on the atom concerned.
You might wonder what it really means to say that the electron has a negative charge. It's a good question, to which the answer is that it is merely a matter of convention. Positive and negative charges—as in the conventional “+” and “−” labels assigned to electrical terminals of a battery—are opposite to one another. Put them together and they cancel out, and there's no overall charge. But it's entirely arbitrary that we say the charge on an electron is “negative” while that on a proton in an atom's nucleus is “positive.” We could have done it the other way round, and nothing would change, any more than if we had decided to call left “right” and vice versa. (Quite aside from the arbitrary nature of the words themselves, whether “left” and “right” are truly equivalent—whether the looking-glass world is really indistinguishable from ours—is a deep question in physics.)
“Every chemical process involves some movements of electrons. They are the glue that holds atoms together in molecules.”
Atoms are neutral—they have no charge—when the number of electrons they contain, dispersed in clouds around the dense central nucleus, is equal to the number of positively charged protons in the nucleus itself. Electrons are much smaller and lighter than protons, but their charge is equal and opposite. When these two numbers are imbalanced, the atom has a positive or negative charge, and is called an ion. That charge is some whole-number multiple of the charge on an electron or proton; it can't lose or gain “half an electron.” (All the same, it's sometimes useful to talk as if atoms or molecules have some fraction of an electron's charge: for example, when a cloud of electrons is smeared out and shared among several of them.)

A microscopic view of copper acetate, formed by the action of vinegar on copper metal

A microscopic view of copper acetate, formed by the action of vinegar on copper metal


We saw earlier that the reaction of a substance with oxygen, often making a compound called an oxide, is called (logically enough) oxidation. However, chemists recognize a more general meaning of “oxidation,” which need not involve oxygen at all. We can only apologize for this. Science is full of terms that arose for historical reasons but then outgrew their origins (the name “oxygen” itself is one of them!). To chemists today, oxidation refers to the process of losing electrons. If an atom or molecule gets relieved of one or more electrons, it has been oxidized—and this means that it acquires a positive charge or that its positive charge increases.

There is a rationale for this nomenclature. Oxygen atoms are among the most avid for electrons of all chemical entities, and this is what makes oxygen such a reactive element: it will seize electrons from wherever it can. We can see this behavior at work, for example, in the rusting of iron. Exposed to air, the surface of iron gets attacked by oxygen, forming red-brown iron oxide. In iron metal, the atoms are electrically neutral, having an equal number of protons and electrons. But in the oxide, the oxygen atoms have stripped some electrons from them. Roughly speaking, each oxygen atom gains two electrons, making oxide ions with a double negative charge, while each iron atom loses three electrons, making iron ions with a triple positive charge. To accommodate that disparity in charge, the ratio of iron to oxygen in the compound must be 2:3: it has the chemical formula Fe2O3. (The actual chemical composition of rust can vary; it forms more readily when there is some moisture present, and some of the water is typically bound into the rust itself: strictly speaking, rust is hydrated iron oxide.) So the iron has been oxidized—which here means that it has reacted with oxygen itself, it's true, but more fundamentally that it has lost electrons.
The converse process of gaining electrons is known as “reduction.” This seems illogical too: how is something “reduced” if it gains? But in its original context a process of reduction did indeed involve a loss—of weight. Most metals, including iron, exist in nature as ores, which are compounds. Iron ore is generally some form of iron oxide, such as the mineral haematite. To make the pure metal, the other elements in an ore (such as oxygen) have to be removed. The Iron Age began (around 1000 BCE; to be more precise with the date, you must specify the region) when humans figured out how to achieve that removal by heating iron ore with charcoal. The oxygen in the ore combines with the carbon in the charcoal to make carbon dioxide, leaving behind molten iron. The discovery was probably made several times over in different parts of the world, and it transformed society and politics: weapons made from brittle bronze were no match for hard-edged iron. The point here is that, by losing oxygen, the ore loses weight—hence “reduction.”
An element or a compound that is good at extracting electrons from others is called an oxidizing agent. Oxygen gas itself is an excellent oxidizing agent; it is the nefarious agent of corrosion, by which iron structures are reddened and ultimately weakened as they are converted into the oxide. But oxygen is not the best of all oxidizing agents—fluorine, for example, is even more hungry for electrons, and indeed may take them from oxygen itself. Ions with a large positive charge—that is, those severely depleted in electrons—may also be good oxidizing agents. One such appears in potassium permanganate, KMnO4, where the manganese atoms can be assigned a positive charge of +7. (Because there is some sharing of electrons between the manganese atom and the four oxygen atoms attached to it, the manganese doesn't truly have such a whopping charge.) The compound is commonly used as a disinfectant to oxidize noxious substances in drinking water.
By the same token, a compound good at donating electrons to others is called a reducing agent. These can include chemical species that already have many electrons, such as the ferrocyanide ion 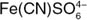 . Alkali metals like lithium and sodium are powerful reducing agents too: they are “eager” to lose an electron. (Remember that this anthropomorphic language simply connotes that the process happens readily because it lowers the overall energy of the system. It's in the same sense that water is “eager” to run downhill.)
. Alkali metals like lithium and sodium are powerful reducing agents too: they are “eager” to lose an electron. (Remember that this anthropomorphic language simply connotes that the process happens readily because it lowers the overall energy of the system. It's in the same sense that water is “eager” to run downhill.)
There can be no oxidation without reduction, and vice versa. It stands to reason: if an electron moves, it has to go from one place to another. Chemical reactions that involve reduction and oxidation are called redox reactions, and they are extremely common in chemistry. The corrosion of iron to form rust is one of them.

The surface of a hot copper plate in the presence of oxygen and acetone vapor: copper first reacts with oxygen to form copper oxide, then the oxide is reduced to copper again, with the cycle repeating until the acetone runs out; the colorful appearance of the oxide layer is caused by light interference

The surface of a hot copper plate in the presence of oxygen and acetone vapor




If chemistry is all about the movement of electrons, so too is electricity. In the crudest terms—a more accurate view is more complicated, as it is so often in science—the flow of an electrical current down a copper wire corresponds to the motion of electrons. Say we attach a wire between the terminals of a battery. Opposite charges attract: electrons are drawn toward the positive terminal. To balance that motion, electrons enter the wire from the negative terminal. This, then, is what a battery is: a source and sink of electrons, whose motion can be tapped to extract power and drive electrical devices.
But if that's so, might we not use electricity—batteries, say—to do chemistry? Yes we can, and this is precisely how electroplating and electrodeposition work. Conversely, batteries themselves harness the electron exchanges of chemical processes by converting the energy of chemical reactions into electrical energy.
The intimate connection between electricity and chemistry was discovered almost as soon as the battery was invented. That invention was sparked by an argument between two Italian scientists in the late eighteenth century. The physician Luigi Galvani had discovered that if a dismembered frog's leg is touched by two different metals, it twitches. Galvani's experiments led some scientists of his time to suspect that life itself was electrical—the result of electricity (at that time envisaged as a kind of fluid) coursing through the nerves of creatures. This idea later impressed the teenaged Mary Godwin, who later married the poet Percy Shelley, and she had it in mind when in 1816 she began to write her most famous novel, Frankenstein.
Galvani's compatriot Alessandro Volta suspected that the frog's leg was not actually producing electricity at all; he thought it came from the metal contacts. To show this was so, he connected two metals—zinc and copper—not via frog's legs but by a piece of cloth or cardboard soaked in salty water. Because it contains electrically charged ions, the salt solution will conduct electricity. Volta showed that this arrangement alone generates an electric current. We can think of it as if the electrons in the two metals have different energies and so will flow energetically “downhill” from one to the other. This makes the assembly a battery. Volta found that he could produce more electricity—a greater voltage, as we'd now say (and you can see where this technical word comes from)—by stacking many paired copper and zinc disks atop one another, each pair separated by a piece of salt-soaked cardboard. The top and bottom disks serve as the two terminals of the battery: the copper disk is the positive (+) terminal, the zinc disk is the negative (−) terminal. This is a voltaic pile, which Volta first reported in 1799.
After Volta described his device in a letter to the Royal Society in London in 1800, the English chemist William Nicholson and the surgeon Anthony Carlisle used it to pass a current through water via brass wires and found that gases began to bubble from the immersed end of the wire attached to the zinc terminal. They used silver rather than copper as the other disk, and saw that the brass wire at this end became blackened by tarnishing. When they used platinum wires in place of brass, gas bubbled from both wires. They deduced that the gas produced at the negative terminal is hydrogen, and that at the positive terminal is oxygen—which reacted with and blackened brass, but not with less-reactive platinum. These two elements, hydrogen and oxygen, are the components of water (H2O), and Nicholson and Carlisle figured that they had split water into its elements—a chemical reaction—using electricity. This process became known as electrolysis, “lysis” meaning splitting in Greek.

The electrolysis of sodium sulfate solution, with bromothymol blue added as a pH indicator
“A battery is a source and sink of electrons, whose motion can be tapped to extract power.”

The electrolysis of sodium sulfate solution using platinum electrodes; hydrogen gas is produced at the left-hand electrode, and oxygen gas at the right
“Chemists figured they might be able to split substances into their elemental components by electrolysis.”
Chemists figured they might be able to split other substances into their elemental components by electrolysis. Volta gave a pile to his friend, the English chemist Humphry Davy at the Royal Institution in London, who studied the electrolysis of the compounds “potash” (a general term for potassium salts like potassium carbonate and potassium hydroxide) and “soda” (sodium carbonate). He found that from molten potash he could collect globules of a soft silvery metal at the negative electrode, which quickly became covered with a white film. This is potassium metal. Soda yielded a similar substance, christened sodium. Both of these pure metals had a tendency to burst into flame if there was any moisture around: they are very reactive, and the reaction with water produces inflammable hydrogen gas.
These experiments, which Davy reported in 1808, were the first sightings of those two highly reactive metals. In that same year Davy went on to discover several other new elements by electrolysis: calcium, boron, barium, strontium, and magnesium.
Several of these elements are very common in natural minerals. But they had never been seen in pure form before because their atoms react so readily with others, losing their electrons in the process to form positively charged ions. If the voltage produced by the voltaic pile was large enough—in effect, if the stack of disks was tall enough—then electrons could be forced back onto the ions present in the molten compounds. (It's no good trying to do that in solutions of the respective salts, because those electrons will more readily impress themselves on hydrogen ions in the water to make hydrogen gas. In fact, Davy found that the same may happen if there are traces of moisture in the molten salts he used, whereupon the hydrogen could ignite as a bright flame at the electrode.) We can write the reaction for potassium (K) like this:
Here e− represents an electron coming from the battery via the metal electrode. We give it the minus symbol to make sure that the electrical charges balance: the negative charge of the electron cancels the positive charge of the potassium ion.
This looks a bit different from chemical equations we've seen earlier, though, because it doesn't seem balanced: where has the electron gone on the right-hand side? Well, it has gone onto the potassium ion itself, producing a neutral potassium atom, as in the pure metal.
That, then, is the process occurring at the negative electrode (called the cathode): the one that dispenses electrons. At the positive electrode (anode), electrons are in effect sucked into the metal wire. In this way they circulate through the entire circuit: out of the cathode, into the stuff in between (this electrically conducting substance between the electrodes is called the electrolyte), then out of the electrolyte and into the anode. So an electric current flows between the two terminals of the battery. If the electrolyte is molten potassium hydroxide, the hydroxide ions are converted at the anode into oxygen and water. It's a somewhat complicated reaction to balance, but looks like this:
These two electrochemical processes—one at the cathode, the other at the anode—are called the half-cell reactions, each taking place in one half of an electrochemical cell. The overall reaction resulting from the two half-cell processes is the conversion of potassium hydroxide to potassium metal, water, and oxygen gas using electricity. And by “using electricity,” we mean that you need a source of electrical power—like a battery—to drive this reaction forward. Making metals like sodium and potassium from their abundant minerals costs energy.
According to the definitions we introduced earlier, turning a potassium ion into a neutral atom in potassium metal is a process of reduction: the adding-on of electrons. The half-cell reaction at the other electrode is then a process of oxidation. It's not immediately obvious what is being oxidized here, but in fact it's the oxygen itself that has this distinction. Remember that a process of oxidation withdraws electrons, and that's the net result here—for every hydroxide ion arriving at the electrode, an electron is being drawn into the circuit.
The electrochemical processes that happen during electrolysis were explained in the early nineteenth century by Michael Faraday, the English scientist who began as Davy's humble assistant at the Royal Institution and rose to become his colleague and successor—not without, it must be said, some chagrin from his mentor. It was Faraday, at the suggestion of his friend William Whewell, who proposed the terms ion, electrode, anode, and cathode, giving electrochemistry its lexicon.
Electrolysis, as we see, can be used to turn metal ions into the pure elemental metal itself. For metals less reactive than sodium, potassium, magnesium, and their ilk, this process can work for electrodes dipped into a simple solution of a metal salt. Stick metal electrodes into copper sulfate, wire them up to a battery, and copper ions will be deposited on the negative electrode (cathode) as a thin film of brownish metal. If you make the other electrode (the anode) from copper metal itself, the half-cell reaction here turns copper atoms into ions, replenishing those lost from solution. In this way, copper is in effect eaten away from the anode and transferred to the cathode. This is the basis of the process called electroplating—in this case, copper-plating.
“Electrolysis can be used to turn metal ions into the pure elemental metal itself.”
You can do it with all kinds of metals, provided they are not so reactive that water immediately reacts with and corrodes them. Metal objects can be silver-plated by using them as the cathode in a solution of a silver salt such as silver nitrate. One method of galvanizing steel—which means giving it a protective coating of zinc to reduce corrosion—uses electroplating in a solution of a zinc salt.
The first use of electroplating, introduced only five years after Volta described his voltaic pile, was to coat items with gold. In this way, jewelry made from relatively cheap metals could be made to look like it was wrought from gold itself—and who would know the difference, so long as their evaluation was literally superficial? (Canny traders and metalsmiths had long known, however, that you could spot gold-covered fakes—they were formerly made by coating with fine gold leaf—by measuring their density, most metals being less dense than gold.)
The Italian inventor Luigi Brugnatelli described the electrochemical process of gold-plating in 1805. By midcentury, gold- and silver-plating was being done on an industrial scale. Even if you were of modest means, you could now serve your dinner guests with what looked like luxurious silverware.
Churches could enthrall worshipers with relatively inexpensive religious icons apparently fashioned from solid gold. By the end of the century, automobile manufacturers were already plating their fenders with the bright, reflective, and rustproof metal called chromium.
“Even if you were of modest means, you could now serve your dinner guests with what looked like luxurious silverware.”
Oddly enough, one application for which electroplating is not used is the silvering of mirrors. This has generally been done with a chemical rather than an electrochemical method. The “silvered” mirrors made from the Middle Ages until the late nineteenth century didn't contain silver at all; their reflective coating, laid onto glass, was an amalgam—a liquid metal mixture—of tin and mercury, which could be smeared onto the glass surface and the mercury allowed to evaporate. But in 1835 the German chemist Justus von Liebig discovered a reaction that converted a silver salt to silver metal, and which could be used to deposit a layer of the metal so thin that it wasn't prohibitively expensive. He improved the recipe in 1856, and in that same year his friend, the German inventor Carl August von Steinheil, began using the process to make mirrors for astronomical telescopes and other optical devices. Reflecting telescopes, in which carefully shaped mirrors gather and focus light onto a point, were best for making instruments with very wide apertures (thereby gathering more light), but the difficulty of making large mirrors of adequate quality had previously limited their scale.
The anode during the electrolysis of a sodium bromide solution; both oxygen bubbles and orange bromine are formed at this electrode

A transparent glass bottle covered by a layer of silver, made by the “silver mirror” reaction

Detailed view of imperfections in the silver layer made by the silver mirror reaction

A close-up view of silverware, showing both smooth and rough surfaces
Liebig's method uses a mixture of silver nitrate and ammonia, plus a small amount of sodium hydroxide. Two ammonia molecules attach themselves to each silver ion, forming so-called silver diammine ions. When this solution is mixed with a sugar such as glucose, the silver oxidizes the sugar and is itself reduced in the process: you might say that it plucks an electron from a sugar molecule, converting the metal ion to the elemental metal. If this reaction mixture is sprayed onto glass, a beautifully smooth and reflective film of silver appears: a redox reaction to delight the senses.
Things don't always go so smoothly—literally. Since the earliest days of electrodeposition, it has been known that metal films made this way can become rough or fluffy: some parts grow faster than others, so the film develops tiny fingers or branches. These irregularities might only be visible under the microscope, but even so they destroy the smooth, shiny finish and make it look dull and matte. Seen close up, the surface might seem less like the mirror-flat surface of a lake and more like a jungle canopy.
These branching processes aren't easy to understand or predict, but it's not so hard to see why such complications can arise. Only the positively charged metal ions are attracted to the cathode where the layer grows—negatively charged ions are repelled. But this means that the two types of ion are distributed unequally close to the surface, and a buildup of positive charge makes the metal ions repel one another. Will the surface attract them more than they repel each other? It all gets rather complicated and finely poised, and the process of film growth becomes prone to “instabilities” of the sort that make ice crystals sprout into snowflakes (chapter 4). Deposition can end up behaving almost like some organic growth process, the metal branches resembling tiny coral reefs.
This isn't just a problem if you're trying to make shiny metal coatings. It may also cause difficulties for batteries. In many traditional rechargeable batteries, the recharging process is a kind of electroplating that regrows metal terminals after they have partly dissolved during discharge. If the metal surfaces sprout branches, the electrodes don't work as well as they did before, and it's possible that the branches might even span from one terminal to the other, short-circuiting the battery.
Batteries, then, are also electrochemical devices. Instead of using electricity to drive chemistry, as in electrolysis, they do the reverse, creating electricity from chemistry. They exploit a chemical reaction that produces energy—like the burning of a fuel or a candle—but the transaction of electrons is harnessed to capture them onto one electrode and dispense them from the other, in two half-cell reactions.
Take the rechargeable lithium-air battery. This is a new type of device that some researchers think will replace the current breed of lithium-ion batteries. It gets its energy from the reaction of lithium metal with oxygen to make lithium oxides, and sometimes other lithium compounds; the exact reaction varies depending on the specific design. The key processes, however, are oxidation of lithium (releasing electrons) at the anode and reduction of oxygen (accepting electrons) at the cathode. When the battery is being discharged, these electrons flow through the circuit.
Because lithium is a very lightweight metal, and its reactions with oxygen are very energetic, lithium-air (or lithium-oxygen) batteries should be able to pack a lot of power into a small, compact device: more so than lithium-ion batteries, and sufficient to give fully electric vehicles a large range before the power fades. If we can make this battery technology work safely, reliably, and cheaply, it could reduce our dependence on polluting fossil fuels for transport.
To recharge a battery, you use an electrical power source to drive the reaction in reverse: in effect to push it “uphill,” just as you might use electrical power to pump water uphill into a dam reservoir. During recharging of a lithium-air battery, lithium ions are deposited back on the negative electrode as the pure metal. It's quite common for this to grow into spiky metal whiskers called dendrites (see chapter 4). On each discharge-recharge cycle, the lithium electrode might become less like a flat plate and more like a thicket of branches. Eventually it'll break up or short-circuit—which, in the worst case, could make the battery catch fire. A lot of work is now going into finding ways to suppress these growth instabilities—for example, by using solid electrolytes (in which ions can move about in a solid lattice), which simply block the branches physically, or by using additives in the electrolytes mixture. Branched, whiskery electrodeposits can look lovely up close, but in technology they can be a headache.
“If we can make lithium-air batteries work safely, reliably, and cheaply, it could reduce our dependence on polluting fossil fuels for transport.”
A silvery glint is one of the hallmarks of a metal, shared by elements from sodium and magnesium to iron and platinum, and even by liquid mercury. That, at least, is how a freshly exposed and smooth metal surface looks. Some metals don't stay looking that way for long, however, thanks to chemical reactions on the surface that cause discoloration as the metal atoms combine with and lose electrons to atoms and compounds in the air: oxygen, carbon dioxide, water vapor. For highly reactive metals like sodium this happens within minutes. For iron it may take days; silver tarnishes over months and years, platinum and gold hardly at all.
But why are they shiny in the first place? What is special about metals that gives them this distinctive sheen, in contrast to the appearance of, say, granite, paper, or skin? Partly it's a question of texture: metals may be mirror-smooth, so when light reflects from them it does so cleanly, whereas for a rougher surface the light will be scattered randomly from pits and bumps. Brushed metals, in acquiring a field of tiny scratches, lose their shine.
But the silveriness is also distinct from the opaque colors that even smooth, shiny surfaces of nonmetallic materials might have. It is a direct result of what truly defines a metal, which is a high electrical conductivity. Metals conduct electricity because they have many electrons that have drifted away from the constituent atoms and are free to move through the crystal lattice. Lone metal atoms are electrically neutral, but put many of them together and they will shed an electron or two into a common pool, a sort of sea of electrons that pervades the material. The metal ions they have escaped are like pebbles stacked in a filled bathtub, with the electron fluid filling the spaces in between like water.
“Inside a gold ring, particles are dancing at close to the speed of light.”
These mobile electrons are sloshy—and when light falls on the surface of a metal, the electrons at the surface are set sloshing. Recall that light is an undulating electromagnetic field, so the electrons will respond to its wavy influence. They too vibrate, setting up their own undulating electric field—and in a way that perfectly cancels out the light's field. This means that the light can barely penetrate the metal at all, and so it is reflected in a mirror-like manner. (If the frequency of the light is high enough, however, the electrons can't vibrate fast enough in response—which is why ultraviolet light may penetrate further.)
A metal that is not silvery but has a colored tint, like copper or gold, does slightly absorb some of the visible light, rather than reflecting it all. In those metals the absorption happens for light of shorter wavelength—in the violet and blue part of the spectrum—leaving a red or yellow bias in what gets reflected.
But here is a peculiar thing. For gold, this color is imparted by the exotic consequences of Einstein's theory of special relativity. According to this theory, objects that move at speeds approaching the speed of light experience an increase in their mass. It's not that they somehow acquire more material from out of empty space; it's just that each particle of the material gains mass.
In gold, the atoms have such a big nucleus, with such a large positive charge, that the electrons closest to it get accelerated by the electric field to very high speeds: big enough to alter their mass due to this “relativistic” effect. The increase in mass makes the electrons orbit the nucleus slightly more tightly, and there is a knock-on effect that alters the energies of electrons further from the nucleus—the ones that make up the mobile electrons in the metal. Those shifted energies leave the metal slightly less shielded from blue light, which can penetrate and be absorbed.

Gold crystals grown by depositing the metal from a gold-rich vapor
The yellow gleam that excited the lust of Cortés, then, was produced by one of the most bizarre phenomena in modern physics: an increase in mass induced by sheer speed. If you're wearing a gold ring, particles are dancing within that dense metal at close to the speed of light, and in the process, they give the metal its hue. It transpires that these relativistic effects also account for gold's low chemical reactivity and its resistance to corrosion—making it a potent symbol of permanence. Who would have guessed that this dangerous allure had such exotic origins?

Gold crystals grown by depositing the metal from a gold-rich vapor
Further reading
- Boynton, H., ed. The Beginnings of Modern Science. Roslyn, NY: Walter J. Black, 1948.
- Hammer, H., and J. Norskov. “Why gold is the noblest of all the metals.” Nature 376, 238–240 (1995).
- Hunt, L. B. “The early history of gold plating.” Gold Bulletin 6, 16–27 (1973).
- Kim, H., et al. “Metallic anodes for next generation secondary batteries.” Chemical Society Reviews 42, 9011–9034 (2013).
- Knight, D. Humphry Davy: Science and Power. Oxford: Blackwell, 1992.
Images are very important to my understanding of chemistry. I have used them throughout my career to show how the phase transition that takes place in a mass spectrometer from solution to gas phase can perturb protein structures. I also use images to convey chemical processes. Here I think you can let your creativity run riot to convey what you think might be happening.
Imagination is very important in science; art and imagery are powerful ways of making your ideas come to life.
Professor Dame Carol Robinson, FRS,