9
ORGANIC: THE CONTORTIONS OF CHEMICAL GARDENS
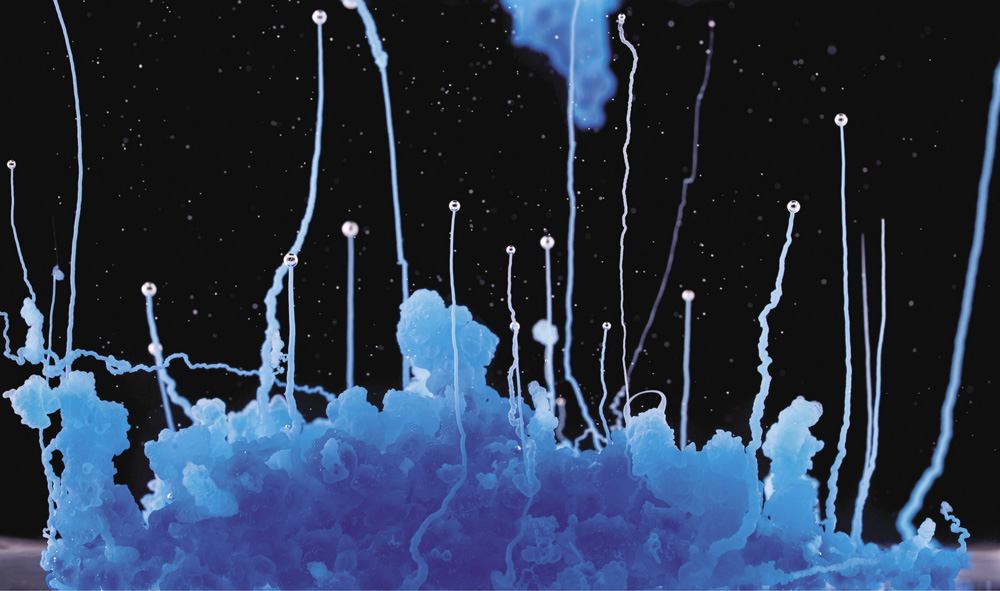
A chemical garden formed from copper nitrate in sodium silicate (water glass) solution

A chemical garden formed from copper nitrate in sodium silicate (water glass) solution
Chemistry is sometimes said to sit between physics and biology, uniting what is inanimate with what is alive: the crystal with the cell, rock with flesh. Both, in the end, are made from substances comprised of atoms joined by the rules of chemical bonding.
It's true. But that's to make chemistry neither quite one thing nor the other: lacking both the mathematical precision and symmetry of physics and the rich, vital profusion of biology.
Actually, chemistry has both of these qualities! But it is not merely a bridge and mediator between these other scientific disciplines. It has its own rules and its own aesthetics. They are informed by physics and they inform biology, but they have a life of their own.
If you want to see that this is so, consider the chemical garden.
That's what you're looking at in these images. The bulbous growths resemble something that has sprouted organically: yeast can look a bit like this under the microscope, and so can fungal spores. If you inspected a meteorite and saw structures like these, you might imagine that the rock has come through space from an inhabited world.
But these marvelous formations are a product of inorganic chemistry alone, with not a living organism in sight. What this tells us is that we have intuitions about what living things look like (vegetative, branching, irregular) and what gets made in nonliving processes (symmetrical, sharp-edged)—and these intuitions can be wrong!
Or are they? Crystal gardens aren't just an example of chemistry mimicking life. Some researchers think they, or something like them, might show us how life on our planet really began: how biology did indeed emerge from nothing more than simple chemicals, maybe as much as four billion years ago when the Earth was young.
“We have intuitions about what living things look like and what gets made in nonliving processes—and these intuitions can be wrong.”



When chemistry as a science was still in its infancy around four centuries ago, intuitions about structure and form were pretty much all that natural philosophers had to go on in trying to interpret the forces that shape our world. So when they discovered chemical gardens, they took a rather casual attitude to whether these systems should be regarded as animal, vegetable, or mineral.
The first detailed description of how to make a chemical garden appears in a book published in 1646 by the German-Dutch chemist Johann Glauber. Like many chemists of that time, he was a practical sort of fellow who made a living producing useful substances like drugs and medicines and studying commercially important chemical processes like winemaking. In his book, Glauber described how to make a metal such as iron “grow like a tree with a body, boughs, branches and twigs.” It required a liquid that Glauber called “liquor of flints” or liquor silicum, which today we recognize as a silicate solution—silicate being a key component of stones and rocks. Stones don't usually dissolve in water, but you can get a mineral like quartz (sand) to do so by boiling it up with a strong alkali. Glauber made his liquor silicum by melting sand and potash in a crucible and grinding up the product. It turns into a viscous liquid when exposed to moist air, later called “water glass”—for it is indeed rather like a liquid form of glass, the silicate ions linking into polymer-like chains.
If you add liquor silicum to a salt made by dissolving a metal like iron in a strong acid, the garden starts to grow. The process is, Glauber wrote, “very pleasant to behold, the growth being very swift, so as within an hour and a half, or two hours at the most, the whole glass is fill'd with little iron trees”—these forms having the familiar rusty red hue of iron compounds. If you used copper instead of iron, the trees were malachite green. Lead and tin made white formations, silver and cobalt produced blues, and gold—if you could afford it—made yellow. With several metal compounds mixed together in a single flask of water glass, you can produce a multicolored chemical garden: a rainbow wonderland of shoots, bulbs, and branches.
It's no wonder they enthralled so many scientists who came after Glauber. Isaac Newton, who was very interested in chemistry (which he thought of as alchemy), wrote a manuscript about the “vegetation of metals” that he believed he was witnessing in the chemical garden. He thought that this “vegetation”—growth that generated organic-looking, twisty branches—was basically the same in metals as it was in plants and animals, driven by a formative agency that is present in all of nature.
You might detect here an echo of the rather mystical ideas that Johannes Kepler invoked to explain the snowflake (chapter 4). But the idea that chemical gardens had something to do with life didn't go away. On the contrary, it got stronger. A German chemist called Moritz Traube, smitten by these beautiful structures in the mid-nineteenth century, figured that they had similarities to biological cells. (Only at the start of that century had biologists begun to suspect that all living things are made of cells.)
True, these branchlike formations are much bigger than cells and have a different shape—but the similarity consists in the fact that both structures are hollow and have an inside and an outside separated by a thin, soft membrane through which water and dissolved substances can permeate. This permeability is important for cells, because it is one way in which chemicals can get in or out. You can see that process in action as a dried fruit swells when soaked in water, the water seeping into its cells by osmosis and blowing them up like water-filled balloons.
“The idea that chemical gardens had something to do with life didn't go away. On the contrary, it got stronger.”


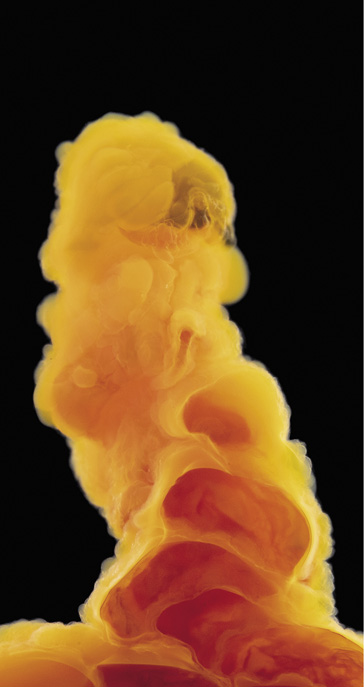




Cobalt(II) chloride; the line dividing the pink and blue regions is where the structure contacts the glass wall of the container
In chemical gardens, the “trees” are tubes of a silicate material, formed by the linking of silicate ions in the water glass solution and tinted in lovely ways by metal ions. It's a very different fabric from the lipid membranes of cells, but both are thin, flexible, permeable, and capable of growth. These silicate membranes blossom and sprout into irregular, branching forms that put us in mind of brightly colored flower beds, fungi, and coral reefs. In the early twentieth century the French biologist Stéphane Leduc was fascinated that ingredients as assuredly inanimate as dissolved rock and metal salts could mimic plants and animals in this way. Thomas Mann witnessed them and—again displaying his scientific sensibility—described them delightfully in one of his most famous novels, Doctor Faustus (1947):
I shall never forget the sight. The vessel of crystallization was three-quarters full of slightly muddy water—that is, dilute water-glass—and from the sandy bottom there strove upwards a grotesque little landscape of variously coloured growths: a confused vegetation of blue, green, and brown shoots which reminded one of algae, mushrooms, attached polyps, also moss, then mussels, fruit pods, little trees or twigs from trees, here and there of limbs. It was the most remarkable sight I ever saw, and remarkable not so much for its appearance, strange and amazing though that was, as on account of its profoundly melancholy nature. For when Father Leverkühn asked us what we thought of it and we timidly answered him that they might be plants: “No,” he replied, “they are not, they only act that way. But do not think the less of them. Precisely because they do, because they try to as hard as they can, they are worthy of all respect.”
They are indeed worthy of respect, but only since the late twentieth century have we begun to understand why—aside from their sheer splendor—that is so.
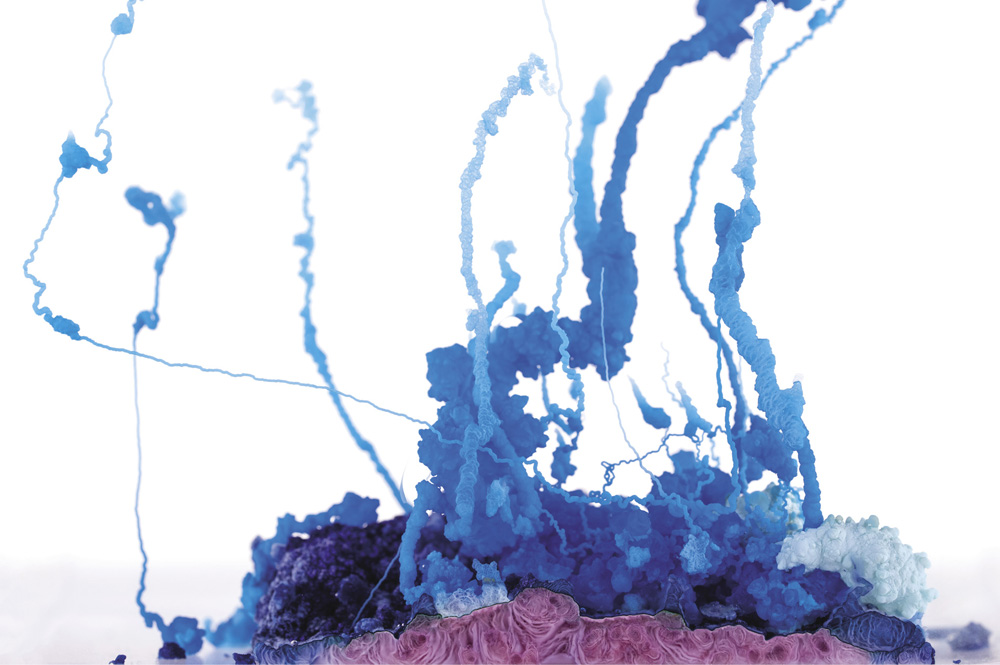
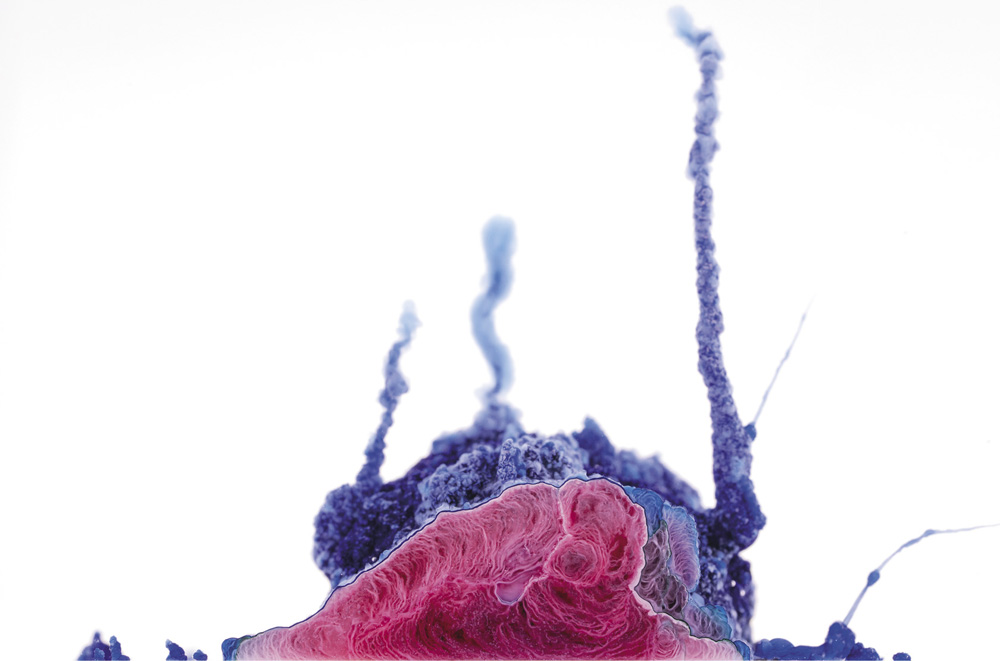
Chemical gardens from a mixture of copper nitrate and cobalt(II) chloride


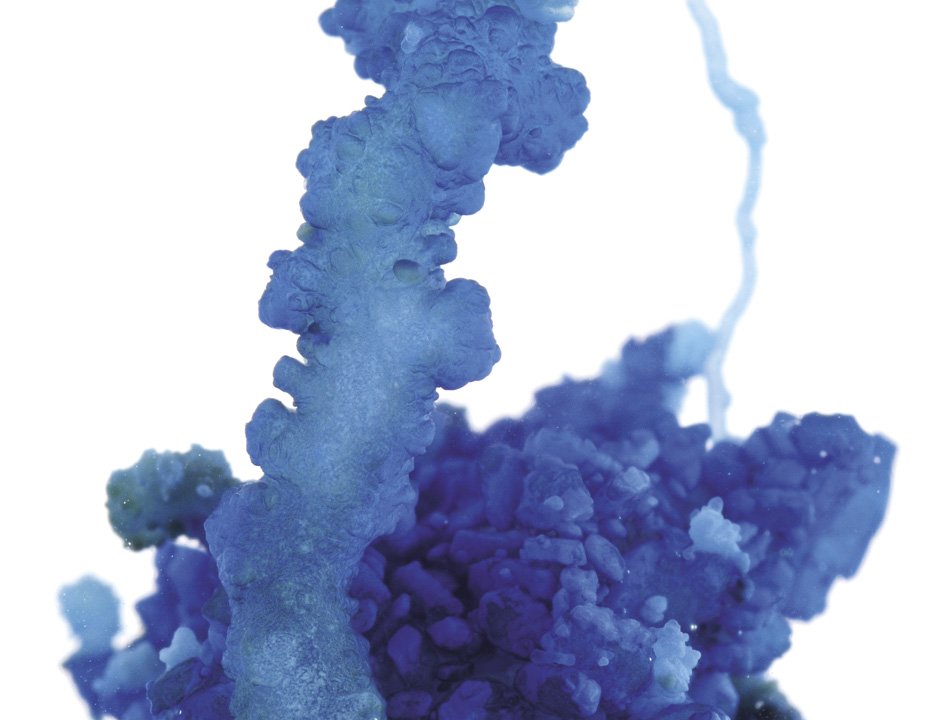
Let's take a closer look at how these gardens grow. The usual recipe today tells you to place a crystal of a metal salt—the “seed”—at the bottom of a flask of water glass. Slowly this seed gets surrounded and encapsulated by a bulging membrane, as dissolved metal ions from the seed combine with silicate ions in the water glass to make an insoluble silicate. It's really just a precipitation reaction of the kind we saw in chapter 3—but because silicate ions can link up into chains and sheets, they form a thin membrane.
Here now is the key. As the metal salt in the seed keeps dissolving, the concentration of metal ions in the water inside the membrane is greater than that in the solution outside it—and so water is drawn in through the membrane by osmosis.
That increases the pressure inside the com-partment, making it swell and bulge rather as plant cells become taut and rigid when their sugar-rich contents draw water inside them. This so-called turgor pressure is what keeps green plant stems rigid. So you see, there really is a genuine similarity between the structures of a chemical garden and the plants they resemble!
It's what happens next that creates the richness of shape. If the pressure of water inside the silicate compartment gets too high, the membrane can rupture. Then the metal-salt solution flows out through the hole into the silicate solution outside. Because the metal-salt solution is generally less dense than water glass, it rises up through the hole in a narrow jet. But as it encounters the silicate solution, almost at once a new membrane forms around it, producing a near-vertical column or wormlike tube. Other columns might sprout from this blob as it ruptures elsewhere, each of them rising up as if seeking the sky. And there's your garden.
“If the pressure gets too high, the membrane can rupture and the metal-salt solution flows out. Almost at once a new membrane forms around it.”
The growth process can produce various shapes, depending on details such as the concentration of the water glass, temperature, and so on. You might get smooth-sided tubes, or ones bumpy with nodules, or thick, lumpy, warty forms. Air bubbles can become attached to the growing tip of a tube, guiding it straight upward like a buoyant balloon. Chemical gardeners may get skilled at producing one shape or another, but there's always a bit of unpredictability. That's gardening for you.
These chemical sculptures challenge the notion that complex form demands complex, perhaps “living,” ingredients. This in turn highlights a theme that has become ever more important in chemistry over the past several decades: simple components—atoms, ions, and molecules—can in the right circumstances organize themselves into complicated structures, sometimes large enough to see with the naked eye. There's a sense here of one thing leading to another, in a hierarchical cascade of structuring processes. Silicate ions have a tendency to link together; granted that, you can form membranes; once you have a membrane, it can make closed compartments; these may then be blown up like balloons by osmotic flow; their rupture leads to chimneys and branches.

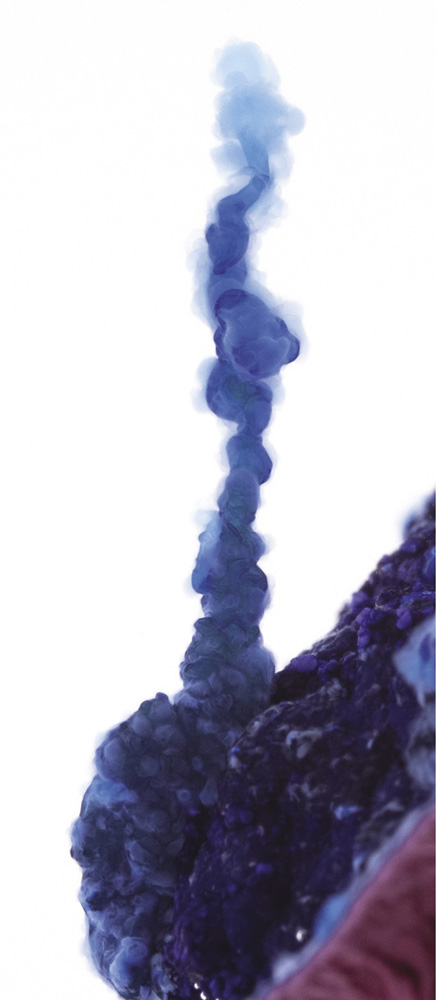
Cobalt(II) chloride; the dark lines are produced by the growth structures meeting the glass wall of the reaction vessel

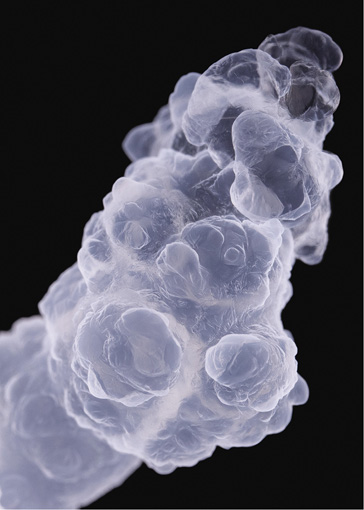

Now we're getting closer to life itself. For what is that but atoms and molecules organizing themselves on an expanding scale? Life on Earth has evolved a neat way of achieving this reliably and repeatedly: by encoding many of the instructions it needs into strands of DNA, to be copied and passed on between generations. But living organisms exploit a variety of other self-organizing chemical processes too, for example in the way lipid molecules can spontaneously gather into layers and hollow vessels (page 43).
But when life began on our planet, there was no DNA around to consult for instructions about how to make and organize the right molecules. There were just simple chemicals, like—well, like salts, minerals, and water.
In other words, like the ingredients of chemical gardens! Is it possible that these self-organized structures might have had something to do with that momentous event?
Structures like chemical gardens can occur in nature. One of the places where they do is at regions on the ocean floor where fluids heated by volcanic activity just beneath the surface dissolve a rich brew of minerals before gushing out under pressure from fissures in the rock. These places are called hydrothermal vents, and many researchers suspect they might be where life began.
“Is it possible that these self-organized structures might have had something to do with the origin of life?”

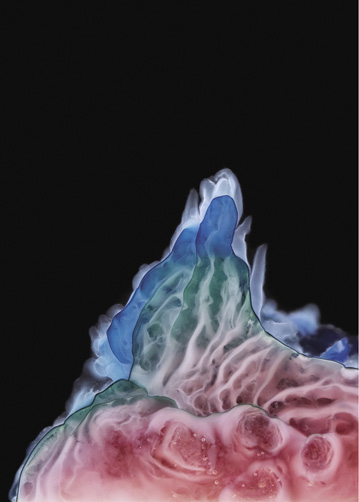
There are several reasons to think that. The early Earth was a fiery place, likely to have been bombarded with meteorites made from the cosmic debris left over after planet formation, which rained down with the force of atomic bombs. It was not a very welcoming environment for something as delicate as the beginning of life. But the seabed would have been sheltered from the worst of that maelstrom.
Life needs a source of energy, and today this comes almost entirely from sunlight, which plants and some bacteria soak up and store by photosynthesis. Animals can eat the plants and tap into those stores. But harnessing sunlight in this way is a complicated biochemical process. Hydrothermal vents offer an alternative energy source: heat, but also chemical energy that comes from the differences between the high concentrations of dissolved salts in the vent fluids and the low concentrations in the water all around. Such an imbalance in concentration is rather like a body of water high on a hillslope: you can tap it to do useful work, such as turning a water wheel to grind corn or make electricity.
Better still, you can store up the energy using sluice gates or dam walls, so that it can be released in a more controlled way. In the same way, an imbalance of chemical concentrations like that found at hydrothermal vents can be controlled and exploited by confining it behind walls and barriers: within membranes.
So chemical gardens of inorganic tubes and compartments at a hydrothermal vent, produced and fed by salt-rich fluids coming from within the crust, could have acted like a chemical battery to power proto-living systems.
This arrangement is after all not so different from how living cells get their energy. Photosynthesis itself ultimately drives the buildup of hydrogen ions on one side of a cell membrane: an imbalance in pH. Rather incredibly, cells do then in fact use something like a water wheel—made of protein and measuring just ten millionths of a millimeter across—to extract energy from this concentration gradient. The protein “wheel” sits in the cell membrane and lets hydrogen ions pass through from the high-concentration to the low-concentration side. In the process, the protein's rotor turns and the motion allows it to make the energy-rich molecules that drive the cell's biochemical reactions.
A chemical garden at a hydrothermal vent, made from a silicate membrane, has nothing so elaborate at its disposal. But it has a ready-made ion concentration gradient from the hydrothermal fluids flowing into the tubes; there's no need to set one up using the energy of sunlight. (That is just as well, for it is completely dark in the depths of the sea.) Researchers have figured out how pH (hydrogen-ion) gradients at vents with alkaline fluids might have powered chemical reactions that resemble some of those involved in the metabolism of real cells. The tubes and compartments growing on the vents might have eventually budded off into little self-contained, membrane-encapsulated reactors: primitive proto-cells. It's still pure chemistry at that point—but on the road to becoming biology.
These are still speculative ideas, but they make sense. And they might even be testable in the chemical laboratory. At any rate, we know that hydrothermal vents can support life even without sunlight, because those on Earth today, surveyed with deep-sea submersibles, sometimes have rich communities of microorganisms, worms, shrimp, and fish.
The question of how chemistry gave rise to biology was precisely what drew researchers like Traube and Leduc to study chemical gardens. But they couldn't get very far toward an answer because rather little was known in their time about what the chemistry of life is. Today we know so much more, and although there are still many questions unsolved, we know that some of the most vital factors involved can—in principle, at least—be supplied by chemical gardens.
So when we look at these remarkable and often beautiful structures, we shouldn't be embarrassed to let our imagination run riot: to see trees, fungi, corals, forests. It may be that our intuition is a good guide after all, whispering to us that here lies a promise, a little foretaste, of life itself. After all, imagination and dreams are what drives science forward, and where its ideas are born.
“The question of how chemistry gave rise to biology was precisely what drew early researchers to study chemical gardens.”



Further reading
- Barge, L. M., et al. “From chemical gardens to chembrionics.” Chemical Reviews 115, 8652–8703 (2015).
- Cartwright, J. H. E., J. M. Garcia-Ruiz, M. L. Novella, and F. Otálora. “Formation of chemical gardens.” Journal of Colloid and Interface Science 256, 351–359 (2002).
- Lane, N. The Vital Question: Why Is Life the Way It Is? London: Profile, 2015.
- Lane, N., and W. Martin. “The origin of membrane bioenergetics.” Cell 151, 1406–1416 (2012).
- Leduc, S. La Biologie synthétique. Paris: A. Poinat, 1912.
- Martin, W., and M. J. Russell. “On the origin of biochemistry at an alkaline hydrothermal vent.” Philosophical Transactions of the Royal Society B 367, 1887–1925 (2007).
- Steinbock, O., J. Cartwright, and L. Barge. “The fertile physics of chemical gardens.” Physics Today 69, 44 (2016).
Chemists have only recently discovered mechanical bonds, but artists have been drawing, painting, and carving them for thousands of years. Take the Borromean rings, originally used to represent the three wings of the Borromeo family, who were bankers in northern Italy during the Renaissance. It was the mechanical and physical beauty of this structure—three rings mechanically interlocked such that the breaking of any one ring results in the whole caboodle falling apart—that inspired us to try to make the first-ever molecular Borromean rings. After a decade of failed attempts, we finally succeeded in 2004 using an approach called dynamic covalent chemistry. The dynamic nature of the synthesis meant that, with a very small change in reaction conditions, we could also make a Solomon knot, in which two of the three rings present form a doubly interlocked catenane. This particular topology contains pairs of mechanically interlocked molecules that are related to each other as an object is to its mirror image. Like a hand and its image, they can't be superimposed, and so we produced a 50:50 mixture of the two forms.
The mechanical bond is an integral part of our everyday lives. A rattle, reminiscent of a rotaxane, is one of the more common examples and undoubtedly played a part in my own design and synthesis in 1991, for the first time, of a “molecular shuttle” based on a rotaxane. By giving the hoop two docking places on the axle, the molecular shuttle became a molecular switch, and ultimately led us toward molecular machines based on the mechanical bond.
The journey from the playpen to being able to play life's games, from understanding nature to an appreciation of art, from connecting the microscopic world to mechanical and electrical engineering, has been a sheer joy for me. Enjoyment is itself a beautiful experience, particularly when you realize that the physics that dictates the workings of micro- and nanomachines is not the physics that defines and controls the macroscopic world we know so well. Beauty is to be found in surprises that force us to think differently about the biological and molecular worlds.
Nobel Laureate in Chemistry, 2016