APPENDIX: MOLECULES AND STRUCTURES

Throughout this book we have sought to convey the wonder and delightfulness of the forms, patterns, and structures that emerge in chemical processes, and which we can see with our own eyes—perhaps with a bit of help from the magnifying glass, the microscope, the thermal camera.
But underneath all this richness, as we have intimated, lie atoms and molecules, the fundamental building blocks of chemistry. “Building blocks” is a clichéd term, but we're using it nonetheless because it is so inescapably apt. Chemistry is all about building with these ingredients. Nature, as Nobel laureate Frances Arnold so eloquently describes (p. 37), builds them into molecules such as proteins and DNA, and from these constructs cells and organisms. We humans are far less adept at molecular construction, but we are getting better all the time. In his novel The Wrench, Primo Levi drew an explicit analogy between the profession of the construction engineer who makes bridges and that of the synthetic chemist who makes molecules. In Levi's story, the narrator tries to describe to the engineer Faussone why their jobs have so much in common:
My profession . . . the profession I studied in school and that has kept me alive so far is the profession of the chemist . . . it's a bit like yours, only we rig and dismantle very tiny constructions . . . the things we handle are too small to be seen, even with the most powerful microscopes: so we've invented various intelligent gadgets to recognize them without seeing them. . . . But we are still blind, even in the best circumstances, that is, with structures that are simple and stable. Blind, and we don't have those tweezers we often dream of at night, the way a thirsty man dreams of springs, that would allow us to pick up a segment, hold it firm and straight, and paste it in the right direction on the segment that has already been assembled. If we had those tweezers (and it's possible that, one day, we will), we would have managed to create some lovely things that so far only the Almighty has made, for example, to assemble—perhaps not a frog or a dragonfly—but at least a microbe or the spore of a mold.
We believe, therefore, that it would be a shame not to give you a glimpse of what these atoms and molecules, on which chemists exercise and extend their constructional skills, look like.
But there is a problem. We can't photograph these objects. It's true that things have come a long way since Levi wrote The Wrench, and today we are creating not only tools a bit like those tweezers his narrator dreamed of but also cameras to show us the atomic world. There are, for example, instruments called scanning probe microscopes that can show us single molecules, revealed as blurry blobs or as frameworks of struts (representing chemical bonds) that look astonishingly like the schematic diagrams of molecules that chemists have drawn for over a century.
Yet is this what molecules look like? Not really. They are images made by computers based on measurements made by sophisticated instruments. Molecules are not ever something we can see in the same way that we can see a flower, or even the cells of a leaf under the microscope. Remember our description of light as an electromagnetic wave? Well, the problem here is that the size of a single wave is much, much bigger than the size of a typical molecule, let alone the individual atoms it contains. This means that the visible light to which our eyes respond can never disclose a molecule, any more than we can paint a miniature portrait the size of a thumbnail with a wallpaper brush. There is no “way a molecule looks” in the normal sense of the term.
What we can nevertheless do is work out how atoms are arranged in molecules. We saw in chapter 2 that X-ray crystallography is one of the oldest and best ways of doing that. Even today it is the standard tool for figuring out the shapes of molecules and crystals, the disposition of their atoms. Once we know how the atoms are positioned, we can use computer graphics to represent that structure. Typically this will involve showing atoms as balls—hard, glistening spheres perhaps, of a particular color that denotes the chemical identity of the atom concerned—as if glued together, or perhaps united by sticklike struts representing the chemical bonds.
These are totally fictitious modes of repre-sentation. Atoms are not exactly colored (although they may give rise to colors, as iron atoms do in the hemoglobin protein molecules of our red blood), and they are certainly not shiny—that notion only makes sense at human scales. They are not hard, and they don't have well-defined surfaces and edges. Still, representations like these offer some sense of the shapes of molecules—and we may find that these shapes are sometimes aesthetically pleasing, even beautiful.
In this appendix we show some of them. For the first and only time in this book, what we are therefore showing is not a “real” picture of the world but more of a cartoon, created artificially by graphics packages, which offers an artist's impression of how we might think about it. We don't apologize for doing that, however, because molecules and atomic-scale structures are a part of the beauty of chemistry too.
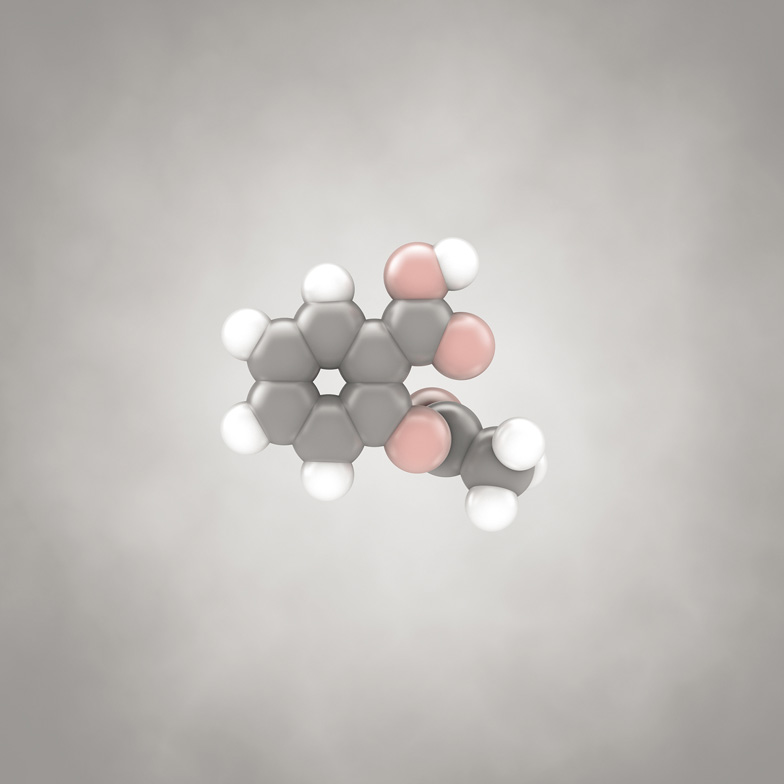
This is the molecule in aspirin that allows it to act as a painkiller. Here (and subsequently, on the whole) the gray spheres denote carbon atoms, the white spheres are hydrogen, and the red spheres are oxygen. The chemical formula is C9H8O4, as you can deduce by counting up the spheres.
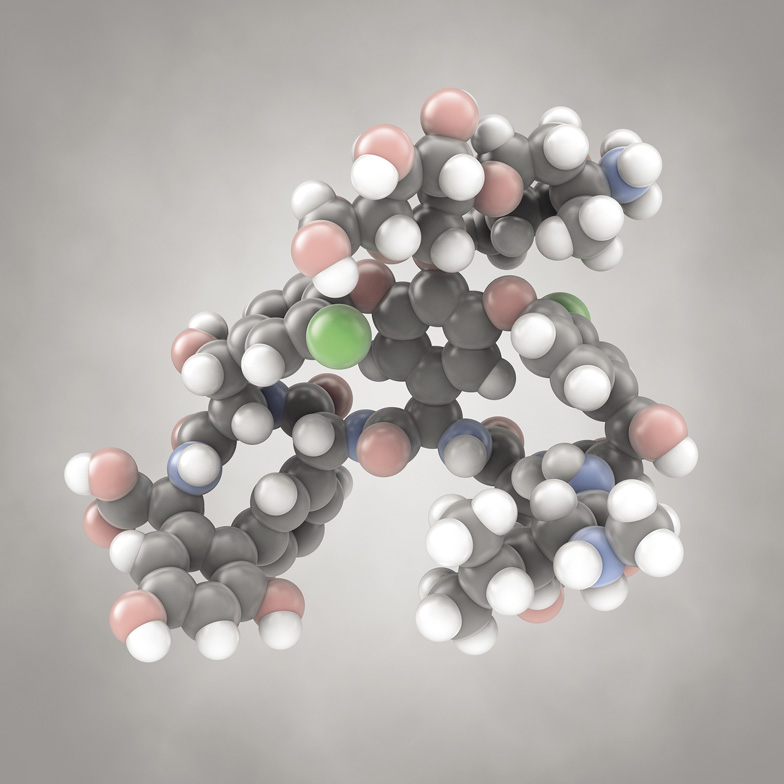
This is vancomycin, a molecule used as a common antibiotic. It is produced by a kind of soil bacterium, and it shows how complicated such “natural product” molecules can be. The light blue atoms are nitrogen, the green ones are chlorine.

This is a molecular structure called a catenane, in which two ring-shaped molecules are interlinked. The colors here don't denote particular types of atom, but instead are used to highlight the two distinct rings. The two rings can't be separated without breaking chemical bonds—which is why chemists aren't completely agreed about whether catenanes should be considered single molecules (linked by “mechanical” as well as chemical bonds) or two linked molecules. These catenanes are artificial molecules made by synthetic chemistry; it was the synthesis and study of such molecules that won Fraser Stoddart the Nobel prize.
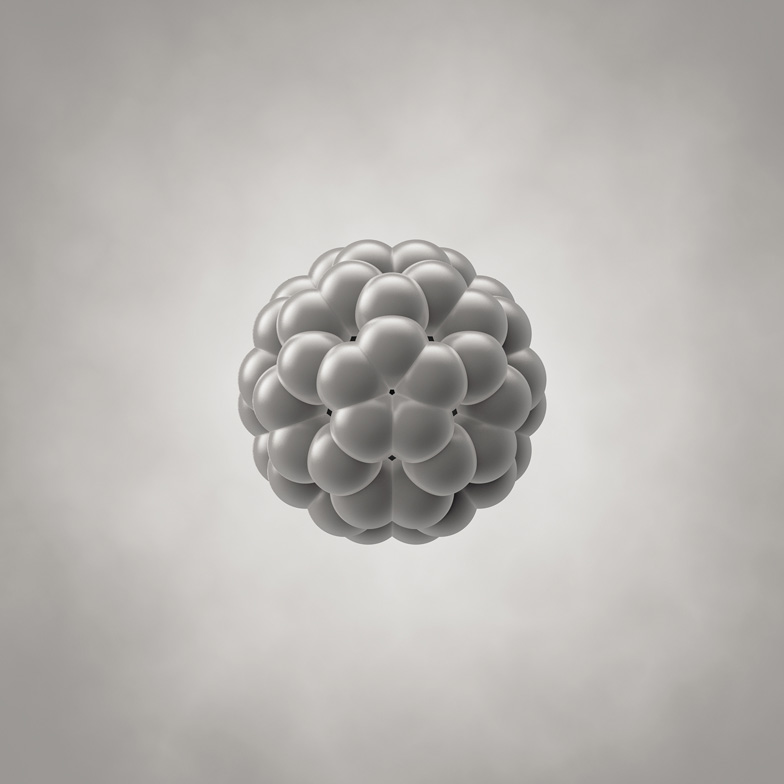
A molecule of buckminsterfullerene (C60), in which precisely 60 carbon atoms are linked into hexagonal and pentagonal rings that make a hollow, more or less spherical shell with the same pattern of hexagons and pentagons as on a soccer ball. The molecule is a little under one millionth of a millimeter in diameter.
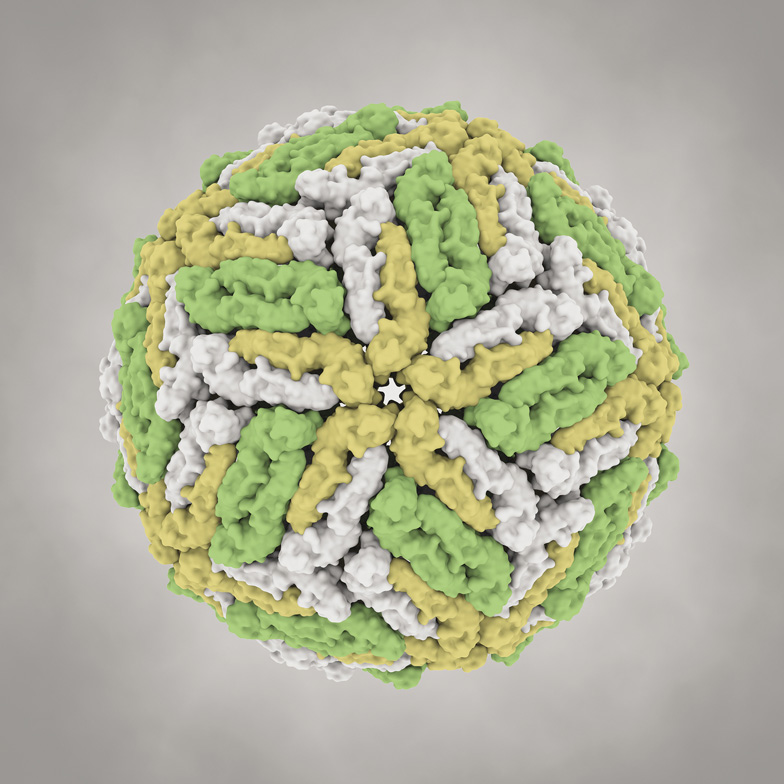
This is a representation of a single Dengue virus—the virus responsible for Dengue fever. Each of the colored blobs is a single protein molecule, and they are packed together in the virus's “coat” in a highly symmetrical way, a structure that is typical of viruses. Inside the coat (and out of sight here) sits the genetic material of the virus, encoded in the nucleic acid RNA.
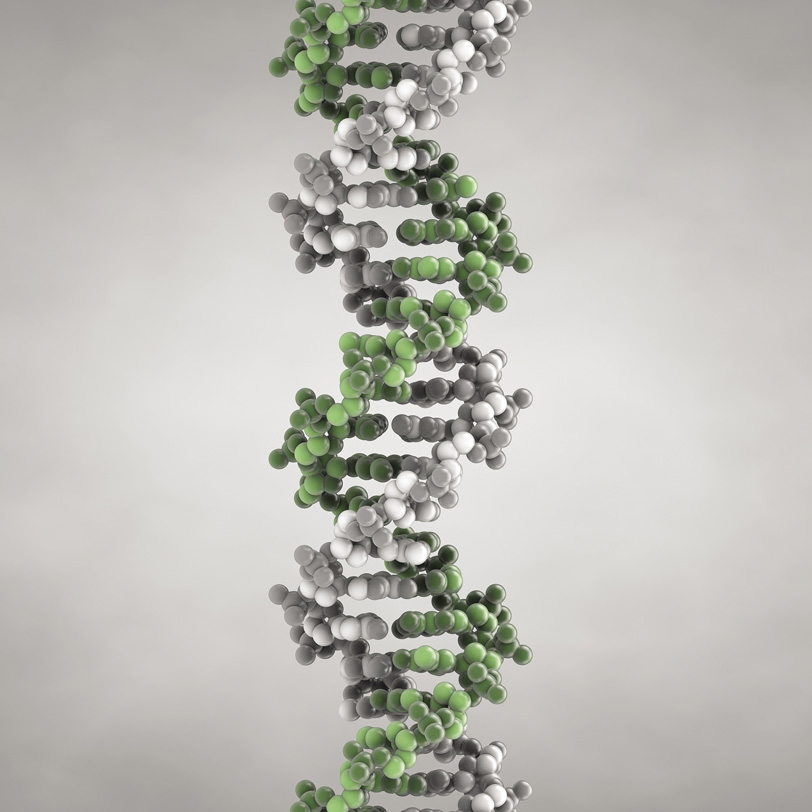
Here is the DNA molecule, which carries the genes that are passed on between generations for all living organisms from bacteria to humans. It contains two strands, each a single chainlike molecule (shown here in green and gray), which twist around one another in the famous double helix. In our cells, the entirety of our genetic material (the genome) is divided into 46 separate pieces of DNA, which are packaged up with protein molecules in the chromosomes.
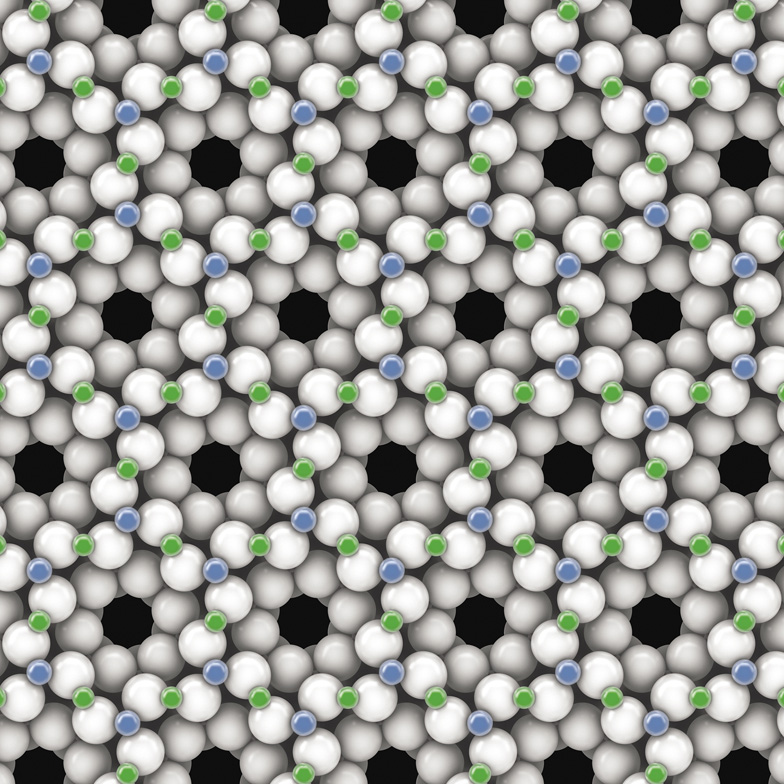
This is one slice through the atomic lattice of an emerald crystal. Here the small blue atoms are aluminum, and the small green ones are beryllium.
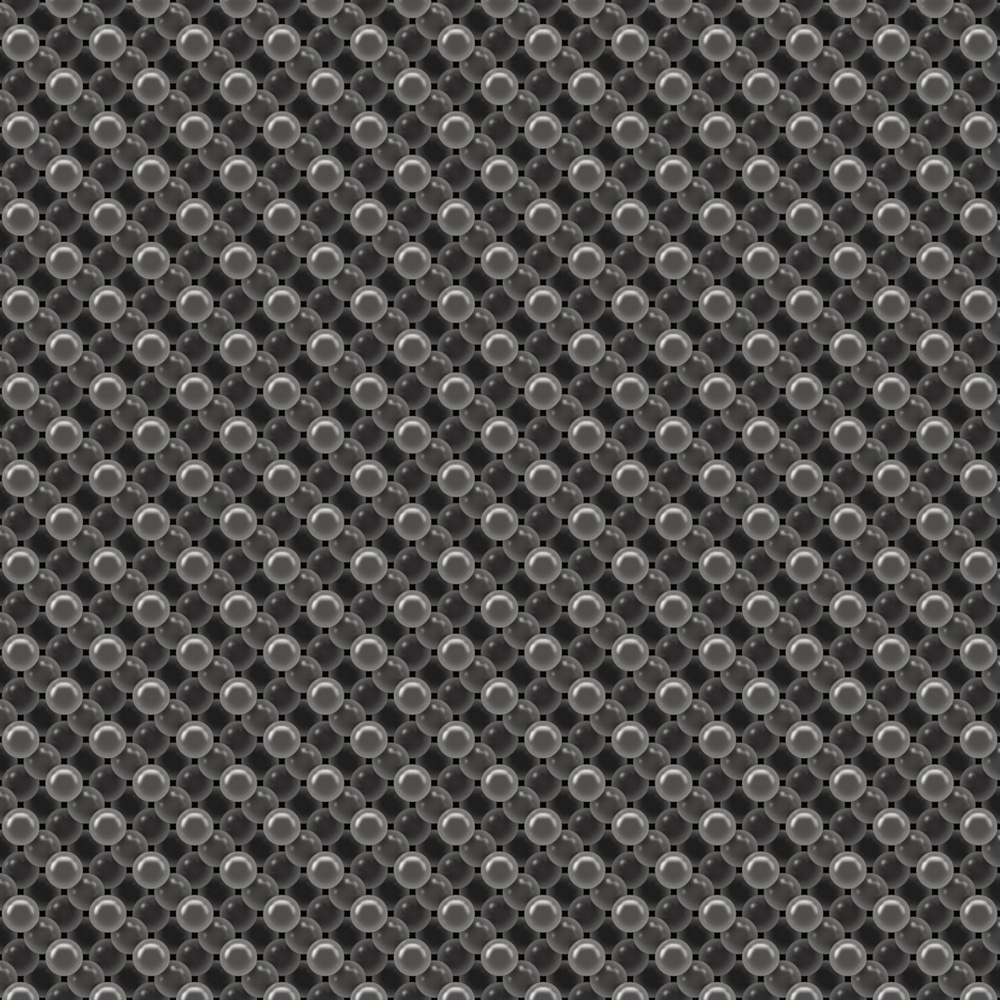
The diamond crystal is elegant simplicity itself. Every atom here is carbon.
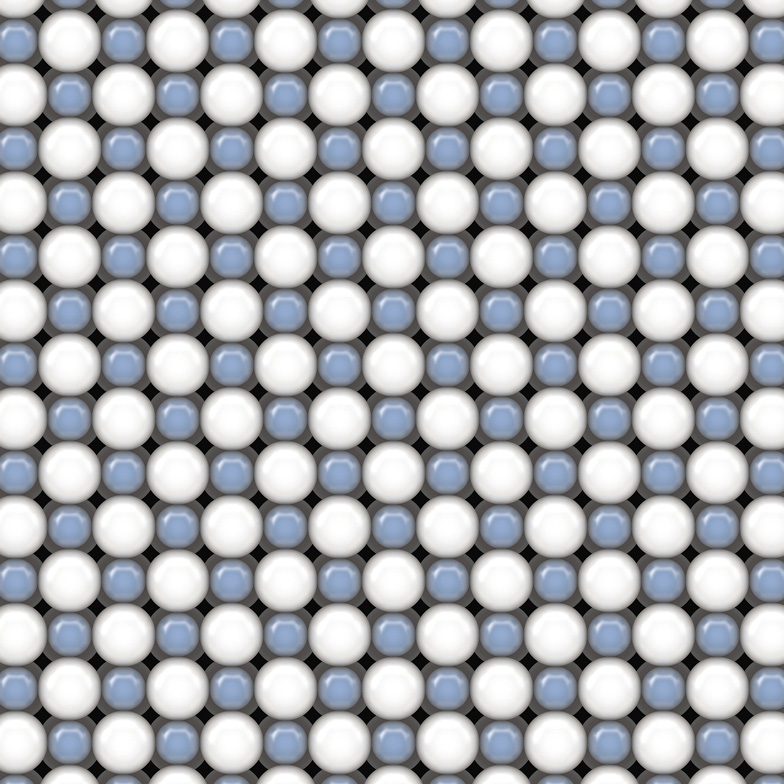
The crystal structure of sodium chloride (table salt). The blue balls are sodium ions, the white are chloride ions. The square symmetry of this structure is reflected in the square facets of the faces of a salt crystal.
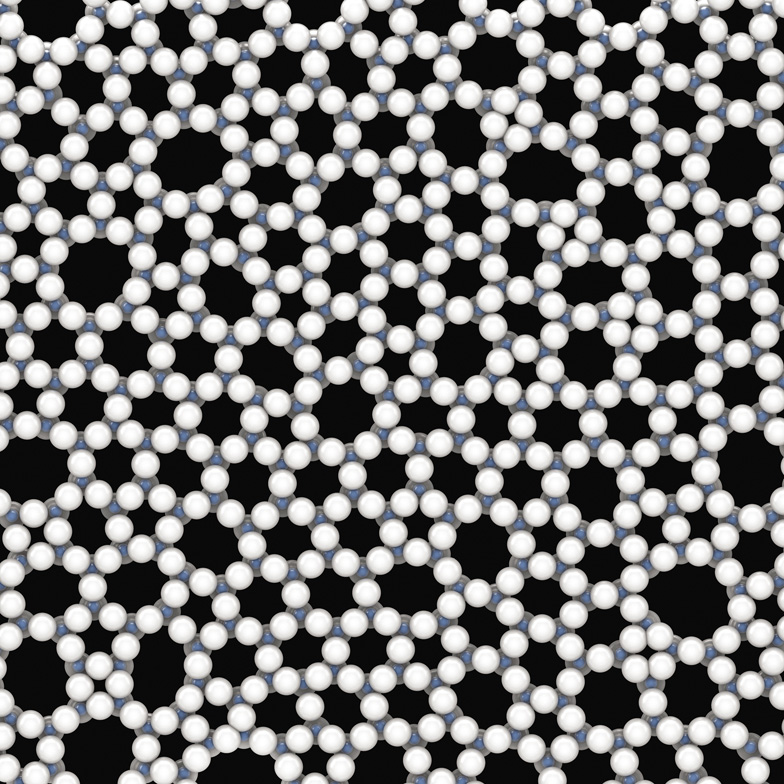
The structure of a thin slice of ordinary glass (silica). White atoms are oxygen, blue are silicon. Here the atoms aren't stacked into an orderly lattice—the structure is disordered. All the same, you might notice that certain motifs, such as rings of five or six atoms, recur throughout.