7 Cell Transport
Gradients are life for a cell. Our must know is based on this idea: a cell without a chemical gradient (meaning it is at equilibrium) is dead. A chemical gradient is a form of potential energy, and without energy … well, that’s no good. Think back to the previous chapter. A cell was able to generate a whole bunch of ATP by using the process of chemiosmosis, or the diffusion of hydrogen ions down their concentration gradient. That concentration gradient of H+ was not easy to maintain! It required a constant flow of electrons to power those little hydrogen ion pumps. Without that gradient, ATP synthase wouldn’t work. These gradients are everything to a cell. What we will discuss now is how, exactly, a cell maintains those gradients (active transport), and what processes are working to undo gradients (diffusion). When we learn about the diffusion of water, keep our second must know in mind: water will diffuse from a region of high water potential to low water potential.
Passive versus Active Transport
If you have a passive personality, it means you just go with the flow and don’t try and force things to happen. Passive transport is similar—it’s letting something just flow without the expenditure of energy. Passive transport is the movement of a substance from a region of high concentration to a region of low concentration. For example:
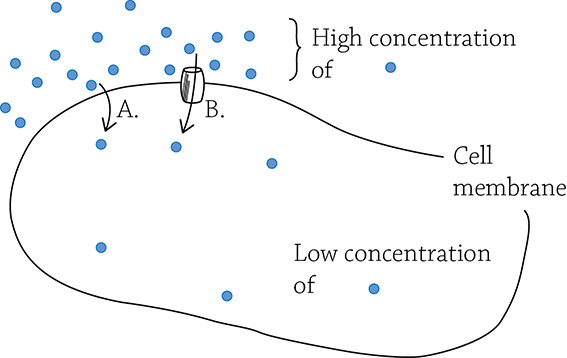
Diffusion. (A) Simple diffusion; (B) facilitated diffusion.
This happens spontaneously without an input of energy. For example, if you stand at one end of the room and spray perfume, your friend at the other end of the room would eventually notice. The scent particles were initially concentrated around you, yet after a relatively short amount of time, the particles diffused to areas of low concentration (the other end of the room). Diffusion (part A in the figure above) is the passive movement of molecules through a gas (like the air) or a fluid. In biology, diffusion is usually from the perspective of a solute moving through water across a semipermeable cell membrane.
Osmosis is the term to use if you’re talking specifically about the diffusion of water. Finally, if a molecule wants to diffuse but is otherwise blocked by the cell membrane, it may need a bit of help with a protein tunnel of some sort. The term facilitated diffusion (part B in the figure above) refers to diffusion of molecules though a transport protein (the tunnel). All three of these processes work to wipe out concentration gradients, and do so without the slightest input of energy.
The term solute refers to something dissolved in a liquid (salt, for example). The solvent is the liquid part, usually water.
Active transport, simply speaking, is the opposite of diffusion: active transport moves things against their concentration gradient (from low concentration to high), and it requires energy, along with a transmembrane protein pump:
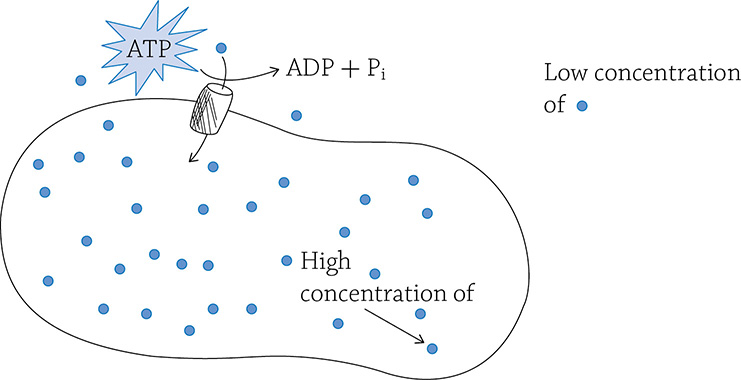
Active transport, involving a transmembrane pump and the expenditure of energy
Active transport is critical to a cell because, as we mentioned before, a cell at equilibrium is unable to generate energy; active transport battles against equilibrium! In a mitochondrion, there needs to be a high concentration of hydrogen ions in the inner membrane space in order to create ATP (chemiosmosis). Active transport created that concentration gradient in the first place. Recall that the energy lost as the electron dropped down the electron transport chain powered the proton pumps embedded in the inner membrane. If not for this concentration of H+, the cell would die.
A common mistake is assuming if there is a transmembrane protein involved, it must be active transport. This is not the case! Facilitated diffusion also requires a protein. The difference is it is only acting as a tunnel—no energy needed—as opposed to the energy-powered protein pump of active transport.

IRL
Once upon a time (early 1900s), there was a new “miracle” diet drug that promised to make people lose weight fast. And sure enough it did … it also caused their body temperatures to spike as high as 110°F, followed quickly by coma and death.
This drug–2,4-dinitrophenol–worked by making the mitochondrial inner membrane leaky, allowing hydrogen ions to sneak back into the matrix without passing through the enzyme ATP synthase. This destroyed the mitochondrion’s ability to create ATP via chemiosmosis. In turn, the body would desperately try harder to create the hydrogen gradient by sending even more electrons down the electron transport chain, as quickly as possible; the cells would burn through all the available glucose in a futile effort to ramp up ATP production. Because the mitochondria are working so hard, body temperature goes through the roof. Furthermore, because for every electron that hits the end of the electron transport chain, there needs to be an O2 to take it away (as water). If the rate of electrons flying down that chain increases, so does the need for O2. Many deaths occurred due to excessive fever (111°F!) and unsustainable respiration rates.
Soon thereafter, this “miracle drug” was designated as dangerous and not safe for human consumption (duh). But here’s the punch line: it’s still being marketed today, even though the consequences of consumption have been studied and widely documented. The moral of these studies? If you hear of some “miracle drug” that is purported to do some magical deed, chances are it’s at best an ineffective waste of money, at worst a dangerous and potentially deadly concoction.
Another type of active transport is on an even greater scale: endocytosis (also called phagocytosis) and exocytosis. In endocytosis, the cell uses energy to rearrange its internal skeleton so it can reach out pseudopods (“fake arms”) in order to engulf a particle and package it within a food vacuole. The thing being engulfed could be a speck of food, an invading bacterium, or even an old and worn-out organelle that needs to be recycled. After the item stuck in the food vacuole fuses with a lysosome (another type of vacuole that contains hydrolytic enzymes), the item is digested into smaller bits. Exocytosis then moves the vacuole over to the cell membrane to expel any waste.
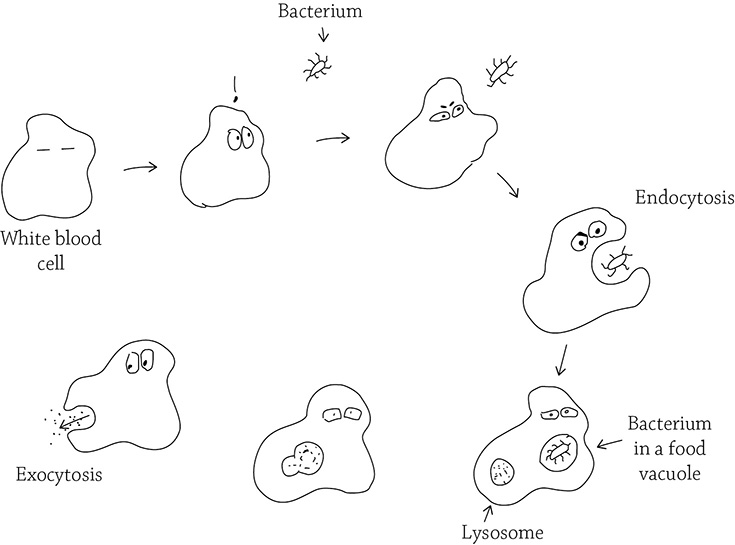
Endocytosis and exocytosis
The white blood cells of your immune system rely on endocytosis in order to phagocytose (“eat”) invading bacteria and other foreign bodies.
Osmosis and a Cell’s Surroundings
The diffusion of water can mean life or death for a cell. Imagine if you were a single-celled critter, like a paramecium, living in a freshwater pond. Your life is composed of happy days filled with feasting on bacteria and scooting about your habitat in search of pockets of the perfect temperature water (in other words, trying to maintain homeostatic conditions). You maneuver into a corner of the pond in which the water has an unusually high salt concentration due to road salt seeping into your habitat. All of a sudden, the water begins to leave your cell body, leached away by the saltier surroundings of the pond water. You respond instinctively (not like there’s a lot of higher reasoning happening in a protists) and zip away from this salty corner and return to your previous perfect water conditions.
What happened here? First of all, a cell needs to maintain a constant amount of water inside itself. Losing too much water can lead to the cell shriveling up, and too much water can cause the cell to expand and explode. The ideal condition for a cell is to live in an aquatic environment where there is an equal rate of water loss and water uptake; this is called an isotonic environment. The paramecium strayed into water with a higher solute concentration than what is in the cell—this is called hypertonic conditions and it leads to water loss from the cell. If instead a cell is surrounded by pure water without a speck of solute (or at least less solute than what is in the cell), this is called a hypotonic solution and might lead to an influx of water into the cell. Below is a table to help you understand what these different solutions mean to a cell living within them:
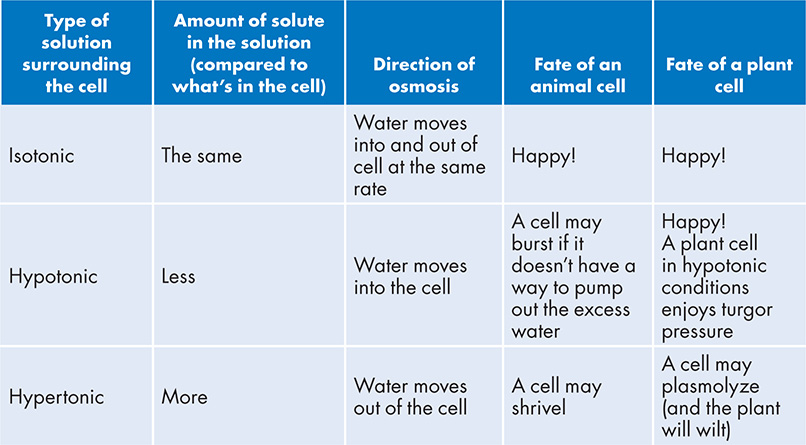
When considering the movement of water across a cell membrane, you need to keep in mind that the selectively permeable cell membrane is preventing the solute from passing through. Therefore, only the diffusion of water (osmosis) can occur across the cell membrane. The diffusion of anything goes from high concentration to low; water will also diffuse from a region of higher concentration water to a lower concentration of water. When a cell is floating in a hypotonic solution, there is a lower concentration of solute surrounding the cell than what is inside the cell. That means there is a higher concentration of water surrounding the cell, and it will diffuse down its concentration gradient (into the cell). The opposite is true for a cell floating in a hypertonic solution. There is a higher concentration of solute surrounding the cell, and less water. The inside of the cell has more water, and so it will diffuse out.
In order to simplify things a bit, we can use our must know term water potential to help us describe the direction of osmosis. It is an actual numerical value that takes two things into consideration: the amount of solute added to the water (Ψs, or solute potential) and the amount of pressure exerted on the system (Ψp, or pressure potential). I know this sounds weird and confusing, but let me try and explain.
1. Water potential (symbolized by this → Ψ) is an actual number (measured in units of pressure called megapascals, or MPa) that can be calculated, and it can help us decide the direction water will flow:
Ψ = Ψs + Ψp
2. Water will move from a region of high water potential to low water potential.
As you can see, the overall value of a solution’s water potential takes two things into consideration: the solute potential (Ψs) and the pressure potential (Ψp). Start with this fact: pure water in an open container has an overall Ψ of zero. “Pure water” means there is nothing dissolved in it (Ψs = 0) and if it’s in an open container, there is no change from normal atmospheric pressure (Ψp = 0). Now let’s consider what happens when you alter either the amount of solute or the pressure.
Pressure Potential Ψp
The pressure potential is the amount of pressure exerted on a solution. Look back at what is required to have an overall zero water potential—there cannot be any pressure other than just atmospheric pressure (saying it’s in an “open container” means just that, only the atmosphere is pushing down on the solution). Once pressure is either added or removed from the solution, the pressure potential can become either negative or positive. If a cell has a positive pressure, that means the cell is very full of water and it’s pressing against the cell membrane, like an overinflated balloon. If, instead, there is a vacuum applied and water is being pulled from the cell, the cell is under a negative pressure.
Solute Potential Ψs
Water potential defines the amount of water in a solution: a high water potential means that there is a lot of water in the mix (and not much stuff dissolved in it). Pure water has the highest water potential possible … because it’s pure water. If you start shoveling solute into the water, you are lowering the water potential because, per volume, some of the water is being displaced by the stuff dissolved in it. Now, if *pure water* has a solute potential of zero, guess what it becomes if you add stuff to it? Negative! You can never have a solute potential larger than zero (can’t make pure water any more watery). As solute concentration increases, the Ψs becomes more negative. In order to calculate the numerical value for the solute potential, you need to use this equation:

It makes sense that you need to know the molar concentration of the solute (C); the more concentrated the solute, the lower the solute potential. The “R” is a pressure constant that never changes, and the temperature must be in kelvin (add 273 to the Celsius temperature). That “i” variable, however, may not be as obvious. It’s the ionization constant, and it takes into consideration how many “pieces” the solute breaks into upon dissolving. Glucose (C6H6O6), for example, will dissolve when mixed with water, but that doesn’t mean the actual molecule breaks up into pieces. If you mix 100 molecules of glucose in a small volume of water, you will still have 100 molecules of glucose. Molecules that ionize, however, are different. Table salt (NaCl) will ionize into Na+ and Cl- when dissolved in water. If 100 molecules of NaCl are dissolved in a small volume of water, you will end up with 200 separate bits once each molecule of NaCl splits into two separate ions. Therefore, the ionization constant for NaCl is two! Finally, notice the entire equation begins with a negative sign. Makes sense, because if you dissolve anything in water, it is no longer pure water and will have a solute potential below zero (i.e., negative).
EXAMPLE
 What is the solute potential of a 0.15 M NaCl solution at 24°C?
What is the solute potential of a 0.15 M NaCl solution at 24°C?
 Below, I will walk you through how to solve the previous problem using the equation for solute potential, Ψs = –iCRT.
Below, I will walk you through how to solve the previous problem using the equation for solute potential, Ψs = –iCRT.

Living with Osmotic Challenges
As you read above, cells tend to be happiest when they are in isotonic conditions. But if cells only lived in such a narrow selection of conditions, imagine all the ecological niches that would remain open and unexplored! Therefore, natural selection has come up with some ways for cells to live in water conditions that would otherwise be dangerous. For a single-celled critter to live in hypotonic conditions (fresh water), it has to have a way to deal with excess water entering the cell. Luckily, a species of paramecium has a special organelle called a contractile vacuole that pumps excess water back out of the cell. In an earlier chapter, we learned that plant cells have a large organelle called a central vacuole that holds and stores excess water. It expands and inflates the outer cell membrane, but it won’t pop; instead, the cell membrane presses up against the plant cell wall, providing the pressure needed to keep the cell wall stiff. This is called turgor pressure, and it is how plants maintain their structure. If, instead, a plant cell is surrounded by a hypertonic solution, the water will seep out and the cell membrane will shrink and pull away from the cell wall. This causes a loss of turgor pressure and the plant will wilt.

IRL
Think about what your fingertips look like after soaking in a bath for a long time—they get pruney and wrinkly, right? And if you’re wrinkling up, it means your cells are losing water and your bath must be a hypertonic environment? But that doesn’t make sense, considering that your bath is freshwater. The truth is, your bath is a hypotonic solution, and because water is moving into your cells, you’re actually puffing up (not shriveling!). There is not a consensus on why this happens, but there’s a couple really good hypotheses. One idea is that the strange, wrinkly look is because your outmost epidermal layer is swelling and increasing its surface area, so it has to become folded in order to fit on the area provided by your fingertip. Another hypothesis is that wrinkling of the surfaces of the fingers and toes provides an evolutionary advantage when hanging on to wet surfaces, because the higher surface area provides a better grip!
REVIEW QUESTIONS
1. What two things are needed in order for active transport across a cell membrane to occur?
2. Compare and contrast diffusion, facilitated diffusion, and osmosis.
3. Is the process of endocytosis passive or active transport?
4. A cell living in hypotonic conditions must deal with osmosis occurring [choose one: out of the cell/into the cell].
5. A paramecium is a single-celled organism that normally lives in a freshwater environment. What would happen if it was transferred to a saltwater habitat?
6. What is the solute potential (Ψs) of a 0.23 M NaCl solution at 20°C?
7. Glucose molecules do not easily pass across a cell membrane, even if there is a higher concentration of glucose molecules on the outside of the cell than on the inside. What type of cell transport would help the passive movement of glucose molecules into the interior of the cell?
8. Why is it bad to disrupt a cell’s chemical gradients?
9. For each of the following, indicate if the solution surrounding the cell is hypertonic, isotonic, or hypotonic:
a. A plant cell floating in pure water:________________.
b. A protist cell normally living in a freshwater lake transferred to a marine environment:________________.
c. A bacterium in a solution that has the same water potential as inside the bacterial cell:________________.
10. Some cells that live in hypotonic conditions have evolved to rely on an organelle called a________________to pump out the extra water that diffuses inward.
11. If a cell is in an open container and has a solute potential of –2.0 MPa, what is its overall water potential?

 KNOW
KNOW Gradients are necessary for a cell to live.
Gradients are necessary for a cell to live.