
The Wonder of Water
Theme: Water displays God’s creative handiwork.
Bible Verse: Fear God and give glory to Him, for the hour of His judgment has come; and worship Him who made heaven and earth, the sea and springs of water. (Revelation 14:7)
Materials Needed:
• 9-volt batteries
• Paper clips
• Salt
• Small clear containers of water
• Tape
Bible Lesson
The book of Genesis tells us that God made everything, including the land, the seas, and the heavens above. All things were made supernaturally, from nothing, by God’s mighty word:
For He spoke, and it was done;
He commanded, and it stood fast. (Psalm 33:9)
Try as we might, we simply cannot comprehend how God created the universe. As Deuteronomy 29:29 explains, “The secret things belong to the LORD.” The supernatural is in God’s realm alone, being by definition outside of nature. For this reason, all attempts to fully explain the origin of the universe, the earth, or the beginning of life itself are futile. In the science world, natural origin theories rise and then fall again, only to be replaced by the latest new theory. Perhaps we should simply praise the Creator for his work and not attempt to understand exactly how he accomplished it.
In studying the present-day creation, we can see some of the building blocks that God used. For example, our verse describes the water that fills the vast seas and that bubbles from the ground as springs. This common chemical called water is not just a simple, formless liquid. Instead, it is made up of two separate elements, hydrogen and oxygen. In the creation of water, God formed these two elements complete with their electron, proton, and neutron components exactly in place. The details of chemistry and physics also were established during the creation week. It is easy to read Revelation 14:7 without giving much thought to the infinite details that were actually put in place. The words from Scripture truly reveal a mighty God.
Science Activity
This activity can be done by small groups. Water is divided into its component elements, hydrogen and oxygen gases, by using electrolysis. This is the name for the electrical separation of the components of any chemical compound, in this case water.
Two partially straightened paper clips are attached with tape to the terminals of a fresh 9-volt battery. This is the common type of rectangular battery used in devices such as smoke detectors. Avoid touching the paper clip wires together since this “short circuit” may result in a rapid heating of the wires. However, there is no serious shock hazard with a small battery.
Dissolve a pinch of salt in a small, shallow container of water; the exact amount of salt is not important. This salt will help the water conduct electricity. Now lower the straightened ends of the paper clips into the water. As the chemical reaction begins, many small bubbles of hydrogen gas should appear around the wire attached to the negative terminal of the battery. The other wire probably will not bubble, but instead will slowly darken as oxygen combines with the metal of the paper clip. By using a clear, shallow container, the bubbles rising from the water electrolysis can be seen.
The battery provides the needed energy to divide the water into its component elements, hydrogen and oxygen. Because the atoms are tightly bonded together, water molecules do not ordinarily break down in nature. Even in the form of steam, water is still stable. God made water molecules to be a permanent blessing for the earth.
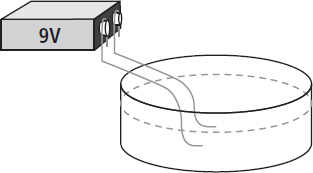
An electric current from a battery will break water down into its component elements, hydrogen and oxygen.
Science Explanation
Many compounds can be divided into smaller components by using electrolysis. The reaction for water is
2H2O → 2H2 + O2
The hydrogen gas is readily seen forming at the negative battery terminal. The oxygen atoms combine with iron on the surface of the paper clip to make iron oxide, FeO:
2Fe + O2 → 2FeO
If one uses a larger battery or several small ones connected together in series, then enough oxygen may be liberated to cause visible bubbling from the positive battery terminal also.