COVID-19 Risk Assessment and Public Health Management Decision Making Algorithm
Resources for State, Local, Territorial and Tribal Health Departments
This page includes information and resources about coronavirus disease 2019 (COVID-19) for state, local, territorial and tribal health departments.
Interim Guidance for Risk Assessment and Monitoring
Interim Guidance for Public Health Personnel Evaluating Persons Under Investigation (PUIs) and Asymptomatic Close Contacts of Confirmed Cases at Their Home or Non-Home Residential Settings – This guidance is intended to address recommended infection prevention and control practices when these activities are performed at a home or non-home residential settings, which warrant additional considerations beyond those described for healthcare settings.
Reporting a Person Under Investigation (PUI) or Laboratory-Confirmed Case for COVID-19 – Information for health departments on reporting and specimen referral for Persons Under Investigation (PUIs) and laboratory-confirmed cases.
Risk Assessment and Public Health Management of Persons with Potential 2019 Novel Coronavirus (COVID-19) Exposure – Interim guidance for assessing risk of potential exposures to COVID-19 and implementing public health actions.
What’s New
Updated Guidance on Evaluating and Testing Persons for Coronavirus Disease 2019 (COVID-19) Sunday, March 8, 2020
Resources for Institutes of Higher Education Sunday, March 8, 2020
COVID-19 and Cruise Ship Travel Sunday, March 8, 2020
Environmental Cleaning and Disinfection Recommendations Sunday, March 8, 2020
People at Risk for Serious Illness from COVID-19 Sunday, March 8, 2020
Communication Resources for Travelers Sunday, March 8, 2020
What Law Enforcement Personnel Need to Know about Coronavirus Disease 2019 (COVID-19) Saturday, March 7, 2020
Preventing COVID-19 Spread in Communities Saturday, March 7, 2020
If you have returned from Hubei Province within the last 14 days, Check and Report Everyday Wednesday, March 4, 2020
Healthcare Professionals
Information for Healthcare Professionals All current interim guidance, and resources on PUIs, clinical care, infection control, home care, and personal protective equipment supplies.
Businesses and Employers
Information for Businesses and Employers to Plan and Response to COVID-19
Travelers
Information for Travel: Information about coronavirus disease 2019 (COVID-19) for travelers
• Travelers to China
• Travelers from China arriving in the U.S.
• Travelers from Countries with Widespread Sustained (Ongoing) Transmission Arriving in the US
Travel Industry
Interim Recommendations for Airlines and Airline Crew: COVID-19 in China
Interim Guidance for Ships on Managing Suspected COVID-2019
Frequently Asked Questions and Answers About:
• Coronavirus Disease 2019
• Travel
• Information for Healthcare Professionals
• Respirators and their Use
• Laboratory Requests for Diagnostic Panels and Virus
• Pregnant Women and COVID-19
• Healthcare Infection Prevention and Control
Additional Resources
• Health Alert Network (HAN) Messages: Primary method of sharing cleared information about urgent public health incidents with public information officers; federal, state, territorial, tribal, and local public health practitioner; clinicians; and public health laboratories.
• CDC Newsroom: CDC Media Telebriefing: Update on COVID-19: CDC provides an update periodically to media on the COVID-19 response.
• Clinician Outreach and Communication Activity (COCA) Calls/Webinars: Subject matter experts present key emergency preparedness and response topics, followed by meaningful Q&A with participants.
• Pandemic Preparedness Resources – Pandemic influenza resources that can be adapted for preparedness for a potential COVID-19 outbreak.
• Stigma and COVID-19 – Resources and information on how to counter stigma associated to COVID-19
• COVID-2019 Publications
• Households with Pets
Interim Guidance for Public Health Personnel Evaluating Persons Under Investigation (PUIs) and Asymptomatic Close Contacts of Confirmed Cases at Their Home or Non-Home Residential Settings
As part of the risk assessment and public health management of persons with potential COVID-19, public health personnel will typically conduct interviews and assess these individuals for fever or other symptoms of COVID-19. In certain circumstances they will also obtain respiratory specimens. This guidance is intended to address recommended infection prevention and control practices when these activities are performed at a home or non-home residential settings, which warrant additional considerations beyond those described for healthcare settings.
For recommendations on the evaluation of PUIs in healthcare settings refer to the Interim Infection Prevention and Control Recommendations for Patients with Confirmed Coronavirus Disease 2019 (COVID-19) or Persons Under Investigation for COVID-19 in Healthcare Settings.
Interviewing and assessing persons with symptoms (PUIs for COVID-19):
• Make every effort to interview the PUI by telephone, text monitoring system, or video conference.
○ Temperature monitoring could be reported by phone or shown to a provider via video conferencing.
• If public health personnel must interview a PUI in their home, the public health personnel should wear recommended personal protective equipment (PPE), including a gown, gloves, eye protection (e.g., goggles, a disposable face shield that covers the front and sides of the face), and respiratory protection that is at least as protective as a NIOSH-approved N95 filtering facepiece respirator, as recommended in the Interim Infection Prevention and Control Recommendations for Patients with Confirmed Coronavirus Disease 2019 (COVID-19) or Persons Under Investigation for COVID-19 in Healthcare Settings.
○ Hand hygiene should be performed before putting on and after removing PPE using alcohol-based hand sanitizer that contains 60 to 95% alcohol.
○ PPE should ideally be put on outside of the home prior to entry into the home.
■ If unable to put on all PPE outside of the home, it is still preferred that face protection (i.e., respirator and eye protection) be put on before entering the home. Alert persons within the home that the public health personnel will be entering the home and ask them to move to a different room, if possible, or keep a 6-foot distance in the same room. Once the entry area is clear, enter the home and put on a gown and gloves.
○ Ask PUI if an external trash can is present at the home, or if one can be left outside for the disposal of PPE.
○ PPE should ideally be removed outside of the home and discarded by placing in external trash can before departing location. PPE should not be taken from the PUI’s home in public health personnel’s vehicle.
■ If unable to remove all PPE outside of the home, it is still preferred that face protection (i.e., respirator and eye protection) be removed after exiting the home. If gown and gloves must be removed in the home, ask persons within the home to move to a different room, if possible, or keep a 6-foot distance in the same room. Once the entry area is clear, remove gown and gloves and exit the home. Once outside the home, perform hand hygiene with alcohol-based hand sanitizer that contains 60 to 95% alcohol, remove face protection and discard PPE by placing in external trash can before departing location. Perform hand hygiene again.
Interviewing and assessing persons without symptoms (asymptomatic close contacts who have been exposed to a lab-confirmed case of COVID-19):
• Make every effort to interview the asymptomatic close contact by telephone, text monitoring system, or video conference.
○ Temperature monitoring could be reported by phone or shown to a provider via video conferencing.
• If public health personnel must interview the asymptomatic close contact in person, the public health personnel should stay at least 6 feet away from the asymptomatic close contact and ask them if they have had fevers or respiratory symptoms. If the interview and assessment is occurring in the home environment, the public health personnel should not enter the home until these questions have been asked and the asymptomatic close contact has been determined to be afebrile by temperature measurement.
○ If the asymptomatic close contact reports fever or symptoms, they should be considered a PUI and referred for further medical evaluation as appropriate. Public health personnel should document temperature measurement and description of symptoms.
• If the asymptomatic close contact does not report fever or symptoms, they should be instructed to take their own temperature and report the result. If the asymptomatic close contact denies symptoms and fever is not detected, it remains appropriate to stay at least 6 feet away during further interactions even if entering the home environment. If they are not able to take their own temperature, the public health personnel should:
○ Perform hand hygiene
○ Put on a facemask and eye protection (consider adding gloves if entering the asymptomatic close contact’s home)
○ Proceed with checking the asymptomatic close contact’s temperature
○ Remove and discard PPE
○ Perform hand hygiene using alcohol-based hand sanitizer that contains 60 to 95% alcohol
Diagnostic respiratory specimen collection for all individuals (i.e., PUIs for COVID-19 or asymptomatic) at home:
• Testing for the virus that causes COVID-19 should be conducted outdoors if climate allows. If conducted in the home, specimen collection should be performed in the area of the house where the individual being tested self-isolates.
○ Only the public health personnel and individual being tested should be in the room when testing is performed.
○ Collecting diagnostic respiratory specimens (e.g., nasopharyngeal swab) is likely to induce cough or sneezing.
○ Non-aerosol-generating procedures should be performed before aerosol-generating procedures. Aerosol-generating procedures should be the last activity performed just before leaving the home.
• Public health personnel collecting specimens should wear recommended PPE, including a gown, gloves, eye protection, and respiratory protection that is at least as protective as a NIOSH-approved N95 filtering facepiece respirator, as recommended in the Interim Infection Prevention and Control Recommendations for Patients with Confirmed Coronavirus Disease 2019 (COVID-19) or Persons Under Investigation for COVID-19 in Healthcare Settings.
○ Hand hygiene should be performed before putting on and after removing PPE using alcohol-based hand sanitizer that contains 60 to 95% alcohol.
○ PPE should ideally be put on outside of the home prior to entry into the home
■ If unable to put on all PPE outside of the home, it is still preferred that face protection (i.e., respirator and eye protection) be put on before entering the home. Alert persons within the home that the public health personnel will be entering the home and ask them to move to a different room, if possible, or keep a 6-foot distance in the same room. Once the entry area is clear, enter the home and put on a gown and gloves.
○ Ask person being tested if an external trash can is present at the home, or if one can be left outside for the disposal of PPE.
○ PPE should ideally be removed outside of the home and discarded by placing in external trash can before departing location. PPE should not be taken from the home of the person being tested in public health personnel’s vehicle.
■ If unable to remove all PPE outside of the home, it is still preferred that face protection (i.e., respirator and eye protection) be removed after exiting the home. If gown and gloves must be removed in the home, ask persons within the home to move to a different room, if possible, or keep a 6-foot distance in the same room. Once the entry area is clear, remove gown and gloves and exit the home. Once outside the home, perform hand hygiene with alcohol-based hand sanitizer that contains 60 to 95% alcohol, remove face protection and discard PPE by placing in external trash can before departing location. Perform hand hygiene again.
Information for Health Departments on Reporting a Person Under Investigation (PUI), or Presumptive Positive and Laboratory-Confirmed Cases of COVID-19
Coronavirus Disease (COVID-19) is a disease caused by the newly emerged coronavirus SARS-CoV-2. To prevent further spread of SARS-CoV-2 and to collect information to better understand the virus and its impact on health outcomes, CDC is working with state and local health departments to identify persons under investigation (PUI) in the United States and test them for the virus that causes COVID-19. To assist our partners, CDC has developed a form that provides a standardized approach to reporting PUIs, presumptive positive cases (individuals with at least one respiratory specimen that tested positive for the virus that causes COVID-19 at a state or local laboratory) and laboratory-confirmed COVID-19 cases (individuals with at least one respiratory specimen that tested positive for the virus that causes COVID-19 at a CDC laboratory). These data are needed to track the impact of the outbreak and inform public health response.
The COVID-19 Persons under Investigation and Case Report Form is designed to collect key information on PUIs, presumptive positive cases, and laboratory-confirmed COVID-19 case-patients, including:
• Demographic, clinical, and epidemiologic characteristics
• Exposure and contact history
• Course of clinical illness and care received
Additional resources to assist in the completion and analysis of the COVID-19 Persons under Investigation and Case Report Form are available:
• COVID-19 Persons Under Investigation and Case Report Form Instructions
Tips to submit a report on a PUI, Presumptive Positive Case, or Laboratory-Confirmed Case
Healthcare providers who are concerned that a patient may have COVID-19 should contact their loca or state health department immediately for consultation and guidance.
CDC is working closely with state and local health departments around the country to support follow-up of PUI, referral of specimens for testing, and follow-up of any presumptive positive or laboratory-confirmed COVID-19 cases. At this time, the following steps should be taken for specimens being tested by CDC:
For jurisdictions that have the capacity to do their own laboratory testing:
For reporting jurisdictions who are able to assign nCoV IDs and monitor PUIs at the jurisdictional level: The jurisdiction should enter all PUIs or Presumptive Cases (persons with a positive test performed locally without CDC lab confirmation) into CDC’s electronic reporting system with the jurisdiction-issued nCoV ID, unless other arrangements to transmit data have been made and approved by CDC. If a specimen is tested locally and is negative, please update the person’s disposition in CDC’s electronic reporting system. If a specimen is tested locally and is positive, please call the CDC EOC Watch desk at 770-488-7100 and notify CDC of the positive result. The specimen will be identified as ‘presumptive positive’ until the result is confirmed at CDC; send the specimen to CDC for confirmatory testing and update the person’s disposition in CDC’s electronic reporting system.
For reporting jurisdictions who are not yet able to assign nCoV IDs and monitor PUIs at the jurisdictional level: The jurisdiction should call the CDC EOC Watch desk at 770-488-7100 to receive a CDC-issued nCoV ID, and to complete a PUI and Case Report Form. If the specimen is tested locally and is positive, please call the CDC EOC Watch desk at 770-488-7100 and notify of the positive result. The specimen will be identified as ‘presumptive positive’ until the result is confirmed at CDC; send the specimen to CDC for confirmatory testing.
For jurisdictions that do not have capacity to do their own testing and are sending specimens to CDC for testing:
For reporting jurisdictions who are able to assign nCoV IDs and monitor PUIs at the jurisdictional level: The jurisdiction should enter all PUIs into CDC’s electronic reporting system with the jurisdiction-issued nCoV IDs, unless other arrangements to transmit data have been made and approved by CDC. Testing results will be returned to the state lab per current protocol.
For reporting jurisdictions who are not yet able to assign nCoV IDs and monitor PUIs at the jurisdictional level: The jurisdiction should call the CDC EOC Watch Desk at 770-488-7100 to obtain a CDC-issued nCoV ID number, and complete a PUI and Case Report Form. Testing results will be returned to the state lab per current protocol.
The nCoV ID should be included on all PUI and Case Report Forms and shipping labels.
Please contact your state or territorial health department for more information on submitting reports of PUI, presumptive positive cases, and laboratory-confirmed COVID-19 cases. State or territorial health departments with questions about investigating COVID-19 PUI or cases should contact eocevent118@cdc.gov.
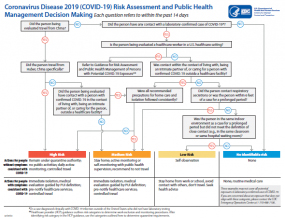
Interim US Guidance for Risk Assessment and Public Health Management of Persons with Potential Coronavirus Disease 2019 (COVID-19) Exposures: Geographic Risk and Contacts of Laboratory-confirmed Cases
Recommendations in this document for actions by public health authorities apply primarily to US jurisdictions that are not experiencing sustained community transmission. CDC will provide separate guidance for US jurisdictions with sustained community transmission.
CDC has provided separate guidance for healthcare settings.
Summary of changes
Revisions were made on March 7, 2020, to reflect the following:
• Defintions for congregate settings and social distancing were revised
Revisions were made on March 5, 2020, to reflect the following:
• Clarified that in jurisdictions without sustained community transmission, decisions for public health action should be based priorities of public health authorities (e.g., surveillance, contact tracing)., In jurisdictions with sustained community transmission, travelers and other potentially exposed individuals should follow local guidance. Also provided a rationale for these changes.
• Updated definitions for self-observation, self-monitoring, and self-monitoring with public health supervision
• Provided exposure risk definitions and recommended management for countries other than China
• Updated recommendations for Crews on Passenger or Cargo Flights
• Removed Workplace section
• Added links to information on discontinuation of isolation for patients with laboratory-confirmed COVID-19
• Clarified that a potentially exposed person’s risk level does not change if symptoms develop
• Reorganized tables
CDC is closely monitoring an epidemic of respiratory illness (COVID-19) caused by a novel (new) coronavirus (SARS-CoV-2) that was first detected in Wuhan, Hubei Province, China. Chinese health officials have reported tens of thousands of illnesses with COVID-19 in China and the virus is spreading from person-to-person in many parts of that country. Cases of COVID-19 are also being reported in a growing number of international locations, several of which are experiencing sustained community-level or widespread person-to-person transmission. Cases of COVID-19 without direct links to travel have been reported in the United States and sustained transmission is occurring in some US communities.
The purpose of this interim guidance to provide public health authorities and other partners in US jurisdictions that are not experiencing sustained community transmission of COVID-19 with a framework for assessing and managing risk of potential exposures to SARS-CoV-2 and implementing public health actions based on a person’s risk level and clinical presentation. Public health actions may include monitoring or the application of movement restrictions, including isolation and quarantine, when needed to delay the introduction and spread of SARS-CoV-2 in these communities.
The recommendations in this guidance apply to US-bound travelers who may have been exposed to SARS-CoV-2 and people identified through contact investigations of laboratory-confirmed cases. CDC acknowledges that state and local jurisdictions may make risk management decisions that differ from those recommended here. Public health management decisions should be based on the situation in the jurisdiction and the priorities of public health authorities. The guidance will be updated based on the evolving circumstances of the epidemic.
The guidance was designed for a “containment” approach in the absence of sustained SARS-CoV-2 transmission in US communities in order to delay introduction and spread of SARS-CoV-2. It focuses on decreasing the risk of unrecognized case importation from international locations with sustained transmission and managing contacts of laboratory-confirmed cases. In US jurisdictions that are not experiencing sustained community transmission, these activities are still important; however, a resource-intense containment approach that focuses on international travelers poses a risk of diverting public health resources from other priority activities, including surveillance and case finding, contact tracing, and preparing for community mitigation measures. Allowing health departments the flexibility to prioritize public health actions in their jurisdictions enables prudent deployment of public health resources where they can have the most benefit based on the local situation. State and local health departments are best positioned to make such decisions within their jurisdictions.
In US jurisdictions with sustained community transmission, shifting from containment to mitigation conserves public health resources and directs them to where they can have the most benefit. In such jurisdictions, residents may have the same exposure risk as international travelers from countries with sustained transmission; therefore, applying stringent containment measures to international travelers (e.g., staying home for 14 days) no longer has a public health benefit and would be arbitrary in the context of similar risk among others in the community. Applying such containment measures (e.g., asking people to stay home) community-wide would have severe detrimental effects on community infrastructure. When SARS-CoV-2 is spreading in a community, it is also not feasible to identify all people with symptoms compatible with COVID-19 or identify all potentially exposed contacts. Applying stringent containment measures to people who are tested and have laboratory confirmation and their contacts, but not to others who are not tested and their contacts, would have no public health benefit. Such an approach could hamper surveillance efforts and ability of public health authorities to make data-driven decisions for the implementation of community mitigation measures. Separate CDC guidance is in development that harmonizes recommendations for people who are tested and confirmed positive for COVID-19 and others in the community who are symptomatic but not tested, as well as their contacts.
Definitions Used in this Guidance
Symptoms compatible with COVID-19, for the purpose of these recommendations, include subjective or measured fever, cough, or difficulty breathing.
Self-observation means people should remain alert for subjective fever, cough, or difficulty breathing. If they feel feverish or develop cough or difficulty breathing during the self-observation period, they should take their temperature, self-isolate, limit contact with others, and seek advice by telephone from a healthcare provider or their local health department to determine whether medical evaluation is needed.
Self-monitoring means people should monitor themselves for fever by taking their temperatures twice a day and remain alert for cough or difficulty breathing. If they feel feverish or develop measured fever, cough, or difficulty breathing during the self-monitoring period, they should self-isolate, limit contact with others, and seek advice by telephone from a healthcare provider or their local health department to determine whether medical evaluation is needed.
Self-monitoring with delegated supervision means, for certain occupational groups (e.g., some healthcare or laboratory personnel, airline crew members), self-monitoring with oversight by the appropriate occupational health or infection control program in coordination with the health department of jurisdiction. The occupational health or infection control personnel for the employing organization should establish points of contact between the organization, the self-monitoring personnel, and the local or state health departments with jurisdiction for the location where personnel will be during the self-monitoring period. This communication should result in agreement on a plan for medical evaluation of personnel who develop fever, cough, or difficulty breathing during the self-monitoring period. The plan should include instructions for notifying occupational health and the local public health authority, and transportation arrangements to a pre-designated hospital, if medically necessary, with advance notice if fever, cough, or difficulty breathing occur. The supervising organization should remain in contact with personnel through the self-monitoring period to oversee self-monitoring activities.
Self-monitoring with public health supervision means public health authorities assume the responsibility for oversight of self-monitoring for certain groups of people. The ability of jurisdictions to initiate or provide continued oversight will depend on other competing priorities (e.g., contact tracing, implementation of community mitigation strategies). Depending on local priorities, CDC recommends that health departments consider establishing initial communication with these people, provide a plan for self-monitoring and clear instructions for notifying the health department before the person seeks health care if they develop fever, cough, or difficulty breathing. As resources allow, health authorities may also check in intermittently with these people over the course of the self-monitoring period. If travelers for whom public health supervision is recommended are identified at a US port of entry, CDC will notify state and territorial health departments with jurisdiction for the travelers’ final destinations.
Active monitoring means that the state or local public health authority assumes responsibility for establishing regular communication with potentially exposed people to assess for the presence of fever, cough, or difficulty breathing. For people with high-risk exposures, CDC recommends this communication occurs at least once each day. The mode of communication can be determined by the state or local public health authority and may include telephone calls or any electronic or internet-based means of communication.
Close contact is defined as:
a) being within approximately 6 feet (2 meters) of a COVID-19 case for a prolonged period of time; close contact can occur while caring for, living with, visiting, or sharing a healthcare waiting area or room with a COVID-19 case
– or –
b) having direct contact with infectious secretions of a COVID-19 case (e.g., being coughed on)
Public health orders are legally enforceable directives issued under the authority of a relevant federal, state, or local entity that, when applied to a person or group, may place restrictions on the activities undertaken by that person or group, potentially including movement restrictions or a requirement for monitoring by a public health authority, for the purposes of protecting the public’s health. Federal, state, or local public health orders may be issued to enforce isolation, quarantine or conditional release. The list of quarantinable communicable diseases for which federal public health orders are authorized is defined by Executive Order and includes “severe acute respiratory syndromes.” COVID-19 meets the definition for “severe acute respiratory syndromes” as set forth in Executive Order 13295, as amended by Executive Order 13375 and 13674, and, therefore, is a federally quarantinable communicable disease.
Isolation means the separation of a person or group of people known or reasonably believed to be infected with a communicable disease and potentially infectious from those who are not infected to prevent spread of the communicable disease. Isolation for public health purposes may be voluntary or compelled by federal, state, or local public health order.
Quarantine in general means the separation of a person or group of people reasonably believed to have been exposed to a communicable disease but not yet symptomatic, from others who have not been so exposed, to prevent the possible spread of the communicable disease.
Conditional release defines a set of legally enforceable conditions under which a person may be released from more stringent public health movement restrictions, such as quarantine in a secure facility. These conditions may include public health supervision through in-person visits by a health official or designee, telephone, or any electronic or internet-based means of communication as determined by the CDC Director or state or local health authority. A conditional release order may also place limits on travel or require restriction of a person’s movement outside their home.
Controlled travel involves exclusion from long-distance commercial conveyances (e.g., aircraft, ship, train, bus). For people subject to active monitoring, any long-distance travel should be coordinated with public health authorities to ensure uninterrupted monitoring. Air travel is not allowed by commercial flight but may occur via approved noncommercial air transport. CDC may use public health orders or federal public health travel restrictions to enforce controlled travel. CDC also has the authority to issue travel permits to define the conditions of interstate travel within the United States for people under certain public health orders or if other conditions are met.
Congregate settings are crowded public places where close contact with others may occur, such as shopping centers, movie theaters, stadiums.
Social distancing means remaining out of congregate settings, avoiding mass gatherings, and maintaining distance (approximately 6 feet or 2 meters) from others when possible.
These categories are interim and subject to change.
CDC has established the following exposure risk categories to help guide public health management of people following potential SARS-CoV-2 exposure in jurisdictions that are not experiencing sustained community transmission. These categories may not cover all potential exposure scenarios. They should not replace an individual assessment of risk for the purpose of clinical decision making or individualized public health management.
All exposures apply to the 14 days prior to assessment.
For country-level risk classifications, see Coronavirus Disease 2019 Information for Travel.
CDC has provided separate guidance for healthcare settings.
Table 1. Risk Categories for Exposures Associated with International Travel or Identified during Contact Investigations of Laboratory-confirmed Cases
|
Risk Level |
Geographic (Travel-associated) Exposures* |
Exposures Identified through Contact Investigation |
|---|---|---|
|
High |
Travel from Hubei Province, China |
Living in the same household as, being an intimate partner of, or providing care in a nonhealthcare setting (such as a home) for a person with symptomatic laboratory-confirmed COVID-19 infection without using recommended precautions for home care and home isolation |
|
Medium (assumes no exposures in the high-risk category) |
• Travel from mainland China outside Hubei Province or Iran • Travel from a country with widespread sustained transmission, other than China or Iran • Travel from a country with sustained community transmission |
• Close contact with a person with symptomatic laboratory-confirmed COVID-19 • On an aircraft, being seated within 6 feet (two meters) of a traveler with symptomatic laboratory-confirmed COVID-19 infection; this distance correlates approximately with 2 seats in each direction • Living in the same household as, an intimate partner of, or caring for a person in a nonhealthcare setting (such as a home) to a person with symptomatic laboratory-confirmed COVID-19 infection while consistently using recommended precautions for home care and home isolation |
|
Low (assumes no exposures in the high-risk category) |
Travel from any other country |
Being in the same indoor environment (e.g., a classroom, a hospital waiting room) as a person with symptomatic laboratory-confirmed COVID-19 for a prolonged period of time but not meeting the definition of close contact |
|
No identifiable risk |
Not applicable |
Interactions with a person with symptomatic laboratory-confirmed COVID-19 infection that do not meet any of the high-, medium- or low-risk conditions above, such as walking by the person or being briefly in the same room. |
|
* In general, geographic exposure categories do not apply to travelers who only transit through an airport. |
||
Recommendations for Exposure Risk Management
State and local authorities have primary jurisdiction for isolation and other public health orders within their respective jurisdictions. Federal public health authority primarily extends to international arrivals at ports of entry and to preventing interstate communicable disease threats.
CDC recognizes that decisions and criteria to use such public health measures may differ by jurisdiction. Consistent with principles of federalism, state and local jurisdictions may choose to make decisions about isolation, other public health orders, and monitoring that exceed those recommended in federal guidance. As the domestic COVID-19 situation evolves, public health authorities should base their decisions about application of individual-level monitoring or movement restrictions on the situation in their jurisdictions, including whether sustained community transmission is occurring and competing priorities.
The issuance of public health orders should be considered in the context of other less restrictive means that could accomplish the same public health goals. People under public health orders must be treated with respect, fairness, and compassion, and public health authorities should take steps to reduce the potential for stigma (e.g., through outreach to affected communities, public education campaigns). Considerable, thoughtful planning by public health authorities is needed to implement public health orders properly. Specifically, measures must be in place to provide shelter, food, water, and other necessities for people whose movement is restricted under public health orders, and to protect their dignity and privacy.
CDC’s recommendations for public health management of international travelers with potential exposure to SARS-CoV-2 and people identified through contact investigations of laboratory-confirmed cases, including monitoring and the application of travel or movement restrictions, are summarized in Table 2.
Additional recommendations in specific groups or settings are provided below.
Crews on Passenger or Cargo Flights
For country-level risk classifications, see Coronavirus Disease 2019 Information for Travel. Regardless of residence or travel history, crew members who have known exposure to persons with COVID-19 should be assessed and managed on a case-by-case basis.
Air carriers have the authority to adopt occupational health policies for their own employees that exceed CDC’s recommendations.
US-based crews who have layovers in countries with sustained (community or widespread) transmission or other international locations
US-based crew members who are on layovers in countries with sustained (community or widespread) transmission should limit their activities in public and their interactions with local populations and practice social distancing while in those countries. Crew members who follow these recommendations, and who have no known exposure to persons with COVID-19, are assessed as low risk. These crew members should self-monitor for 14 days after their layovers under the supervision of the air carrier’s occupational health program. These crew members have no movement restrictions while in the United States and may continue to work on passenger or cargo flights as long as they remain asymptomatic. Air carriers should coordinate with health departments of jurisdiction for crew members’ residences to establish plans for managing crew members identified as symptomatic. If individualized planning with health departments is infeasible based on volume of crew members or priorities of health departments, air carriers should at a minimum ensure crew members know how to contact their local health departments. If they develop fever, cough, or difficulty breathing, crew members should self-isolate and be excluded from work on flights immediately until cleared by public health authorities.
At this time, CDC recommends that US-based flight crews consider practicing social distancing during layovers or other travel at all international destinations and self-observation at all times.
Crews based in countries without sustained (community or widespread) transmission who have had layovers in countries with sustained (community or widespread) transmission
Crew members who are based in other countries not known to have sustained transmission and who practice social distancing during layovers in a country with sustained transmission in the past 14 days are assessed as low risk. The crew members should self-monitor for 14 days after their layovers under the supervision of the air carrier’s occupational health program while on layovers in the United States. These crew members have no movement restrictions while in the United States and may continue to work on passenger or cargo flights as long as they remain asymptomatic. Air carriers should coordinate with health departments of jurisdiction for airports where they operate to establish plans for managing crew members identified as symptomatic while in the United States. If they develop fever, cough, or difficulty breathing, crew members should self-isolate and be excluded from work on commercial flights immediately until cleared by public health authorities.
Crews based in countries with sustained community transmission
Crew members who are based in countries with sustained community transmission and who are in the United States for layovers are assessed as medium risk but may continue to work on passenger or cargo flights to and within the United States as long as they remain asymptomatic. The crew members should self-monitor under the supervision of the air carrier’s occupational health program. These crew members are also recommended practice social distancing while in the United States. Air carriers should coordinate with health departments of jurisdiction for airports where they operate to establish plans for managing crew members identified as symptomatic while in the United States. If they develop fever, cough, or difficulty breathing, crew members should self-isolate and be excluded from work on commercial flights immediately until cleared by public health authorities.
Crews based in countries with widespread sustained transmission
Crew members who are based in countries with widespread sustained transmission and who are in the United States for layovers are assessed as medium risk but may continue to work on passenger or cargo flights to and within the United States as long as they remain asymptomatic. These crew members should self-monitor under the supervision of the air carrier’s occupational health program. These crew members are also recommended to remain in their hotel rooms, limit activities in public, and practice social distancing while in the United States. Air carriers should coordinate with health departments of jurisdiction for airports where they operate to establish plans for managing crew members identified as symptomatic while in the United States. If they develop fever, cough, or difficulty breathing, crew members should self-isolate and be excluded from work on commercial flights immediately until cleared by public health authorities.
People with Laboratory-Confirmed COVID-19 and Symptomatic People Under Investigation for COVID-19
CDC has established criteria for determining when an individual can be considered non-infectious to guide discontinuation of transmission-based precautions for hospitalized patients or home isolation. While individuals are considered infectious, local or long-distance travel should occur only by medical transport (e.g., ambulance or air medical transport) or private vehicle. Isolation and travel restrictions are removed upon determination by public health authorities that the person is no longer considered to be infectious.
Symptomatic people who meet CDC’s definition of Persons Under Investigation (PUI) should be evaluated by healthcare providers in conjunction with local health authorities. PUIs awaiting results of rRT-PCR testing for COVID-19 should remain in isolation at home or in a healthcare facility until their test results are known. Depending on the clinical suspicion of COVID-19, PUIs for whom an initial rRT-PCR test is negative may be candidates for removal of any isolation and travel restrictions specific to symptomatic people, but any restrictions for asymptomatic people according to the assigned risk level should still apply. Management decisions of PUIs who are not tested should be made on a case-by-case basis, using available epidemiologic and clinical information, in conjunction with CDC guidance.
Contacts of Asymptomatic People Exposed to COVID-19
CDC does not recommend testing, symptom monitoring or special management for people exposed to asymptomatic people with potential exposures to SARS-CoV-2 (such as in a household), i.e., “contacts of contacts;” these people are not considered exposed to SARS-CoV-2.
Table 2. Summary of CDC Recommendations for Management of Exposed Persons with by Risk Level and Presence of Symptoms
The public health actions recommended in the table below apply to people who have been determined to have at least some risk for COVID-19. People who are being managed as asymptomatic in a particular risk level who develop signs or symptoms compatible with COVID-19 should be moved immediately into the symptomatic category in the same risk level and be managed accordingly. The risk level does not change if symptoms develop.
|
Risk Level |
Management if Asymptomatic |
Management if Symptomatic1 |
|---|---|---|
|
High risk |
• Quarantine (voluntary or under public health orders) in a location to be determined by public health authorities. • No public activities. • Daily active monitoring, if possible based on local priorities • Controlled travel |
• Immediate isolation with consideration of public health orders • Public health assessment to determine the need for medical evaluation; if medical evaluation warranted, diagnostic testing should be guided by CDC’s PUI definition • If medical evaluation is needed, it should occur with pre-notification to the receiving HCF and EMS, if EMS transport indicated, and with all recommended infection control precautions in place. • Controlled travel: Air travel only via air medical transport. Local travel is only allowed by medical transport (e.g., ambulance) or private vehicle while symptomatic person is wearing a face mask. |
|
Medium risk |
Close contacts in this category: • Recommendation to remain at home or in a comparable setting Practice social distancing • Active monitoring as determined by local priorities • Recommendation to postpone long-distance travel on commercial conveyances |
• Self-isolation • Public health assessment to determine the need for medical evaluation; if medical evaluation warranted, diagnostic testing should be guided by CDC’s PUI definition • If medical evaluation is needed, it should ideally occur with pre-notification to the receiving HCF and EMS, if EMS transport indicated, and with all recommended infection control precautions in place. • Controlled travel: Air travel only via air medical transport. Local travel is only allowed by medical transport (e.g., ambulance) or private vehicle while symptomatic person is wearing a face mask. |
|
Travelers from mainland China (outside Hubei Province) or Iran • Recommendation to remain at home or in a comparable setting Practice social distancing • Self-monitoring with public health supervision as determined by local priorities • Recommendation to postpone additional long-distance travel on commercial conveyances after they reach their final destination |
||
|
Travelers from other country with widespread transmission • Recommendation to remain at home or in a comparable setting, • Practice social distancing • Self-monitoring • Recommendation to postpone additional long-distance travel on commercial conveyances after they reach their final destination |
||
|
Travelers from country with sustained community transmission • Practice social distancing • Self-observation |
||
|
Low risk |
• No restriction on movement • Self-observation |
• Self-isolation, social distancing • Person should seek health advice to determine if medical evaluation is needed. • If sought, medical evaluation and care should be guided by clinical presentation; diagnostic testing for COVID-19 should be guided by CDC’s PUI definition. • Travel on commercial conveyances should be postponed until no longer symptomatic. |
|
No identifiable risk |
None |
• Self-isolation, social distancing • Person should seek health advice to determine if medical evaluation is needed. • If sought, medical evaluation and care should be guided by clinical presentation; diagnostic testing for COVID-19 should be guided by CDC’s PUI definition. • Travel on commercial conveyances should be postponed until no longer symptomatic. |
|
EMS = emergency medical services; HCF = healthcare facility; PUI = Person Under Investigation for COVID-19 1 For the purpose of this document: subjective or measured fever, cough, or difficulty breathing |
||
Note: The public health management recommendations made above are primarily intended for jurisdictions not experiencing sustained community transmission. In jurisdictions not experiencing sustained community transmission, CDC recommends that post-exposure public health management for asymptomatic exposed individuals continue until 14 days after the last potential exposure; however, these decisions should be made based on the local situation, available resources, and competing priorities. These factors should also guide decisions about managing symptomatic exposed individuals.
International travelers and other potentially exposed individuals in jurisdictions experiencing sustained community transmission should follow local guidance.
For country-level risk classifications, see Coronavirus Disease 2019 Information for Travel.
CDC has provided separate guidance for healthcare settings.
Pandemic Preparedness Resources
While the content at the links provided below was developed to prepare for, or respond to, an influenza (“flu”) pandemic, the newly emerged coronavirus disease 2019 (COVID-19) is a respiratory disease that seems to be spreading much like flu. Guidance and tools developed for pandemic influenza planning and preparedness can serve as appropriate resources for health departments in the event the current COVID-19 outbreak triggers a pandemic.
• Pandemic Planning and Preparedness Resources
• Pandemic Influenza Plan (UPDATED 2017)[1 MB, 52 pages]
• Community Mitigation Guidelines to Prevent Pandemic Influenza — United States, 2017
• Nonpharmaceutical Interventions (NPIs)
• NPI 101: An Introduction to Nonpharmaceutical Interventions (NPIs) for Pandemic Influenza CDC TRAIN course
Interim Guidance for Public Health Professionals Managing People With COVID-19 in Home Care and Isolation Who Have Pets or Other Animals
Purpose:
This interim guidance is for public health professionals managing the home care and isolation of people with COVID-19 who have pets or other animals (including service or working animals) in the same home. The intent of this guidance is to facilitate preparedness and establish practices that can help people and animals stay safe and healthy. At this time, there is no evidence that companion animals, including pets, can spread COVID-19. States may have their own specific requirements for these circumstances; this guidance provides recommendations for a conservative approach due to the unknown risks to pets and other animals. Guidance is based on the limited available data and general recommendations for zoonotic disease infection prevention and control. This is a rapidly evolving situation. Guidance will be updated as new information becomes available.
Considerations for COVID-19 patients under home care and isolation who have pets or other animals:
People with COVID-19 should be advised to tell their public health point of contact that they have pets or other animals in their home.
In addition to other prevention measures, people with COVID-19 who are identified by public health officials as requiring home care and isolation should be advised to limit interaction with pets and other animals. Specifically, while these people are symptomatic, they should maintain separation from pets as they would with other household members, and avoid direct contact with pets, including petting, snuggling, being kissed or licked, and sharing food. Service animals should be permitted to remain with their handlers.
If possible, a household member should be designated to care for pets in the home. If the individual in home care and isolation must care for pet(s), including service animals, they should ensure they wash their hands before and after caring for pets and wear a facemask while interacting with pets, until they are medically cleared to return to normal activities.
At this point there is no evidence that companion animals, including pets, can spread COVID-19.
When a public health professional is notified of a pet, or other animal, in the home of a person with COVID-19, they should notify the state public health veterinarian or other designated animal health professional.
State public health veterinarians who have been contacted about pets or other animals potentially exposed to COVID-19 can consult with the CDC One Health Team 24/7 by calling CDC’s Emergency Operations Center at 770-488-7100.
This is a rapidly evolving situation and information will be updated as it becomes available.
Resources
CDC’s up-to-date information on COVID-19:
• Interim US Guidance for Risk Assessment and Public Health Management of Persons with Potential 2019 Novel Coronavirus (COVID-19) Exposure in Travel-associated or Community Settings
• Interim Guidance for Implementing Home Care of People Not Requiring Hospitalization for 2019 Novel Coronavirus (COVID-19)
• Frequently Asked Questions (including COVID-19 and Animals)
NASPHV Compendium of Veterinary Standard Precautions for Zoonotic Disease Prevention in Veterinary
CDC’s Healthy Pets, Healthy People Website
World Health Organization (WHO) Website
World Organisation for Animal Health (OIE) Q & A Website
• Burke RM, Midgley CM, Dratch A, et al. Active Monitoring of Persons Exposed to Patients with Confirmed COVID-19 — United States, January–February 2020. MMWR Morb Mortal Wkly Rep 2020;69:245–246. DOI: http://dx.doi.org/10.15585/mmwr.mm6909e1.
• Guan WJ, Ni ZY, Hu Y, et al. Clinical Characteristics of Coronavirus Disease 2019 in China. N Engl J Med. February 28, 2020. DOI: 10.1056/NEJMoa2002032.
• Morens DM, Daszak P, Taubenberger JK. Escaping Pandora’s Box – Another Novel Coronavirus. N Engl J Med. February 26, 2020. DOI: 10.1056/NEJMp2002106.
• Rasmussen SA, Smulian JC, Lednicky JA, et al. Coronavirus Disease 2019 (COVID-19) and Pregnancy: What obstetricians need to know. Amer J Ob Gyn. Available online February 24, 2020. DOI: https://doi.org/10.1016/j.ajog.2020.02.017.
• Wu Z, McGoogan JM. Characteristics of and Important Lessons From the Coronavirus Disease 2019 (COVID-19) Outbreak in China: Summary of a Report of 72 314 Cases From the Chinese Center for Disease Control and Prevention. JAMA. Published online: February 24, 2020. DOI:10.1001/jama.2020.2648.
• Yang X, Yu Y, Xu J, et al. Clinical course and outcomes of critically ill patients with SARS-CoV-2 pneumonia in Wuhan, China: a single-centered, retrospective, observational study. The Lancet. Published online: February 24, 2020. DOI: https://doi.org/10.1016/S2213-2600(20)30079-5.
• Shi H, Han X, Jiang N, et al. Radiological findings from 81 patients with COVID-19 pneumonia in Wuhan, China: a descriptive study. The Lancet. Published online February 24, 2020. DOI: https://doi.org/10.1016/S1473-3099(20)30086-4.
• Xu XW, Wu XX, Jiang XG, et al. Clinical findings in a group of patients infected with the 2019 novel coronavirus (SARS-Cov-2) outside of Wuhan, China: retrospective case series. BMJ 2020;368:m606. DOI: https://doi.org/10.1136/bmj.m606.
• Zou L, Ruan F, Huang M, et al. SARS-CoV-2 Viral Load in Upper Respiratory Specimens of Infected Patients. N Engl J Med. February 19, 2020. DOI: 10.1056/NEJMc2001737.
• Xu Z, Shi L, Wang Y, et al. Pathological findings of COVID-19 associated with acute respiratory distress syndrome. The Lancet. Published online February 18, 2020. DOI: https://doi.org/10.1016/S2213-2600(20)30076-X.
• Ping Y, Jiang Z, Zhengdong Z, et al. A familial cluster of infection associated with the 2019 novel coronavirus indicating potential person-to-person transmission during the incubation period. The Journal of Infectious Diseases, jiaa077. February 18, 2020. DOI: https://doi.org/10.1093/infdis/jiaa077.
• Novel Coronavirus Pneumonia Emergency Response Epidemiology Team. [The Epidemiological Characteristics of an Outbreak of 2019 Novel Coronavirus Diseases (COVID-19) in China]. Zhonghua Liu Xing Bing Xue Za Zhi. 2020;41(2):145–151. DOI:10.3760/cma.j.issn.0254-6450.2020.02.003.
• Qiao J. What are the risks of COVID-19 infection in pregnant women? The Lancet. February 12, 2010. DOI: https://doi.org/10.1016/S0140-6736(20)30365-2
• To KKW, Tsang OTY, Yip CCY, et al. Consistent detection of 2019 novel coronavirus in saliva. Clinical Infectious Diseases, ciaa149. February 12, 2020. DOI: https://doi.org/10.1093/cid/ciaa149
• Liu YC, Lio CH, Chang CF, et al. A Locally Transmitted Case of SARS-CoV-2 Infection in Taiwan. N Engl J Med. February 12, 2020. DOI: 10.1056/NEJMc2001573.
• Pongpirul WA, Pongpirul K, Ratnarathon AC. Journey of a Thai Taxi Driver and Novel Coronavirus. N Engl J Med. February 12, 2020. DOI: 10.1056/NEJMc2001621.
• Huijun C, Guo, J, Wang, C, et al. Clinical characteristics and intrauterine vertical transmission potential of COVID-19 infection in nine pregnant women: a retrospective review of medical records. The Lancet. Published online February 12, 2020. DOI: https://doi.org/10.1016/S0140-6736(20)30360-3.
• Ki M, -nCoV TFF. Epidemiologic characteristics of early cases with 2019 novel coronavirus (2019-nCoV) disease in Republic of Korea. Epidemiol Health. February 9, 2020:e2020007. doi: 10.4178/epih.e2020007.
• Bajema KL, Oster AM, McGovern OL, et al. Persons Evaluated for 2019 Novel Coronavirus — United States, January 2020. MMWR Morb Mortal Wkly Rep. ePub: February 7, 2020. DOI: dx.doi.org/10.15585/mmwr.mm6906e1.
• Wang D, Hu B, Hu C, et al. Clinical Characteristics of 138 Hospitalized Patients with 2019 Novel Coronavirus-Infected Pneumonia in Wuhan, China. JAMA. Published online February 7, 2020.
• Chang D, Lin M, Wei L, et al. Epidemiologic and Clinical Characteristics of Novel Coronavirus Infections Involving 13 Patients Outside Wuhan, China. JAMA. Published online February 7, 2020. DOI:10.1001/jama.2020.1623.
• Kampf G, Todt D, Pfaender S, Steinmann E. Persistence of coronaviruses on inanimate surfaces and its inactivation with biocidal agents. J Hosp Infect. February 6, 2020. pii: S0195-6701(20)30046-3. doi: 10.1016/j.jhin.2020.01.022.
• Patel A, Jernigan DB. Initial Public Health Response and Interim Clinical Guidance for the 2019 Novel Coronavirus Outbreak — United States, December 31, 2019–February 4, 2020. MMWR Morb Mortal Wkly Rep 2020;69:140–146. DOI: dx.doi.org/10.15585/mmwr.mm6905e1.
• Holshue M, DeBolt C, Lindquist S, et al. First Case of 2019 Novel Coronavirus in the United States. N Engl J Med. January 31, 2020. DOI: 10.1056/NEJMoa2001191.
• Lu R, Zhao X, Li J, et al. Genomic characterization and epidemiology of 2019 novel coronavirus: implications for virus origins and receptor binding [1.35 MB, 10 pages]. The Lancet. Published online January 29, 2020. https://doi.org/10.1016/S0140-6736(20)30251-8
• Chen N, Zhou M, Dong X, et al. Epidemiological and clinical characteristics of 99 cases of 2019 novel coronavirus pneumonia in Wuhan, China: a descriptive study [7 pages]. The Lancet. Published online January 29, 2020. https://doi.org/10.1016/ S0140-6736(20)30211-7
• Phan L, Nguyen T, Luong Q, et al. Importation and Human-to-Human Transmission of a Novel Coronavirus in Vietnam. N Engl J Med. January 28, 2020. DOI: 10.1056/NEJMc2001272
• The Lancet. Emerging understandings of 2019-nCoV [1 page]. The Lancet. Published online January 24, 2020. https://doi.org/10.1016/ S0140-6736(20)30186-0
• Heymann D. Data sharing and outbreaks: best practice exemplified [2 pages]. The Lancet. Published online January 24, 2020. https://doi.org/10.1016/S0140-6736(20)30184-7
• Huang C, Wang Y, Li X, et al. Clinical features of patients infected with 2019 novel coronavirus in Wuhan, China [1.5 MB, 10 pages]. The Lancet. Published online January 24, 2020. https://doi.org/10.1016/S0140-6736(20)30183-5
• Chan JF, Yuan S, Kok K, et al. A familial cluster of pneumonia associated with the 2019 novel coronavirus indicating person-to-person transmission: a study of a family cluster[1.4 MB, 10 pages]. The Lancet. Published online January 24, 2020. https://doi.org/10.1016/S0140-6736(20)30154-9
• Wang C, Horby P, Hayden F, Gao G. A novel coronavirus outbreak of global health concern [4 pages]. The Lancet. Published online January 24, 2020. https://doi.org/10.1016/S0140-6736(20)30185-9
• Paules C, Marston H, Fauci A. Coronavirus Infections – More Than Just the Common Cold [2 pages]. JAMA. Published online January 23, 2020. doi:10.1001/jama.2020.0757