
Ernest Rutherford (1871–1937), left, and Hans Geiger (1882–1945), right, in their laboratory at Manchester University in about 1908. They are seen with the equipment they used to detect alpha particles from a radioactive source.
Ernest Rutherford is a very unusual example of an experimental physicist who made his most important discovery after he had received the Nobel Prize for something else. The prize, awarded in 1908, was actually the Nobel Prize in Chemistry, for his work on what was called ‘the disintegration of the elements’. Following up Becquerel’s discovery of radioactivity (see here), Rutherford had shown that the process involves an atom spitting out a part of itself and being transformed into another kind of atom. Along the way, he identified two kinds of radiation, which he called alpha rays and beta rays; alpha rays were soon identified as helium atoms lacking their electrons, and beta rays as electrons. He later found and named a third kind of atomic radiation, gamma rays, which turned out to be like X-rays but with even shorter wavelengths (higher frequency) and therefore carrying even more energy.
Not content with just identifying and classifying different kinds of atomic radiation, Rutherford realized that he could use these fast-moving particles, in particular alpha particles, to probe the structure of matter. At the beginning of the twentieth century, although electrons had been identified as parts of an atom (see here), nobody was sure how they were arranged inside an atom, or where the positive charge needed to balance the electrons’ negative charge was located. The most popular idea was proposed in 1904 by J. J. Thomson, who envisaged the atom as a cloud of positive charge in which negative electrons were embedded. This was sometimes known as the ‘plum pudding’ model, but a better analogy would be with a watermelon, where the flesh of the melon is the positive charge and the pips are electrons.

In 1909, Rutherford was Professor of Physics at the University of Manchester, where he devised an experiment that was carried out, under his direction, by Hans Geiger and Ernest Marsden. Alpha particles produced by natural radioactivity were fired towards a target in the form of a thin sheet of gold foil. Using a detector that could be moved to different places around the foil (the forerunner of the detector named after Geiger), they planned to find out how the alpha rays were affected as they passed through the foil. They anticipated that the beam of particles might be deflected slightly, like a beam of light passing from air into a glass prism. But to their surprise they found that most of the alpha particles went straight through the foil as if it were not there, while some of them were being deflected at large angles. Even when they moved the detector round to the front of the foil, on the same side that the alpha particles were coming from, some particles were detected, having been bounced back off the foil almost in the direction they came from. It was as unexpected as if you threw a brick at a sheet of wet tissue paper hung up on a line and it bounced back to hit you in the face.
Rutherford described it as ‘quite the most incredible event that has ever happened to me in my life’, but soon worked out what was going on. The atom must actually be composed of a very small, positively charged nucleus (a term first used in this context by Rutherford, in 1912), surrounded by a cloud of electrons. An alpha particle has eight thousand times as much mass as an individual electron, and most alpha particles in the experiment shoot through the electron clouds almost entirely unaffected. But alpha particles, like the nuclei of atoms, have positive charge, and if one happens to pass close by a nucleus it is deflected by a large angle, because like charges repel each other. On rare occasions, an alpha particle heading straight towards a nucleus is bounced back the way it came, because a nucleus of gold has 49 times the mass of an alpha particle and cannot be brushed aside.
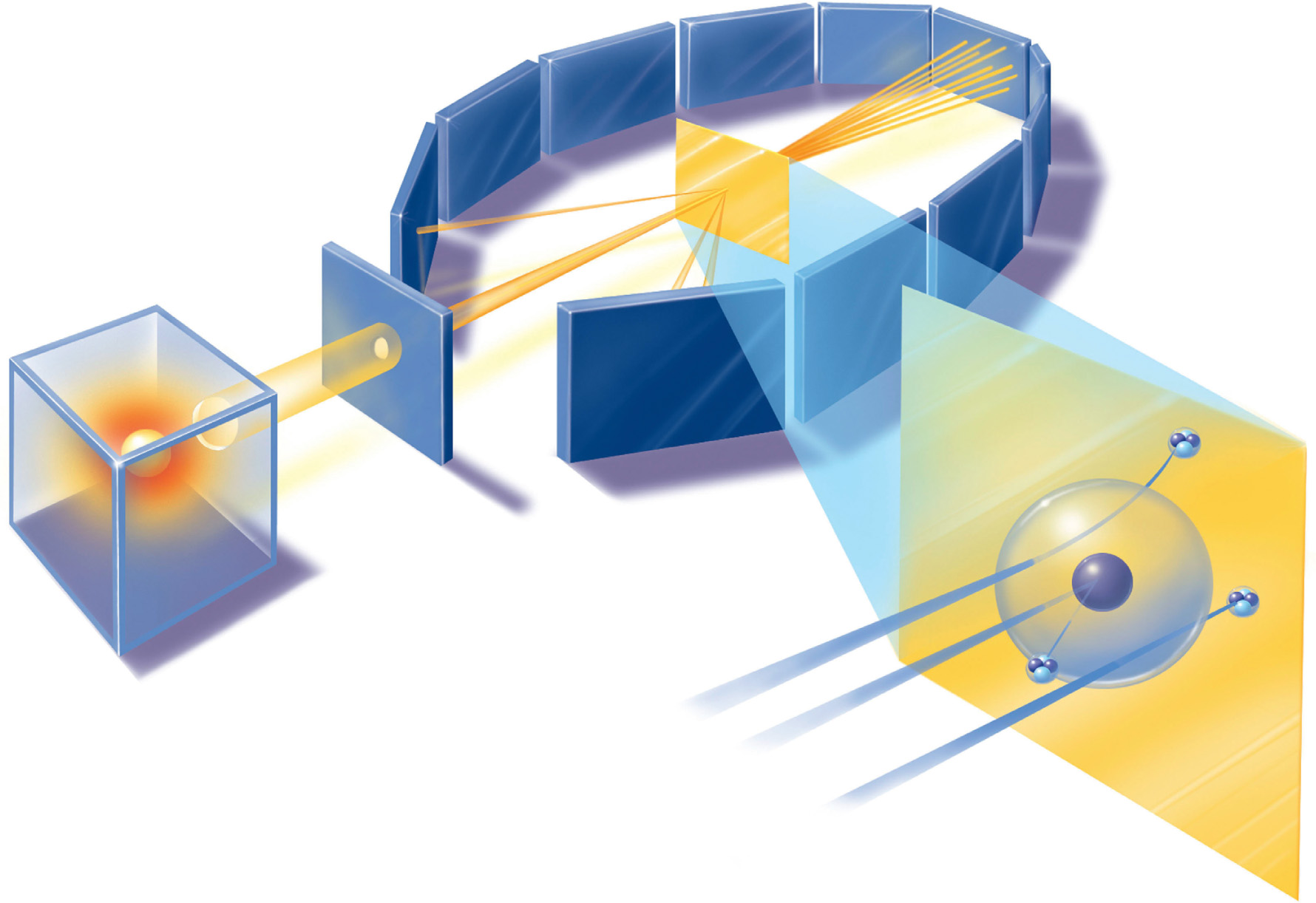
By counting the number of particles deflected at different angles in a long run of experiments and applying an appropriate statistical analysis, Rutherford was even able to work out roughly the size of the nucleus. Using more modern measurements, a typical nucleus 10–13 centimetres across is surrounded by a cloud of electrons 10–8 centimetres across. The proportions are roughly those of a grain of sand (the nucleus) placed in the centre of the Albert Hall (the electron cloud). Atoms, Rutherford had discovered, are mostly empty space, in particle terms, filled with a web of electric and magnetic fields linking tiny particles. The nucleus itself, later experiments showed, is made up of positively charged protons (hydrogen nuclei), one for each electron in the cloud of an atom, and electrically neutral particles similar to protons, called neutrons. Alpha particles are nuclei of helium, made up of two protons and two neutrons.