1 Introduction
Limited supply of oil, natural gas and coal, as well as the environmental impact, caused by the combustion of fossil hydrocarbons, like global warming and the acidification of the oceans, calls for a substitution of fossil feedstocks. In the field of green chemistry, biomass is seen as a promising renewable resource that can be converted into fuels or chemicals for further processing. However, chemical processes cannot simply be termed as “green” or “sustainable”, because of the use of biomass (Gustafsson and Börjesson 2007). Environmental impacts induced by the consumption of (metal-based) catalysts, energy and other auxiliaries have to be assessed as well (Benavides et al. 2016). It is important to detect whether burdens of the process itself are reduced or simply shifted. Thus, for a holistic overview of environmental impacts of chemical processes, a Life Cycle Assessment (LCA) is recommended (Burgess and Brennan 2000; Tufvesson et al. 2013).
This study uses LCA methods and process simulation for a first estimation of environmental impacts of an innovative process on an early development stage based on current literature. This gives the chance to support further development, highlight chances to improve the environmental performance and evaluate different scenarios for the implementation of the process into industrial practice (Azapagic 1999). For a case study, the OxFA-process is applied as a typical representative for a process using biogenic feedstock, which aims at substituting a conventional process based on fossil resources.

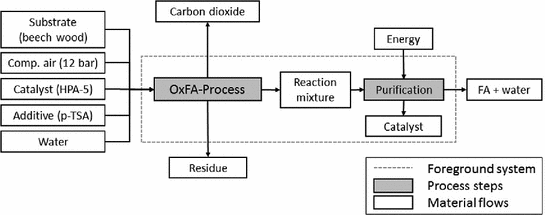
Simplified flowsheet of the OxFA-Process
A Keggin-type heteropoly acid with the molecular formula H8PV5Mo7O40 (HPA-5) currently shows the best catalytic performance in terms of activity and selectivity to formic acid (Albert 2014; Reichert and Albert 2017). The reaction proceeds in two steps: First, the homogeneous catalyst oxidizes the substrate. Thereby the catalyst is reduced. Afterwards, the catalyst has to be reoxidized using molecular oxygen at the lab-scale.
To avoid a reaction rate reduction, the reoxidation of the catalyst has to be faster than the oxidation of the biomass. The former is influenced by the partial pressure of oxygen in the reactor, while the latter is governed by the chemical structure of the substrate. In the literature an oxygen pressure of 20 bar is suggested (Albert and Wasserscheid 2015). The use of complex biomass results in a slower reaction rate, thus a system pressure of 12 bar is assumed to be sufficient. On industrial scale, compressed air is used instead of pure oxygen, because of economic and ecological reason.
After the reaction, the reaction mixture is purified to a saleable product via distillation. The formation of a high-boiling azeotrope, close-boiling components and the thermal sensitivity of formic acid (FA) complicate the separation via rectification. First, a water rich phase is removed as low boiler overhead until the desired concentration of FA is attained. In a second step, the product is separated from the residue and the catalyst, which remain in the bottom and will be recycled subsequently.
2 Materials and Methods
Goal and Scope: The goal of this study is providing support for development of the OxFA-Process by identifying significant parameters that influence the environmental performance. Therefore the target audience are scientists improving this process. An attributional LCI modelling framework is used.
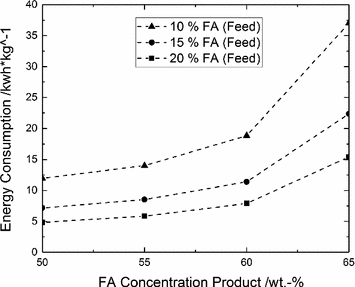
Energy consumption of the purification depending on the feed and product concentration
Life Cycle Inventory: Relevant data for the LCI are the amount of consumed oxygen and substrate, the amount of residue in the case of incomplete conversion, as well as the loss of additive and catalyst. Furthermore, the energy consumption of the purification step is taken into account. The required equipment and emissions of the OxFA-process itself into air, water and ground are not included in this inventory, because at the current development stage their uncertainty is too high.
Biomass and oxygen consumption are calculated according to the conversion and structural composition of the substrate biomass. Therefore it is assumed that the residue after the reaction has the same composition as the substrate and that oxygen is consumed in a stoichiometric ratio. Accordingly, a high specific yield of formic acid results in lower substrate demand per formed kg formic acid and consequently less residue. The selectivity of the formic acid formation influences the oxygen consumption, because the less carbon dioxide is formed the less oxygen is stoichiometrically consumed with regard to the functional unit of 1 kg FA. From the wide range of possible biomass feedstocks, beech wood from sustainable forestry was chosen as substrate in this study. With respect to the carbon atoms, the yield of formic acid is 29% (Albert 2014). In the literature, no details regarding the loss of catalyst and additive are provided. Therefore, the assumption is made that 10 g of additive and 1 g catalyst are consumed per kilogram of formic acid. This assumption has to be checked in a long-time study.
The catalyst is synthesized from phosphoric acid, hydrogen peroxide, molybdenum(VI)-oxide and vanadium(V)-oxide (Odyakov and Zhizhina 2008). Vanadium(V)-oxide is not included in the ecoinvent-database. For this reason a dataset for the production of vanadium(V)-oxide from vanadium-slag by the roast-leach-process was created. Vanadium-slag, a by-product of iron mining, is converted to vanadium(V)-oxide with sodium carbonate and ammonium sulfate (Goso et al. 2016; Nkosi 2017). The allocation of the environmental impacts of iron mining is done by mass, because the vanadium price is subject to strong fluctuations (Mokalyk and Alfantazi 2013).
3 Results and Discussion
Inventory data of the OxFA-process related to1 kg FA
Component | Amount/kg FA |
|---|---|
Substrate (beech wood) | 2.03 kg |
Residue | 0.96 kg |
Comp. air (12 bar) | 3.87 kg |
Catalyst (HPA-5) | 0.001 g |
Additive (p-TSA) | 0.01 kg |
Energy | 11.4 kWh |
Concerning energy supply, three different scenarios are evaluated. In the scenario “Market”, electricity is received from the German power market, while the scenario “PV” makes use of electricity from a photovoltaic system.
In addition, the utilization of thermal energy from a cogeneration unit is investigated in the scenario “Heat”. Furthermore a transport distance of 50 km for the feedstock is taken into account. The idea of the latter scenario is the centralized processing at an industrial area with existing heat network, while the first two scenarios represent the idea of a decentralized biomass processing at a farm (Albert et al. 2016).
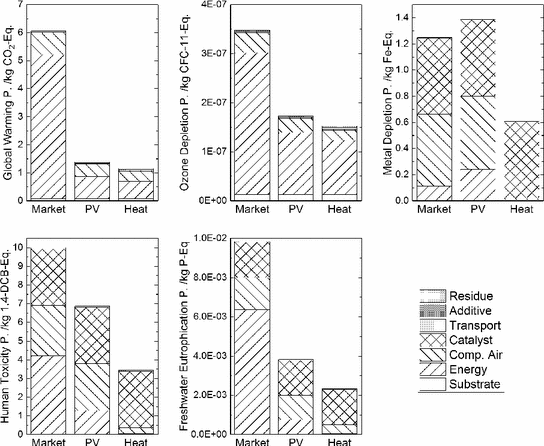
Midpoint indicator results for the three scenarios “Market”, “PV” and “Heat”
Catalyst synthesis and consumption, compressed air production and energy consumption for the purification step are the key contributors to the environmental footprint according to this evaluation. Therefore, process improvements concerning these elements will have the largest benefits. Additive and substrate consumption as well as the treatment of residue are of little relevance. The same results for biomass transport in the scenario “Heat”. However, changing the energy source can significantly reduce the environmental impact (Fig. 3).
Electricity and heat consumption for the purification step and compressed air production predominantly contribute to GWP. Emissions from lignite- and coal-fired power stations are main contributors to the GDP of the scenario “Market”. A similar result is found for the Ozone Depletion Potential, which is by far a factor of two higher for the “Market” scenario compared to “PV” and “Heat”.
Compressed air production strongly contributes to the Metal Depletion Potential in the decentralized scenarios “Market” and “PV”, but not in the scenarios “Heat”. This contribution is caused by the wear of the compressor. In the centralized scenario “Heat” a compressor with a higher capacity is used, which reduces the wear in relation to the amount of compressed air (Steiner and Frischknecht 2007). Molybdenum and Vanadium, which are used for the synthesis of the catalyst also contribute to the MDP. In a similar way, they affect Human Toxicity Potential. In particular mining operations contribute to negative effects on human health and freshwater eutrophication.
In the next step, the potential environmental impacts of the three scenarios of the OxFA-process are compared with the conventional process for the production of formic acid via the methyl formate route. The feedstock for this process is carbon monoxide and water (Reutemann and Kieczka 2005). Because carbon monoxide is mainly produced from coal or gas, this process depends on fossil resources. Inventory data is taken from Sutter (2007). Not included in the evaluation of the reference process is the compression of the gases to 45 bar and the consumption of the typically employed catalyst (sodium methoxide). Both factors which show a significant influence on the environmental balance of the OxFA-process. However, a formic acid concentration of up to 98 wt% is reached.
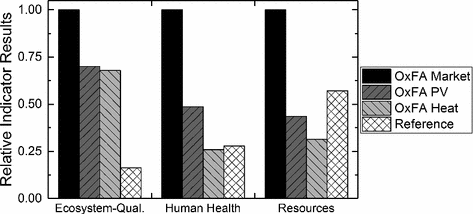
Comparison of the three OxFA-scenarios and the methyl formate route at the endpoint level
Regarding ecosystem-quality, the impacts of the different OxFA-process scenarios are significantly higher compared to those of the reference process. The ecosystem-quality is mainly affected by provision of the substrate (beech wood), which requires a large area for cultivation. Therefore, using waste biomass for the OxFA-process, would reduce the environmental impact significantly. However, waste biomass is only available in a limited amount and increased competition for its use also has to be considered.
Impacts on human health of the scenario “Heat” are comparable with those of the reference process, while the impact on human health of the other scenarios is somewhat higher. Thus, it has to be concluded that the competitiveness of the OxFA-process depends on the energy source chosen. The same applies for resource depletion. Using heat or solar power reduces resource consumption. Under these circumstances, the OxFA-process shows a better performance than the state-of-the-art process. Suggested improvements of the OxFA-process, viz. the in situ extraction of FA with 1-hexanol can further reduce the energy consumption and enable the production of highly concentrated formic acid (Reichert et al. 2015). Another potential improvement is the heterogenization of the homogeneous HPA-5 catalyst, which is currently under investigation.
4 Conclusions
In this study, an evaluation of the environmental footprint of a chemical process at an early stage of development was attempted. Therefore, assumptions had to be made regarding the consumption of auxiliaries like additive and catalyst. The amount of oxygen used had to be calculated based on the assumed stoichiometry laws and, thus, describes the technical optimum, while emissions of the OxFA-process itself were not taken into consideration. Nevertheless, a first estimation with respect to the key contributions to the environmental footprint was made. In our case, the catalyst preparation, energy consumption and compressed air production are the most significant contributors. Improvements which focus on these aspects seem to be the most promising approaches for further development. Finally, it can be assumed that the OxFA-process can compete with the state-of-the-art technology, when the energy supply is based on renewable resources such as photovoltaics or heat cogeneration.