Chapter 11
Prostate Brachytherapy
Overview
- Prostate brachytherapy is a form of targeted radiotherapy
- Brachytherapy offers a day-case radical treatment option for localised prostate cancer with a favourable morbidity profile
- Data beyond 12 years demonstrate the efficacy of prostate brachytherapy
- Modern techniques allow patients with large prostates or prior transurethral resection of the prostate to be treated
- Brachytherapy may be combined with external beam radiotherapy for higher risk patients, and may be used as a salvage treatment option after failed radiotherapy
Brachytherapy is a form of radiotherapy. Rather than apply the energy in the form of X-rays from outside the body as in external beam radiotherapy (EBRT), radioactive sources that emit gamma rays are placed directly in, or in close proximity to the prostate gland. Data from several longitudinal studies inform us that oncological control is directly dependent on prescribed dose. Doses from EBRT, however, are limited by toxicity to the surrounding organs at risk (bladder, urethra and rectum)—also related to dose. The inverse square law governs the dose from a brachytherapy source such that there is a rapid fall-off of dose a short distance from the origin. Multiple sources, each with a ‘cloud’ of radiation, can be placed in such a configuration so as to provide a high dose to the prostate whilst minimising dose, and toxicity, to the surrounding structures.
Prostate brachytherapy sources may be permanent in the form of seeds, or temporary (also known as high dose rate) with iridium wires. Temporary prostate brachytherapy is currently considered developmental. The seeds used in permanent low dose rate implants consist of titanium capsules, 4.5 by 0.8 mm, which encapsulate the radioactive sources. Palladium-123, Caesium-131 and Iodine-125 isotopes are all used although none have been shown to be superior in terms of either toxicity or disease control. For practical purposes, Iodine-125 is used in the UK. Iodine-125 has a half-life of approximately 60 days and so an implant is considered active for ten months, or five half-lives. A typical Iodine-125 implant with a prescribed dose of 145 Gy will deliver the biological equivalent of up to 90 Gy of EBRT. Approximately 70 sources are used for a 40 cm3 gland.
Technique development
The development of trans-rectal ultrasound in the early 1980s allowed accurate placement of brachytherapy sources via the trans-perineal route under image guidance. The early technique was developed in Seattle as a two-stage procedure. At first, the patient is placed in a lithotomy position and images of the prostate, urethra and rectum are captured. Those images are then used to plan the implant in relation to a brachytherapy grid placed over the perineum. At a later date, the patient returns for the implant: under anaesthetic the patient's position is replicated as accurately as possible to match the pre-plan study; the sources, usually on strands, are placed according to the plan using preloaded needles inserted through the grid (Figure 11.1). A post-operative CT scan is commonly utilised in order to detect the seed positions in relation to the prostate and assess the dosimetry.
Figure 11.1 Needles are placed transperineally through a brachytherapy grid, parallel to a trans-rectal ultrasound probe and under image guidance. Aerosolized gel helps visualize the urethra. The patient is in an extended lithotomy position.

The technique has evolved to a single-stage procedure with the use of nomograms and subsequent computerised planning systems that avoid issues with reproducing the pre-plan position. An inverse technique refers to the placement of needles before planning so that difficulties in reaching areas of the prostate (such as behind the pubic arch) can be overcome by adjusting the position intraoperatively. Sources are placed individually in an ‘after-loaded’ manner. Modern techniques, known as dynamic dose-feedback, not only use computer software to create the inverse plan intraoperatively, but also record each seed position as it is placed in real time, and update the dosimetry accordingly (Figures 11.2 and 11.3). This allows on-table adjustments to be made before the patient leaves the operating room.
Figure 11.2 Three-dimensional representation of implant. The prostate is in red, urethra, green, and rectum, blue. The discrete green cylinders are radioactive seeds.
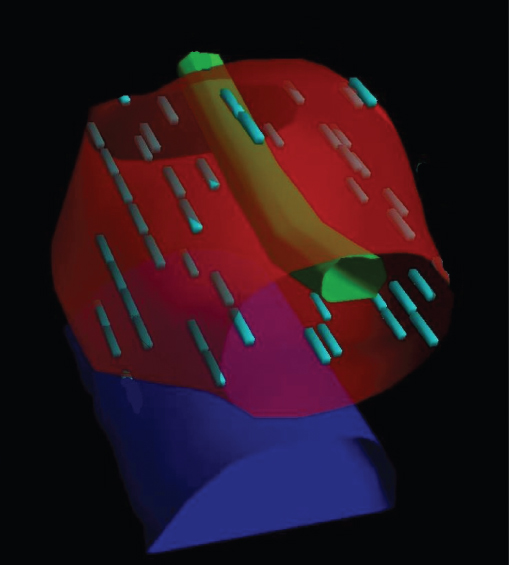
Figure 11.3 Plain radiograph of pelvis with implant in situ.

Side effects
The treatment is usually carried out as a day-case procedure and patients report a rapid return to usual daily activity. The implant is radioactive and bearing this in mind, men are asked to avoid prolonged contact with pregnant women and small children for a period of two months. Similarly, seeds may be emitted in the semen with ejaculation and so a condom is recommended for the first three emissions.
A degree of urinary symptoms occur in the majority of men: initially there may be a reduction of flow due to swelling of the gland during the procedure. Retention rates are low (approximately 5%) and usually recover after a short period of catheterisation. In men who require catheterisation for longer (1%), definitive therapy (such as transurethral resection) should be delayed for a year in order to allow the implant to treat the cancer and for resolution of radiation-associated symptoms. During this time, men may be managed with either intermittent self-catheterisation or a supra-pubic catheter.
Radiation effects cause irritative urinary symptoms that peak at four to six weeks, but usually settle to baseline by nine months (Figure 11.4). Alpha-blockers are routinely prescribed and anticholinergics along with fluid advice are often helpful. Radiation effects on the bowel may cause diarrhoea in 5% of men and, rarely, bleeding occurs; this is self-limiting. Erectile function is commonly preserved following brachytherapy implant, at least for the first few years, with or without the use of phosphodiesterase-5 inhibitors.
Cancer control
Oncological surveillance is managed by serial PSA measurements. As with EBRT, the PSA can take up to two years and sometimes longer to fall to its nadir level. One-third of patients experience a ‘PSA bounce’, whereby the PSA rises before falling again towards its nadir. The dosimetry review, either by post-operative CT images or on-table dynamic assessment, will ensure satisfactory coverage. The D90 refers to the minimum dose that 90% of the prostate receives; a D90 of more than 130Gy has been associated with improved outcomes. Data up to 12 years have shown biochemical control in the order of 90% of patients with low risk disease. A cohort study concerning men with T1 to T2 disease treated without hormones, compared radical prostatectomy (n = 746), EBRT to a median dose of 74 Gy (n = 340) and permanent prostate brachytherapy (n = 733) demonstrated similar seven-year freedom from biochemical recurrence results of 74–79% (statistically insignificant differences).
Patient assessment
As with any treatment for prostate cancer, patient selection is important. NICE guidelines recommend prostate brachytherapy for low and intermediate risk localised disease. Higher risk patients may be treated with a combination of brachytherapy and EBRT: this allows maximum dose to the prostate as well as treatment doses to a margin (in case of extracapsular extension) while still protecting surrounding tissues. Transperineal sector prostate biopsies are particularly useful for disease stratification when planning treatment options. Since the patient position is similar to that of the implant, the accessibility of the gland can be ascertained. The co-ordinates of higher grade or volume of disease can be recorded and these areas targeted with extra dose during the implant procedure.
Urinary symptom score and uroflowmetry are important aspects of pre-brachytherapy assessment. High symptom scores and poor flow are predictors of urinary morbidity following the implant. Patients with equivocal flow rates ought to undergo further urodynamic evaluation. Men with low-risk and low-volume disease who would otherwise be suitable for active surveillance but who have bladder outflow obstruction secondary to prostatic disease may be managed by transurethral resection of the prostate followed by implant six months later. The surgery is best carried out by a urologist familiar with brachytherapy implants.
In the past, large prostate sizes were considered a relative contraindication to brachytherapy implant due to the increased urinary symptoms following the procedure and difficulties with access to the anterior part of the gland (Figure 11.4). Downsizing has been achieved with LHRH analogues prior to implant, but with associated morbidity. Inverse plan techniques overcome pubic arch interference, however, and providing there are no signs of bladder outflow obstruction, prostate glands larger than 50 cm3 may be implanted with satisfactory dosimetric and urinary outcomes.
Figure 11.4 Urinary symptoms following prostate brachytherapy implant: mean international prostate symptom scores.

Similarly, patients with prior bladder outflow surgery were previously considered poor candidates for implant due to historical data reporting incontinence in these men and difficulties in achieving dose coverage. Modern techniques and equipment, however, allow more targeted placement of seeds so that a history of transurethral resection of the prostate need not be a contraindication, providing there is an adequate rim of prostate tissue to implant.
More recently, the indications for brachytherapy have extended to its use as a salvage treatment for radiotherapy failure with promising results. As our understanding of radiation biology evolves and imaging and targeting of dose continue to improve, the indications for and results of this treatment will likely expand. At present, prostate brachytherapy offers a radical treatment option with relatively low morbidity and the convenience of a day-case procedure. Table 11.1 presents a comparison of radical treatments for localised prostate cancer.
Table 11.1 Comparison of radical treatments for localised prostate cancer.

Further reading
National Institute for Health and Clinical Excellence. Prostate Cancer: Diagnosis and Treatment. London: NICE, 2008 (www.nice.org.uk/CG058).
Potters L, Calugaru E, Jassal A, Presser J. Is there a role for postimplant dosimetry after real-time dynamic permanent prostate brachytherapy? Int J Radiat Oncol Biol Phys 2006;65(4):1014–19.
Potters L, Klein EA, Kattan MW, Reddy CA, Ciezki JP, Reuther AM, Kupelian PA. Monotherapy for stage T1-T2 prostate cancer: Radical prostatectomy, external beam radiotherapy, or permanent seed implantation. Radiother Oncol 2004;71(1):29–33.
Prostate Brachytherapy Advisory Group UK and Ireland. (www.prostatebrachytherapyinfo.net).
Stock RG, Stone NN, Cesaretti JA, Rosenstein BS. Biologically effective dose values for prostate brachytherapy: Effects on PSA failure and posttreatment biopsy results. Int J Radiat Oncol Biol Phys 2006;64(2):527–33.