Chapter 15
Recent Advances in Hormonal Therapy
Overview
- Hormonal therapy plays a key role in the treatment of prostate cancer
- Castration can be achieved with surgical orchidectomy or medical therapy with LHRHagonists or GnRH antagonists
- Castration-based therapy remains first line and mainstay treatment for men with advanced disease. It relieves symptoms and delays progression
- After a variable period of time, tumour growth can become resistant to testosterone depletion and other hormone manipulations can be added sequentially. These currently include anti-androgens, oestrogens and steroids
- New hormone therapies are under investigation and are showing great promise for castration-resistant prostate cancer. These include abiraterone acetate and MDV3100
Hormonal therapy plays a key role in the treatment of prostate cancer. Huggins and Hodges first recognised the role of testosterone in stimulating prostate cancer cells in the early 1940s. Treatments aimed at lowering the serum testosterone then rapidly became established as effective management of advanced (metastatic) prostate cancer by reducing symptoms and slowing disease progression.
Since these early beginnings, there have been many advances in the hormone treatment of prostate cancer. Hormonal therapy now has an important role in the management of earlier stages of the disease, including its neoadjuvant and adjuvant combination with radical radiotherapy. New pharmacological approaches for targeting endocrine signalling pathways are in development.
In the early days, endocrine treatment of prostate cancer required the surgical removal of the testes, bilateral orchidectomy, thereby depleting circulating testosterone. Some 95% of serum testosterone is produced by the testes, without which low levels of androgens are maintained by the adrenal glands.
The first bilateral orchidectomy was performed in 1941 and still remains the gold standard against which all other hormonal therapies are judged. This surgical operation produces a rapid and sustained reduction in circulating testosterone within 12 hours. Although a very effective therapy, bilateral orchidectomy is non-reversible and can have an adverse psychological impact on many men.
The development of ‘medical castration’ using luteinising hormone-releasing hormone agonists (LHRHagonists) was a major breakthrough in the management of prostate cancer, introduced into clinical practice in the 1980s. Endogenous LHRH is produced by the neuroendocrine cells of the hypothalamus and causes the anterior pituitary to release luteinising hormone (LH) which in turn acts upon the Leydig cells of the testes, stimulating testosterone synthesis (Figure 15.1). Randomised studies have established that LHRHagonists are as effective as surgical castration, giving patients an important choice in the modality of their treatment.
Figure 15.1 The hypothalamic-pituitary-gonadal axis.
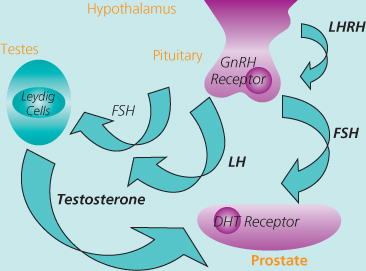
The initial effect of treatment with LHRHagonist is a rise in serum testosterone. With ongoing or continuous treatment, the pituitary becomes depleted of LH and consequently testosterone falls to castrate levels by two weeks. This initial testosterone surge may theoretically result in a tumour flare. Until castrate levels are reached, the effect of testosterone can be dampened by preloading an anti-androgen (i.e. an androgen receptor blocker) prior to the first LHRHa injection, and continued for one to two weeks afterwards.
A recent development in castration-based therapy has been the introduction of the gonadotrophin releasing hormone (GnRH) antagonists. These drugs bind competitively to GnRH receptors and produce a direct and rapid decline of LH, FSH and testosterone. When initiating treatment, there is no stimulation of GnRH, and therefore no testosterone surge and no clinical flare, so concurrent anti-androgen treatment is not required.
Anti-androgens such as bicalutamide and flutamide block the androgen receptor but not the production of testosterone. They have been shown to be equally effective as LHRHagonists injections for the treatment of locally advanced disease and have a different side effect profile.
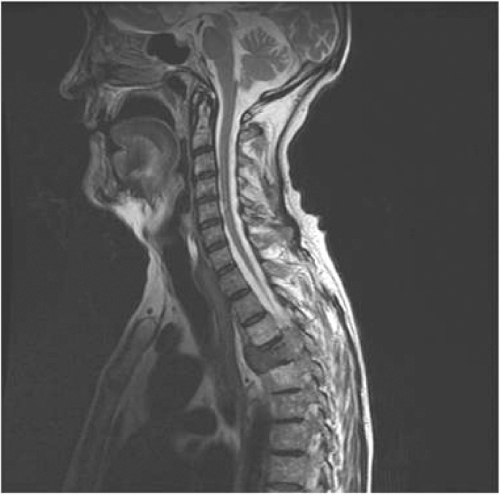
Hormonal manipulation with castration-based therapy remains first line and mainstay treatment for men with advanced (metastatic) disease. It relieves symptoms and delays progression. Response rates of over 85% can be expected for up to three years. The Medical Research Council (1997) study of immediate versus delayed hormonal therapy for metastatic prostate cancer (Figure 15.2) showed advantages for early treatment. Men treated with delayed hormones had significantly higher rates of spinal cord compression (Figure 15.3), pathological fracture, ureteric obstruction and extra-skeletal metastases. These findings support the introduction of early hormonal therapy prior to symptomatic progression.
Figure 15.2 Bone scan showing bone metastases from prostate cancer. Source: Courtesy of Mr. John Anderson, Consultant Urologist, Royal Hallamshire Hospital, Sheffield.
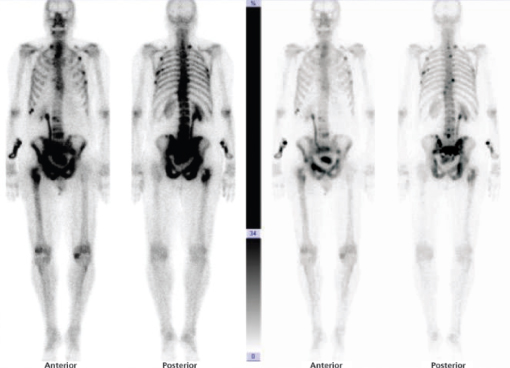
Figure 15.3 MRI scan showing spinal cord compression.
After a variable period of time, tumour growth can become resistant to testosterone depletion. The addition of an anti-androgen to achieve combined androgen blockade (CAB) blocks the effect of residual testosterone on the androgen receptor with response rates of up to 30–50% for an average duration of six months. Subsequent withdrawal of the anti-androgen after relapse on CAB can result in further responses of 20–30%, with an overall duration of four to five months.
Other hormonal manipulations may be administered as sequential third and fourth line therapies. Diethylstilboestrol and or glucocorticoids can sometimes achieve further remission for up to six months or longer. Oestrogen therapy is associated with gynaecomastia, fluid retention and thrombo-embolism. Cardiovascular events can be prevented with aspirin or anticoagulants. By these means, various endocrine signalling pathways can be targeted to delay progression of advanced prostate cancer, indicating hormone-dependent activity in spite of low levels of circulating androgen.
There are many new hormone compounds under investigation which are showing great promise for the future. Tumour resistance to testosterone depletion develops in the course of malignant progression through phenotypic selection of the most aggressive cells. In spite of their so-called hormone resistance to androgen deprivation therapy, prostate cancer often continues to be androgen-driven. High concentration of androgens can be demonstrated in tumour tissue in spite of castrate levels of serum testosterone, indicating androgen synthesis within the tumour.
Abiraterone acetate is a potent, selective inhibitor of CYP17, key enzymes in androgen synthesis, and completely blocks the production of testosterone. Clinical trials have shown encouraging results in men with castrate-resistant prostate cancer (CRPC), with up to 67% demonstrating a PSA response.
It is also known that the androgen receptor may be activated despite very low levels of testosterone. Prostate cancer progression is associated with androgen receptor amplification or over-expression, and mutation; either or both of these mechanisms may increase tumour sensitivity to androgens and anti-androgens. The functional activity of the testosterone-bound androgen receptor is also regulated by binding of co-activators and co-pressors. In advanced malignancy, androgen receptor activity may be enhanced through altered availability of these co-factors and abnormal intracellular signalling. Mechanisms also exist for androgen independent androgen receptor activation.
MDV3100 is a new oral androgen receptor antagonist that inhibits its nuclear translocation and blocks its binding with DNA. This drug has shown great promise in trials of men with CRPC, and PSA responses of 55% have been seen in chemotherapy naïve patients and 36% in those previously treated with docetaxel. These two new compounds, Abiraterone and MDV3100, are currently being investigated in large randomised Phase III studies and the results of these trials are awaited with great interest.
Neoadjuvant and adjuvant hormonal therapy
Hormonal therapy has evolved to play a critical role in the management of prostate cancer that is locally advanced or clinically localised but so-called ‘high risk’ (Gleason Grade ≥ 8, PSA ≥ 20), as combination treatment with radical radiotherapy. In these patients the therapeutic challenges reflect the need to control local disease as well as subclinical microscopic distant metastases. Traditional treatment with external beam radiotherapy alone frequently fails to prevent distant progression, despite improvements in radiotherapy dose and delivery, owing to undetectable micro-metastatic disease at diagnosis. There is a wealth of data to support the addition of systemic hormonal therapy which is now considered standard of care for men with high risk prostate cancer.
Neoadjuvant hormonal therapy, (with a LHRHagonist) is commonly used for two to three months prior to definitive radiotherapy This reduces the size of the prostate by an average of 25–30% which potentially allows smaller fields of radiotherapy to be used with some sparing of the rectum and bladder volumes and potentially reduced toxicity. There are reports that there may also be a sensitising effect between hormonal therapy and radiation treatment. This combination has been shown to demonstrate a significant improvement in disease-free survival in clinical studies.
There is strong supporting evidence for continued adjuvant hormone treatment after radical radiotherapy. A study by Bolla et al. (2008) reported that, in patients with locally advanced prostate cancer, LHRHagonist (goserelin) therapy initiated with radiotherapy and continued for three years improved overall survival compared with hormonal therapy deferred until disease progression. A recent update of this trial reported an 18.3% improvement in 10-year overall survival (58.1% vs 39.8% respectively). There were similar significant improvements for progression free survival and clinical or PSA relapse. Other randomised Phase III studies of adjuvant LHRHagonists have demonstrated similar benefit, and adjuvant antiandrogen therapy with bicalutamide 150 mg has also been shown to improve survival. It is now standard practice for hormone treatment to be continued for two to three years after radiotherapy.
Side-effects of hormonal therapy
The main toxicities of hormone treatment with LHRHagonists are shown in Table 15.1. These side-effects need to be balanced with the benefits of therapy as described above. The potential risks of treatment should be discussed with all men embarking on hormonal therapy for early detection of toxicities and intervention. The anti-androgens (e.g. bicalutamide 150 mg) have some advantages over castration-based therapy in that they can maintain physical capacity and bone mineral density (through aromatisation of available testosterone to oestrogen in bone) with lesser risk of hot flushes and loss of sexual function. However, they carry a greater risk of gynaecomastia and mastalgia. The different side-effect profiles of these two types of hormonal therapy can allow clinicians and patients to choose the best approach to maintain quality of life while assuring effective oncological therapy for the individual.
Table 15.1 Side-effects of hormonal therapy.
| Erectile dysfunction |
| Reduced libido |
| Osteopaenia/osteoporosis |
| Reduced muscle mass |
| Breast swelling and mastalgia |
| Weight gain |
| Hot flushes |
| Lethargy |
| Anaemia |
| Mood swings and depression |
| Metabolic complications, e.g. insulin resistance, hypertension and alterations in lipid levels |
Hormonal therapy has remained a gold standard in the management of prostate cancer for more than 60 years. This has evolved from surgical to medical castration with the introduction of the LHRH agonists and more recently GnRH antagonists. Through advances in the use of hormonal therapy, treatment intent has evolved from purely palliative in advanced (metastatic) prostate cancer to now potentially curative by combination with radiotherapy in locally advanced disease. The therapeutic possibilities are likely to increase in the near future as we await the results of clinical trials of new and promising hormone therapies.
References
Bolla M, Collette L, Van Tienhoven G et al. Ten year results of long term adjuvant androgen deprivation with goserelin in patients with locally advanced prostate cancer treated with radiotherapy: A phase III EORTC study. Int J Radiat Oncol Biol Phys 2008;72(1 Suppl1): S30-S31.
The Medical Research Council Prostate Cancer Working Party Investigators Group Immediate versus deferred treatment for advanced prostatic cancer: initial results of the Medical Research Council Trial. Br J Urol 1997;79:235–46.
Further reading
Attard G et al. Selective inhibition of CYP17 with arbiraterone acetate is highly active in castrate resistant prostate cancer. J Clin Oncol 2009;27(23):3742–8. Epub 2009 May 26.
McLeod DG, Iversen P, See WA, Morris T, Armstrong J, Wirth M, on behalf of the Casodex Early Prostate Cancer Trialists' Group. Bicalutamide 150mg plus standard care vs standard care alone for early prostate cancer. BJU International 2005;97:247–54.
Pilepich MV, Winter K, Lawton CA, Krisch RE, Wolkov HB, Movsas B et al. A ndrogen suppression adjuvant to definitive radiotherapy in prostate carcinoma—long-term results of phase III RTOG 85-31. Int J Radiat Oncol Biol Phys 2005;61(5):1285–90.