CHAPTER 3
IGNITERS
0201. SUGAR-CHLORATE
a. Description.
- This item consists of a mixture of granulated sugar and potassium chlorate or sodium chlorate. It can be used to ignite all the incendiaries listed in chapter 4 except Thermite (0307). It may be used directly as an incendiary on readily flammable material such as rags, dry paper, dry hay, or in the combustible vapor above liquid fuels.
- The igniter can be initiated by Fuse Cord (0101), Improvised String Fuse (0102), or Concentrated Sulfuric Acid (0103).
- This simple sugar-chlorate mixture closely resembles granulated sugar and should not ordinarily arouse suspicion. It is an excellent igniter.
Caution: This mixture is poisonous and must not be eaten.
b. Material and Equipment.
Granulated sugar (do not use powdered or confectioners sugar.)
Potassium chlorate or sodium chlorate (no coarser than granulated sugar).
Spoon (preferably nonmetallic).
Container with tight-fitting lid.
Rolling pin or round stick.
c. Preparation.
- Using a clean, dry spoon, place granulated sugar in the container to one-quarter container volume. Wipe the spoon with a clean cloth.
- If the potassium or sodium chlorate is lumpy, remove all lumps by crushing with a rolling pin. Using the spoon, add an equal quantity of chlorate to the container.
Caution: If this mixture is carelessly handled with excessive bumping and scraping, It could be a fire hazard to the user. As a precaution, remove any mixture adhering to the lip or edge of the jar before tightening the lid.
- Tighten the lid of the jar, turn the jar on its side and slowly roll until the two powders are completely mixed. The mixture is now ready for use. It may be stored for months in a tightly sealed container.
d. Application.
- Carefully pour or spoon the mixture, in a single pile, on the incendiary. Prepare the mixture for ignition with Fuse Cord (0101) or Improvised String Fuse (0102) in the normal manner. The fuse cord should terminate near the center of the igniter mixture. Concentrated Sulfuric Acid (0103) can be used as an initiator, but is generally less convenient. Ignition takes place almost immediately on contact with the acid. Acid is recommended for use with specific delay mechanisms found in chapter 5.
- If only battery-grade sulfuric acid is available, it must be concentrated before use to a specific gravity of 1.835 by heating it in an enameled, heat-resistant glass or porcelain pot until dense, white fumes start to appear. See paragraph 0103 for details.
- When used to ignite flammable liquids, wrap a quantity of the mixture in a nonabsorbent material and suspend it inside the container near the open top. The container must remain open for easy ignition and combustion of the flammable liquid.
- To minimize the hazard of premature ignition of flammable liquid vapors, allow at least two feet of fuse length to extend from the top edge of an open container of flammable liquid before lighting the fuse.
0202. FIRE FUDGE
a. Description.
- This item consists of a mixture of sugar and potassium chlorate in a hot water solution which solidifies when cooled to room temperature. It can be used to ignite all the incendiaries listed in chapter 4 except Thermite (0307). It may be used directly as an incendiary on readily flammable material, such as rags, dry paper, dry hay, or in the combustible vapor above liquid fuels.
- The igniter can be initiated by Fuse Cord (0101), Improvised String Fuse (0102), or Concentrated Sulfuric Acid (0103).
- Fire fudge resembles a white sugar fudge having a smooth, hard surface. The advantage of this igniter material over Sugar-Chlorate (0201), is its moldability. The procedure for preparation must be followed closely to obtain a smooth, uniform material with a hard surface.
Caution: This material is poisonous and must not be eaten.
b. Material and Equipment.
Granulated sugar (do not use powdered or confectioners sugar).
Potassium chlorate (no coarser than granulated sugar) .
Metallic, glass or enameled pan.
Measuring container (size of this container determines quantity of finished product).
Spoon (preferably nonmetallic).
Thermometer (to read in the range 200° F. to 250° F.)
Heat source.
c. Preparation.
- Clean the pan by boiling some clean water in it for about five minutes. Discard the water, pour one measureful of clean water into the pan and warm it. Dry the measuring container and add one measureful of sugar. Stir the liquid until the sugar dissolves.
- Boil the solution until a fairly thick syrup is obtained.
- Remove the pan from the source of heat to a distance of at least six feet and shut off heat. Rapidly add two measurefuls of potassium chlorate. Stir gently for a minute to mix the syrup and powder, then pour or spoon the mixture into appropriate molds. If the mold is paper, it can usually be peeled off when the fire fudge cools and hardens. Pieces of cardboard or paper adhering to the igniter will not impair its use. Pyrex glass or ceramic molds can be used when a clear, smooth surface if desired. It is recommended that section thickness of molded fire fudge be at least one-half inch. If desired, molded fire fudge can be safely broken with the fingers.
- This material is moderately hard immediately after cooling. It will become harder after 24 hours. When kept in a tightly sealed container, it will retain its effectiveness for months.
Caution: If this igniter material is carelessly handled with excessive bumping -or scraping, it could be a fire hazard to the user.
d. Application.
- Place a piece of fire fudge on top of the incendiary. Minimum size should be about one inch square and one-half inch thick Prepare the fire fudge for ignition with Fuse Cord (0101) or Improvised String Fuse (0102) in the normal manner. Concentrated Sulfuric Acid (0103) can be used as an initiator but is generally less convenient. Acid is recommended for use with specific delay mechanisms found in chapter 5.
- If only battery-grade sulfuric acid is available it must be concentrated before use to a specific gravity of 1.835 by heating it in an enameled, heat resistant glass or porcelain pot until dense, white fumes start to appear. See paragraph 0103 for details.
- When used to ignite flammable liquids, wrap a quantity of the igniter mixture in a non-absorbent material and suspend it inside the container near the open top. The container must remain open for easy ignition and combustion of the flammable liquid.
- To minimize the hazard of premature ignition of flammable liquid vapors, allow at least two feet of fuse length to extend from the top edge of an open container of flammable liquid before lighting the fuse.
0203. SUGAR-SODIUM PEROXIDE
a. Description.
- This item consists of a mixture of sodium peroxide and granulated sugar. It can be used to ignite all the incendiaries listed in chapter 4 except Thermite (0307). It may be used directly as an incendiary on readily flammable material such as rags, dry paper dry hay, or in the combustible vapor above liquid fuels.
- The igniter can be initiated by Fuse Cord (0101), Improvised String Fuse (0102), Concentrated Sulfuric Acid (0103), or Water (0104).
Caution: This mixture is unstable and can ignite at high humidity or when wet slightly by drops of water, perspiration, etc.
b. Material and Equipment.
Granulated sugar (do not use powdered or confectioners sugar).
Sodium peroxide (no coarser than granulated sugar).
Spoon.
Container with tight fitting lid for mixing and storage.
c. Preparation.
- Using a clean, dry spoon, place granulated sugar in the container to one-quarter container volume.
- Wipe the spoon with a clean, dry cloth, and add an equal amount of sodium peroxide to the dry mixing container. Tighten the lid on the sodium peroxide container, and remove it at least six feet from the working area.
- Tighten the lid on the mixing container. Turn the container on its side and slowly roll until the two powders are completely mixed. The mixture is now ready for use.
- A good practice is to keep the granulated sugar and sodium peroxide in separate airtight containers and mix just before use.
Caution: Do not store this mixture longer than three days because decomposition may occur and cause spontaneous combustion. Be sure that the storage container is air-tight.
d. Application.
- Carefully pour or spoon the mixture, in a single pile, on the incendiary. Prepare the mixture for ignition with Fuse Cord (0101) or Improvised String Fuse (0102) in the normal manner. The fuse cord should terminate near the center of the igniter mixture. Concentrated Sulfuric Acid (0103) and Water (0104) can be used as initiators, but are generally less convenient. Ignition takes place almost immediately on contact with the acid or water. These liquid initiators are convenient for use with specific delay mechanisms found in chapter 5.
- When used to ignite flammable liquids, wrap a quantity of the mixture in a non-absorbent material and suspend it inside the container near the open top. The container must remain open for easy ignition and combustion of the flammable liquid.
- To minimize the hazard of premature ignition of flammable liquid vapors, allow at least two feet of fuse length to extend from the top of an open container of flammable liquid before lighting the fuse.
0204. ALUMINUM POWDER—SODIUM PEROXIDE
a. Description.
- This item consists of a mixture of sodium peroxide and powdered aluminum. It can be used to ignite all the incendiaries listed in chapter 4 except Thermite (0307). It may be used directly as an incendiary on readily flammable material, such as rags, dry paper, dry hay or in the combustible vapor above liquid fuels.
- The igniter can be initiated by Fuse Cord (0101), Improvised String Fuse (0102), Concentrated Sulfuric Acid (0103), or water (0104).
Caution: This mixture is unstable and can ignite at high humidity or when wet slightly by drops of water, perspiration, etc.
b. Material and Equipment.
Powdered aluminum (no coarser than granulated sugar).
Sodium peroxide no coarser than granulated sugar) .
Spoon.
Container with tight fitting lid for mixing and storage.
c. Preparation.
- Using a clean, dry spoon, place powdered aluminum in the container to one-quarter container volume.
- Wipe the spoon with a clean, dry cloth, and add an equal amount of sodium peroxide to the dry mixing container. Tighten the lid on the sodium peroxide container, and remove it at least six feet from the working area.
- Tighten the lid of the mixing container. Turn the container on its side and slowly roll until the two powders are completely mixed. The mixture is now ready to use.
- A good practice is to keep the powdered aluminum and sodium peroxide in separate containers and mix just before use.
Caution: Do not store this mixture longer than three days because decomposition may occur and cause spontaneous combustion. Be sure that the storage container is air-tight.
d. Application.
- Carefully pour or spoon the mixture, in a single pile, on the incendiary. Prepare the mixture for ignition with Fuse Cord (0101) or Improvised String Fuse (0102) in the normal manner. The fuse cord should terminate near the center of the igniter mixture. Concentrated Sulfuric Acid (0103) and Water (0104) can be used as initiators, but are generally less convenient. Ignition takes place almost immediately on contact with the acid or water. These liquid initiators are convenient for use with specific delay mechanisms found in (chapter 5.)
- When used to ignite flammable liquids, wrap a quantity of the mixture in a nonabsorbent material and suspend it inside the container near the open top. The container must remain open for easy ignition and combustion of the flammable liquid.
- To minimize the hazard of premature ignition of flammable liquid vapors, allow at least two feet of fuse length to extend from the top edge of an open container of flammable liquid before lighting the fuse.
0205. MATCH HEAD
a. Description.
- This item consists of a quantity of match heads, prepared by breaking the heads off their match sticks and grouping the match heads together to form the desired quantity of igniter. Any kind of friction match will do. It can be used to ignite the following incendiaries listed in chapter 4: Napalm (0301), Gelled Gasoline (exotic thickeners) (0302), Gelled Gasoline (improvised thickeners) (0303), Paraffin-Sawdust (0304), and Flammable Liquids (0308). It may be used directly as an incendiary on readily flammable material such as rags, dry paper, dry hay or in the combustible vapor above liquid fuels.
- The igniter can be initiated by a match flame, Fuse Cord (0101), Improvised String Fuse (0102), or Concentrated Sulfuric Acid (0103).
b. Material and Equipment.
Razor blade or knife.
Container with tight-fitting lid.
Matches, friction.
c. Preparation.
- Using a knife or razor blade, cut off the match heads.
- Prepare the desired quantity of igniter and store it in an airtight container until ready for use.
d. Application.
- Pour or spoon the match heads, in a single pile, on the incendiary. Prepare the match heads for ignition with Fuse Cord (0101) or Improvised String Fuse (0102) in the normal manner. The fuse cord should terminate near the center of the match head pile. Concentrated Sulfuric Acid (0103) or a match flame can also be used as an initiator. Ignition takes place almost immediately on contact with the acid or the match flame. Acid is recommended for use with specific delay mechanisms found in chapter 5.
- If only battery-grade sulfuric acid is available, it must be concentrated before use to a specific gravity of 1.835 by heating it in an enameled, heat-resistant glass or porcelain pot until dense, white fumes start to appear. See paragraph 0103 for details.
- When used to ignite flammable liquids, wrap a quantity of the match heads in a non-absorbent material and suspend it inside the container near the open top. The container must remain open for easy ignition and combustion of the flammable liquid.
- To minimize the hazard of premature ignition of flammable liquid vapors, allow at least two feet of fuse length to extend from the top edge of an open container of flammable liquid before lighting the fuse.
0206. POTASSIUM PERMANGANATE—GLYCERIN
a. Description.
- This item consists of a small pile of potassium permanganate crystals which are ignited by the chemical action of glycerin on the crystals. It can be used to ignite all the incendiaries listed in chapter 4 except Thermite (0307). It may be used directly as an incendiary on readily flammable material, such as rags, dry paper; dry hay, or in the combustible vapor above liquid fuels.
- Ignition is accomplished by causing a few drops of glycerin to contact the potassium permanganate crystals. A hotter flame is produced when powdered magnesium or powdered aluminum is mixed with the the potassium permanganate crystals.
- Ignition time, after addition of the glycerin, increases as temperature decreases. This igniter is not reliable below 50° F.
b. Material and Equipment.
Potassium permanganate crystals (no coarser than granulated sugar).
Glycerin.
One small container with tight-fitting lid for the glycerin.
One larger container with tight-fitting lid for the potassium permanganate crystals.
Powdered magnesium or powdered aluminum (no coarser than granulated sugar).
Preparation.
- Put some glycerin in the small containers and cap tightly.
- Fill the larger container with potassium permanganate crystals and cap tightly.
- If powdered magnesium or powdered aluminum is available, mix 85 parts potassium permanganate crystals and 15 parts powdered magnesium or powdered aluminum and store this mixture in the large bottle.
- Keep these containers tightly sealed and the material in the containers will remain effective for a long period of time.
d. Application. Pour out a quantity of the potassium permanganate crystals (with or without powdered aluminum or powdered magnesium), in a single pile on the incendiary. Manual ignition is accomplished by causing a few drops of glycerin from a medicine dropper to come in contact with the potassium permanganate crystals. Keep hands and clothing clear of the igniter; ignition may take place almost instantly with addition of the glycerin. This igniter is convenient for use with specific delay mechanisms found in chapter 5.
0207. POWDERED ALUMINUM—SULFUR PELLETS
a. Description.
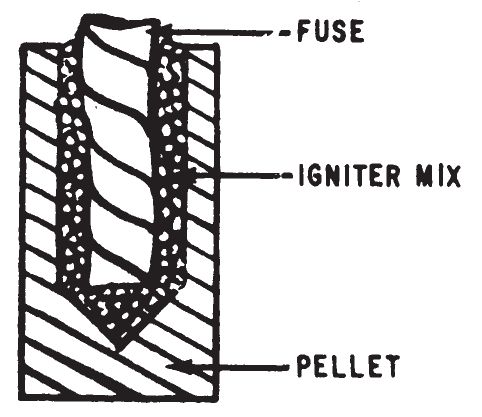
- This igniter consists of finely powdered aluminum, sulfur and starch which have been thoroughly mixed and shaped into hardened cylindrical pellets. It can be used to ignite all the incendiaries listed in chapter 4. It is an excellent igniter for Thermite (0307). It may be used directly as an incendiary on readily flammable material such as rags, dry paper, dry hay, or in the combustible vapor above liquid fuels.
- The igniter can be initiated by Fuse Cord (0101) or Improvised String Fuse (0102). A hole is made in one pellet to receive a fuse and a small quantity of another more easily started igniter mixture. A number of unmodified pellets are attached to the first pellet to increase the quantity of heat after combustion occurs.
b. Material and Equipment.
Finely powdered aluminum (no coarser than cake flour).
Finely powdered sulfur (no coarser than cake flour).
Finely powdered starch (no coarser than cake flour) .
Water.
Cylindrical tube about 4 inches long and ¾ inch inside diameter made of metal, wood, glass or plastic.
Rod which fits into the above tube.
Rod about ⅜ inch in diameter (should be about one-half the inside diameter of the 4-inch long tube).
Mixing bowl.
Tablespoon.
Teaspoon.
Stove or hot plate.
Knife.
Measuring container.
c. Preparation.
- Place six tablespoons of aluminum powder in a mixing bowl then add 15 tablespoons of powdered sulfur.
- Mix the two powders gently with the spoon for a few minutes until no unmixed particles of sulfur are visible.
- In a separate pot add two teaspoons of laundry starch to about 6 ounces of water and boil gently for a few minutes. Stir until the starch is dissolved and allow the solution to cool to room temperature.
- When cool, take about one-half of the starch solution and add it to the mixture of aluminum and sulfur powder.
- Mix with a spoon until the whole mass is a smooth, evenly mixed, putty-like paste.
- Fill the cylindrical tube with this paste, place one end of this tube on a hard surface and tamp the paste with the ⅜ inch diameter rod to squeeze out the air bubbles and consolidate the paste.
- Push the paste out of the tube with the larger rod, which just fits the tube, so that it forms a cylinder, then cut the damp cylinder into 1½ inch lengths using the knife.
- Dry these pieces at 90° F. for at least 24 hours before using. The drying time can be reduced by using a drying oven at a maximum temperature of 150° F.
- Form a hole at least ½ inch in diameter approximately half-way into one end of an igniter pellet.
- Put one of the following igniters into the cavity to roughly one-half its depth:
Sugar-Chlorate (0201)
Sugar—Sodium Peroxide (0203)
Aluminum Powder—Sodium Peroxide (0204)
Silver Nitrate—Magnesium Powder (0208)
- Insert a length of fuse into the hole so that it makes contact with the igniter mix. Fill the remainder of the hole with igniter mix and tamp down to hold the fuse firmly.
- Tape the fuse cord in place to prevent it from working loose and falling out.
- Tape two or more pellets without holes to the one with the fuse.
- Store all the pellets in a dry, closed container until required for use.
d. Application.
- For ignition of thermite, a cluster of at least three pellets should be used. Bury the cluster of igniter pellets just below the surface of the thermite, with the fuse extending for ignition by a match flame. Large quantities of thermite may require a cluster of more than three pellets for satisfactory ignition.
- For use as an igniter of a solid incendiary, place a cluster of pellets on top of the incendiary.
- When used to ignite flammable liquids, wrap a cluster of igniter pellets in a nonabsorbent material and suspend it inside the container near the open top. The container must remain open for easy ignition and combustion of the flammable liquid.
- To minimize the hazard of premature ignition of flammable liquid vapors, allow at least two feet of fuse length to extend from the top edge of an open container of flammable liquid before lighting the fuse.
0208. SILVER NITRATE—MAGNESIUM POWDER
a. Description.
- This item consists of a mixture of silver nitrate crystals and magnesium powder. It can be used to ignite all the incendiaries listed in chapter 4 except Thermite (0307). It may be used directly as an incendiary on readily flammable material such as rags, dry paper, dry hay, or in the combustible vapor above liquid fuels.
- The igniter can be initiated by Fuse Cord (0101), Improvised String Fuse (0102), Concentrated Sulfuric Acid (0103), or Water (0104).
Caution: This mixture is unstable and may ignite at high humidity or when wet slightly by drops of water, perspiration, etc.
b. Material and Equipment.
Silver nitrate crystals (no coarser than granulated sugar).
Magnesium powder or filings (no coarser than granulated sugar).
Spoon.
Container with tight-fitting lid.
c. Preparation.
- Using a clean, dry spoon, place magnesium powder or filings into the dry mixing container to one-quarter container volume. If magnesium filings are used, they should be free of grease.
- Wipe the spoon with a clean, dry cloth, then add an equal quantity of silver nitrate crystals to the dry mixing container. Tighten the lid on the silver nitrate container, and remove it at least six feet from the working area.
- Tightly close the lid on the mixing container. Turn the container on its side and slowly roll until the two powders are completely mixed. The mixture is now ready for use.
- A good practice is to keep the silver nitrate crystals and the magnesium powder or filings in separate air-tight containers and mix just before use.
Caution: This mixture should be kept out of direct sunlight to avoid decomposition of the silver nitrate which could render this igniter mixture ineffective.
d. Application.
- Carefully pour or spoon the mixture, in a single pile, on the incendiary. Prepare the mixture for ignition with either Fuse Cord (0101) or Improvised String Fuse (0102) in the normal manner. The fuse cord should terminate near the center of the igniter mixture. Concentrated Sulfuric Acid (0103) and Water (0104) can be used as initiators but are generally less convenient. Ignition takes place almost immediately on contact with the acid or water. These liquid initiators are convenient for use with specific delay mechanisms found in chapter 5.
- When used to ignite flammable liquids, wrap a quantity of the mixture in a nonabsorbent material and suspend it inside the container near the open top. The container must remain open for easy ignition and combustion of the flammable liquid.
- To minimize the hazard of premature ignition of flammable liquid vapors, allow at least two feet of fuse length to extend from the top edge of an open container of flammable liquid before lighting the fuse.
0209. WHITE PHOSPHORUS
a. Description.
- This item consists of white phosphorus dissolved in carbon disulfide. It can be used to ignite the following incendiaries listed in chapter 4: Napalm (0301), Gelled Gasoline (exotic thickeners) (0302), Gelled Gasoline (improvised thickeners) (0303), and Paraffin-Sawdust (0304). It may be used directly as an incendiary on readily flammable material such as rags, dry paper, dry hay, or in the combustible vapor above liquid fuels.
- Ignition is achieved when the volatile solvent, carbon disulfide, evaporates and the white phosphorus comes in contact with air.
Caution: Never touch white phosphorus directly or allow any of its solutions to touch the skin. Painful burns which heal very slowly may result. White phosphorus sticks must always be stored completely under water. If any of the phosphorus solution is accidently spilled on the skin, immediately flush the affected area with water; then decontaminate the affected area by dabbing with copper sulfate solution.
b. Material and Equipment.
White phosphorus sticks (sometimes called yellow phosphorus).
Carbon disulfide.
Copper sulfate solution.
Tweezers or tongs.
Two glass containers about 8-ounce capacity with lids or stoppers made of glass, earthenware, or metal. Do not use a rubber lid or stopper (carbon disulfide will attack rubber).
c. Preparation.
- Prepare some copper sulfate solution by adding one spoonful of copper sulfate crystals to one of the glass containers. Fill the container with water, place the stopper in the open mouth of the bottle and shake until the crystals dissolve.
- Pour carbon disulfide into the other glass container to one-quarter container volume.
Caution: Carbon disulfide fumes are poisonous. Always cap an open container of carbon disulfide as soon as possible. Work in a well ventilated area.
- With a pair of tweezers remove some sticks of white phosphorus from their storage container. Totally submerge them immediately in the carbon disulfide to bring the level up to one-half full. Be sure that all the phosphorus left in the original container is completely submerged in water before putting the container away. Wash the tweezers immediately in the copper sulfate solution.
- Securely stopper the bottle containing the white phosphorus and carbon disulfide and allow to stand until the white phosphorus dissolves. This usually takes about eight hours. The time required to dissolve white phosphorus can be reduced by shaking the bottle. Be sure that the bottle top does not come off.
- Do not store in direct sunlight because the solution will become ineffective. This solution should never be stored more than three days.
Note. If carbon disulfide is not available, benzene (benzol) may be used to dissolve the phosphorus. It requires considerable shaking and overnight soaking to get an appreciable amount of phosphorus dissolved in benzene. Do not attempt to use red phosphorus for preparing this igniter because it does not behave like white phosphorus.
d. Application.
- To ignite readily flammable material, pour the white phosphorus solution directly onto the material; it will ignite when the solvent evaporates, exposing the white phosphorus to the air. Once the solution is poured, the empty bottle should be discarded immediately because any solution remaining on the bottle will ignite when the solvent evaporates. Do not cover the soaked flammable material because the carbon disulfide must evaporate for ignition to occur.
- The incendiaries mentioned under Description above can be initiated by first impregnating crumpled paper or absorbent paper towels with the white phosphorus solution and placing the impregnated paper on the material to be ignited.
- Delay times of the phosphorus solution may be varied by the addition of gasoline or toluene (toluol). Add a small quantity of either solvent to the original white phosphorus solution and test the solution each time until the desired delay time is achieved. Delay times of 20 to 30 minutes may be obtained in this manner.
- Check the delay time under conditions expected at the target. Air currents hasten the evaporation of the solvent and decrease delay time. A high ambient temperature will also decrease delay time whereas a low ambient temperature will increase the delay time. This igniter is not reliable at or below freezing temperatures (32° F.)
- To make incendiary paper, soak strips of ordinary writing paper in the phosphoruscarbon disulfide for a few minutes. Remove the paper with a pair of tweezers or tongs and place in a vial filled with water. Be sure to wash off the tweezers immediately in copper sulfide solution. Cap the vial and store until ready to use. To use this incendiary paper, remove the strips of paper with a pair of tweezers, and place among the material to be ignited.
0210. MAGNESIUM POWDER—BARIUM PEROXIDE
a. Description.
- This item consists of a mixture of finely powdered magnesium and finely powdered barium peroxide. It can be used to ignite all the incendiaries listed in chapter 4 and is particularly suited for ignition of thermite. It may be used directly as an incendiary on readily flammable material such as rags, dry paper, dry hay, or in the combustible vapor above liquid fuels.
- The igniter can be initiated by Fuse Cord (0101) or Improvised String Fuse (0102).
b. Material and Equipment.
Magnesium powder (no coarser than table salt).
Barium peroxide (no coarser than table salt).
Spoon.
Container with tight-fitting lid.
c. Preparation.
- Using a clean, dry spoon, place powdered magnesium into the dry mixing container to one-quarter container volume.
- Wipe the spoon with a clean, dry cloth, then add powdered barium peroxide to the dry mixing container to three-quarters container volume. Tighten the lid on the barium peroxide container, and remove it at least six feet from the working area.
- Tightly close the lid on the mixing container. Turn the container on its side and slowly roll until the two powders are completely mixed. The mixture is now ready for use.
- A good practice is to keep the powdered magnesium and powdered barium peroxide in separate containers and mix just before use.
d. Application.
- Carefully pour or spoon the mixture, in a single pile, onto the incendiary. Prepare the mixture for ignition with Fuse Cord (0101) or Improvised String Fuse (0102) in the normal manner. The fuse cord should terminate near the center of the igniter mixture.
- In ignition of thermite, spread the igniter mixture to a depth of at least
 inch on the top surface of the thermite which is held in an assembly described under Application of Thermite incendiary (0307). The fuse cord will initiate the thermite igniter which will, in turn, ignite the thermite.
inch on the top surface of the thermite which is held in an assembly described under Application of Thermite incendiary (0307). The fuse cord will initiate the thermite igniter which will, in turn, ignite the thermite. - When used to ignite flammable liquids, wrap a quantity of the mixture in a nonabsorbent material and suspend it inside the container near the open top. The container must remain open for easy ignition and combustion of the flammable liquid.
- To minimize the hazard of premature ignition of flammable liquid vapors, allow at least two feet of fuse length to extend from the top edge of an open container of flammable liquid before lighting the fuse.
0211. SUBIGNITER FOR THERMITE
a. Description.
- This item consists of a mixture of a metal powder and an oxidizing agent. Two metal powder alternates and four oxidizing agent alternates are specified. In the combustion process, the metal powder is oxidized, resulting in the liberation of a large quantity of heat.
- This subigniter is a substitute for Magnesium Powder Barium Peroxide Igniter (0210), and should be used only if that Igniter is not available. The disadvantage of this subigniter is that it cannot be directly initiated by fuse cord. To use this subigniter for initiating thermite, it is necessary to use another igniter mixture to initiate the subigniter, preferably Sugar-Chlorate (0201). The fuse cord will initiate the sugar-chlorate, which will, in turn, ignite the subigniter and, thereby, initiate the thermite.
- This subigniter can be directly initiated by all the igniters listed in chapter 3 except White Phosphorus (0209).
b. Material and Equipment.
Either aluminum or magnesium filings or powder (no coarser than granulated sugar).
Any one of the following oxidizing agents: sodium dichromate, potassium permanganate, potassium nitrate, or potassium dichromate (no coarser than granulated sugar).
Container with tight-fitting lid.
c. Preparation.
- Using a clean, dry spoon, place one of the metal powders or filings in the container to one-third container volume. If metal filings are used, they should be free of grease.
- Wipe the spoon with a clean, dry cloth and add an equal quantity of one of the above oxidizing agents.
- Tighten the lid on the mixing container, turn the container on its side and slowly roll until the two powders are completely mixed. The mixture is now ready to use and may be stored for months in this tightly sealed container.
d. Application.
- To use this subigniter, spread the material to a depth of at least ¼ inch on the top surface of the thermite which is held in an assembly described under Application of Thermite Incendiary (0307). Spread another igniter, preferably Sugar-Chlorate (0201) on top of this subigniter to about the same depth. Prepare the mixture for ignition with Fuse Cord (0101) or Improvised String Fuse (0102) in the normal manner. The fuse cord should terminate near the center of the igniter mixture. The fuse cord initiates the sugar-chlorate igniter which ignites the thermite subigniter which then ignites the thermite.
- For delay times longer than those conveniently obtained with fuse cord in ignition of thermite by this subigniter method, refer to chapter 5.
Caution: Never attempt to ignite thermite subigniter without at least a few seconds delay fuse. It burns extremely fast and hot, and the user could be seriously burned if he were too close when ignition occurred.