CHAPTER 5
DELAY MECHANISMS
0401. CIGARETTE
a. Description.
- This item consists of a bundle of matches wrapped around a lighted cigarette. It is placed directly on easily ignited material. Ignition occurs when the lighted portion of the burning cigarette reaches the match heads. This delay mechanism can be used to initiate all igniters listed in chapter 3 except Magnesium Powder—Barium Peroxide (0210) and Powdered Aluminum—Sulfur Pellets (0207). A cigarette delay directly ignites the following incendiaries: Napalm (0301), Gelled Gasoline (exotic thickeners) (0302), and Gelled Gasoline (improvised thickeners) (0303).
- The following dry tinder type materials may also be directly ignited by the cigarette delay mechanism: Straw, paper, hay, woodshavings and rags.
- Usually this delay will ignite in 15 to 20 minutes, depending on length of cigarette, make of cigarette, and force of air currents. A duplicate delay mechanism should be tested to determine delay time for various ambient conditions.
- The cigarette must be placed so that the flame will travel horizontally or upward. A burning cigarette that is clamped or held will not burn past the point of confinement. Therefore, the cigarette should not contact any object other than matches.
b. Material and Equipment.
Cigarette.
Matches (wooden).
Match box.
String or tape.
c. Preparation.
- Picket-fence delay.
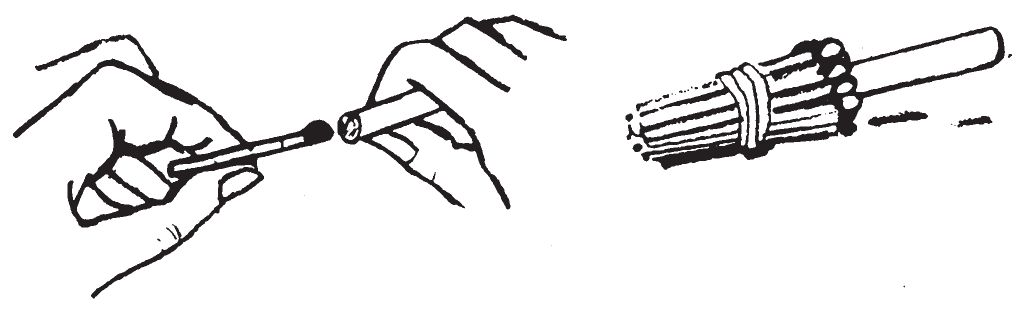
- (a) Push one wooden match head into a cigarette a predetermined distance to obtain the approximate delay time.
- (b) Tie or tape matches around the cigarette with the match heads at the same location as the first match in the cigarette.
- Match box delay.
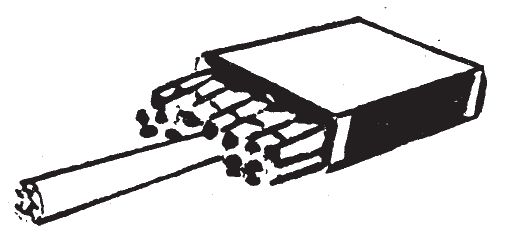
Tear out one end of the inner tray of a box of matches (the end next to the match heads). Push one match into the cigarette. Insert this cigarette into the bunch of matches and parallel to the matches at the center of the pack. Slide the tray out of the inner box, leaving the match heads and the cigarette exposed. The head of the match in the cigarette should be even with the exposed match heads.
d. Application.
- Picket-fence delay.
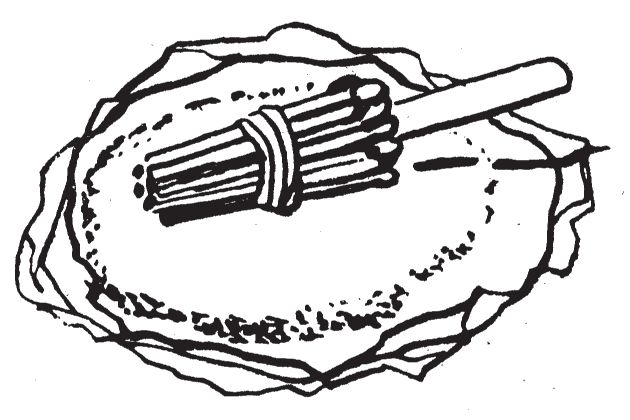
- (a)Light the cigarette and place the delay mechanism on a pile of igniter mixture, paper, straw, or other dry tinder type material. Be sure that the portion of the cigarette between the lit end and the match heads is not touching anything.
- (b)Pile tinder material all around the cigarette to enhance ignition when the match heads ignite.
- Match box delay.
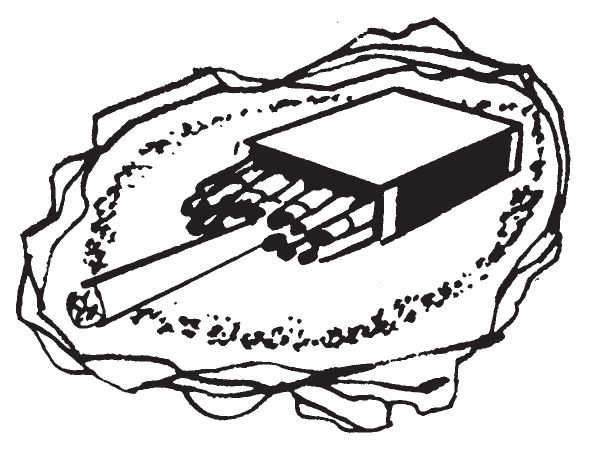
- (a)Place the delay so that the cigarette is horizontal and on top of the material to be ignited. Light the cigarette.
- (b)Be sure ignitable material such as paper, straw, flammable solvents, or napalm is placed close to the match heads. When using flammable solvents, light the cigarette away from the area of solvent fumes.
- (c)To assure ignition of the target, sprinkle some igniter material on the combustible material. The match box delay is then placed on top of the igniter material.
0402. GELATIN CAPSULE
a. Description.
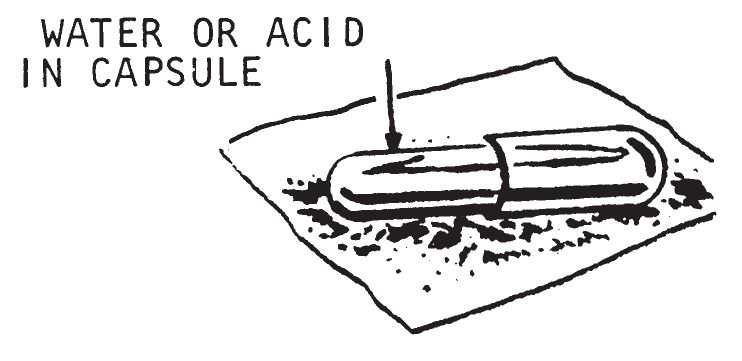
Gelatin capsule delays work by the action of either water or concentrated sulfuric acid on the gelatin. When the liquid dissolves the gelatin, it contacts and reacts with an igniter mix. These delays can be used with various igniters, are easily prepared and easily carried. The disadvantage is that the delay times vary with temperature and they will not work at or below 32° F. Gelatin capsule delays will work with the following igniters:
- Water actuated igniters such as Sugar—Sodium Peroxide (0203), Silver Nitrate—Magnesium Powder (0208), and Aluminum Powder—Sodium Peroxide (0204).
- Concentrated sulfuric acid actuated igniters such as Sugar-Chlorate (0201), Fire Fudge (0202), Sugar—Sodium Peroxide (0203) Aluminum Powder—Sodium Peroxide (0204), Match Head (0205), and Silver Nitrate—magnesium Powder (0208).
b. Material and Equipment.
Concentrated sulfuric acid or water.
Gelatin capsules (1 fluid ounce capacity).
Igniter mixture.
Glass jar or bottle with glass or plastic stopper for carrying acid.
c. Preparation.
- Fill the gelatin capsule with either water or sulfuric acid, depending on which igniter is being used. Use a medicine dropper to fill the capsule. Wipe the outside of the capsule carefully and place it on a quantity of igniter mixture.
- Gelatin will slowly dissolve in either water or concentrated sulfuric acid, usually faster in water than in acid. Sulfuric acid should be handled carefully and only in glass or unchipped enamel containers.
d. Application.
- Fill a gelatin capsule with one of the igniter mixes listed under Description above. Once the liquid is added to the capsule, the next operations should be done quickly. Pile the igniter mixture on and around the capsule. Then place incendiary material in contact with the igniter mixture. (In damp weather this method should not be used with water activated igniters because premature ignition may be caused by humidity in the air.)
- Use the following method in damp weather. Fill a gelatin capsule with one of the igniter mixes listed above. Be sure that both halves of the capsule fit tightly and that no igniter mix is clinging to the outside of the capsule. Place the capsule in a shallow glass or porcelain dish filled with water or concentrated sulfuric acid, depending on which type of igniter mix is used. Make sure the capsule is touching one edge of the bowl and quickly pile incendiary material close to the capsule so that when the capsule ignites, the incendiary will also ignite.
- The gelatin capsule delays work slowly in cold weather and will not work at or below 32° F. Capsule thickness also affects delay time. In water at 77° F.. a delay time of approximately 20 minutes can be expected, while the same type of capsule in concentrated sulfuric acid at 77° F. will give a delay time of approximately one hour. At a temperature of 50° F., the same type of capsule will give a 6 to 8 hour delay time in water and about 24 hours delay time in concentrated sulfuric acid. Delay times become less accurate at lower temperatures.
- The above listed delay times are given for one type of gelatin capsule only. Various types of capsules will give various delay times. Therefore, always check delay times for the capsule to be used.
- The sulfuric acid must be concentrated. If only battery-grade sulfuric acid is available, it must be concentrated before use to a specific gravity of 1.835 by heating it in an enameled, heat resistant glass or porcelain pot until dense, white fumes appear. See paragraph 0103 for details.
0403. RUBBER DIAPHRAGM
a. Description.
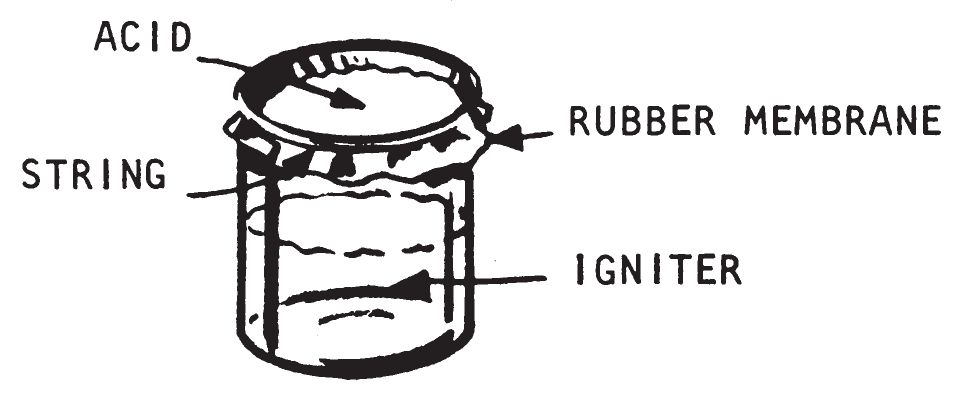
- This delay operates by the action of concentrated sulfuric acid on a thin rubber diaphragm. As the acid eats through the diaphragm, it drips onto the igniter mix and combustion results. This delay can be used to initiate the following igniters listed in chapter 3: Sugar-Chlorate (0201), Fire Fudge (0202), Sugar—Sodium Peroxide (0203), Aluminum Powder—Sodium Peroxide (0204), Match Head (0205), Silver Nitrate—Magnesium Powder (0208), and Fire Bottle (0306).
- The delay does not burn or glow, a very desirable feature where premature detection may occur. The main disadvantages of this type of delay are—
- (a)Delay time fluctuates with temperature changes.
- (b)Delay is not reliable below 40° F.
- (c)Sulfuric acid involves hazards to the operator.
b. Material and Equipment.
Concentrated sulfuric acid.
Thin rubber (such as balloons or condoms).
String, tape, or rubber bands.
Glass jar with glass stopper for carrying acid.
Wide-mouthed jar or can (approximately 1 pint capacity).
c. Preparation.
- Fill the wide mouth container three-quarter full with any one of the following igniter materials:
Sugar-Chlorate (0201).
Fire Fudge (0202).
Sugar—Sodium Peroxide (0203).
Aluminum Powder—Sodium Peroxide (0204).
Match Head (0205).
Silver Nitrate—Magnesium Powder (0208).
- Place the rubber diaphragm over the open end of the container and leave it loose enough to sag slightly into the jar. Either tie in place or secure with a rubber band.
- Pour about 1 fluid ounce of concentrated sulfuric acid into a small glass jar with a glass stopper and seal tightly.
d. Application.
- Place the jar with the rubber membrane at the desired target. Pile the material to be ignited around this jar so that when the flames issue from the jar, they will ignite the incendiary materials. Do not put any of this igniter material on the rubber membrane. Pour the 1 fluid ounce of concentrated sulfuric acid onto the rubber membrane. When the acid penetrates the rubber and drips onto the igniter mix, a chemical reaction occurs and combustion results.
- The time delay of this device depends on the kind and thickness of rubber used, and on the ambient temperature. Test a similar device before actual use on the target.
- Using a thin rubber membrane such as a condom at a temperature of 77° F., a delay time of 15 to 20 minutes is normal. This same delay when tested at 40° F. may take as long as eight hours to penetrate the rubber membrane. Do not use this delay at temperatures below 40° F.
- Another simple method of using this type of delay is to first fill a small jar half full of concentrated sulfuric acid. Tie or tape a rubber membrane over the open end of the jar. BE SURE NO ACID CAN LEAK OUT. Place the bottle on its side, on top of a small pile of igniter material which will ignite on contact with the acid. When the acid penetrates the membrane, combustion will occur as before. If thicker rubber is used, stretch the rubber tightly over the mouth of the jar. This will decrease the delay time because the acid will attack the stretched rubber more effectively.
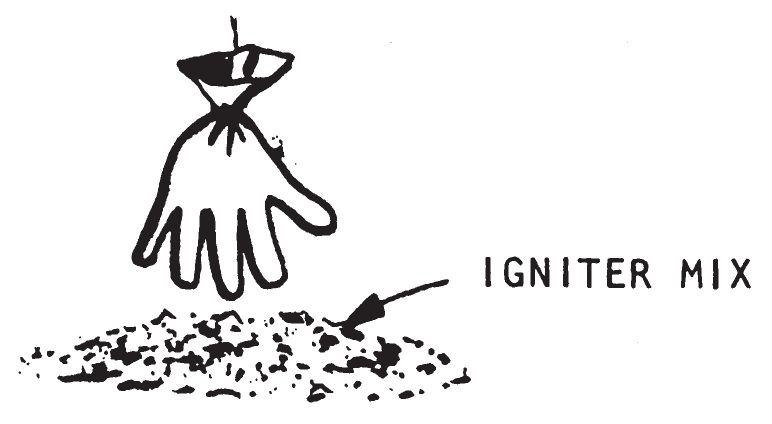
- A rubber glove may also be used as a membrane for this delay. Pour some concentrated sulfuric acid into the glove and suspend the glove over a pile of igniter material. When the acid eats through the glove, it will drip onto the igniter and start a fire. A rubber glove will give a longer delay time than a condom because the material is thicker.
- The rubber membranes for use in this delay must be without pin holes or other imperfections. The sulfuric acid must be concentrated. If only battery-grade sulfuric acid is available, it must be concentrated before use to a specific gravity of 1.835 by heating it in an enameled, heat-resistant glass or porcelain pot until dense, white fumes appear. See paragraph 0103 for details.
0404. PAPER DIAPHRAGM (SULFURIC ACID)
a. Description.
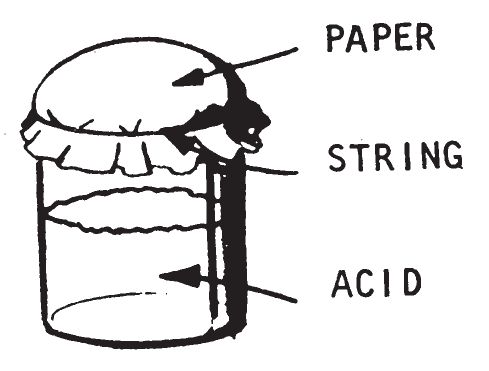
This device consists of a half-full jar of concentrated sulfuric acid, and a paper diaphragm. The paper diaphragm is a piece of paper tied securely over the mouth of the jar. When the jar is placed on its side, the acid soaks through or corrodes the paper. The acid then contacts the igniter material and causes it to burts into flames. This delay can be used for initiating the following igniters listed in chapter 3: Sugar-Chlorate (0201), Fire Fudge (0202), Sugar—Sodium Peroxide (0203), Aluminum Powder—Sodium Peroxide (0204), Match Head (0205), Silver Nitrate—Magnesium Powder (0208).
b. Material and Equipment
Wide-mouthed jar.
Sulfuric acid (concentrated).
Paper.
String.
c. Preparation. Remove the cap from a wide-mouthed jar Fill about half-full with concentrated sulfuric acid. Tie the paper securely over the mouth of the jar.
d. Application.
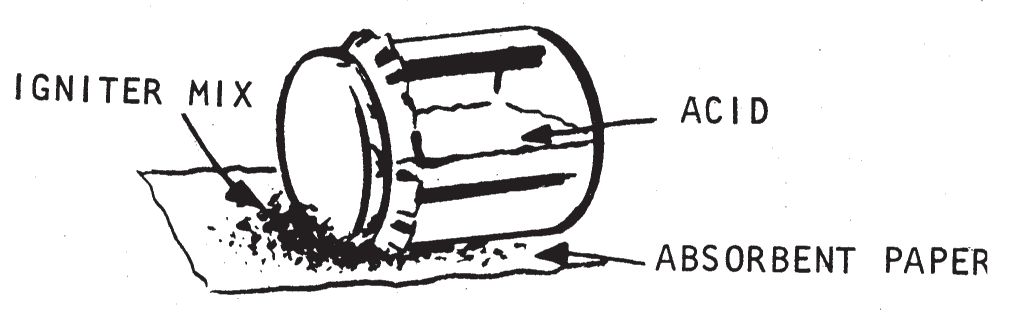
- Make a pile of dry flammable material such as rags, papers, empty boxes, or cartons. Spread out a piece of absorbent paper on this material. Spread igniter material on the absorbent paper and place the jar (on its side) on top of the igniter material. Make certain the jar does not leak. When the acid soaks through or corrodes the paper, it will contact the igniter material and cause it to burst into flame.
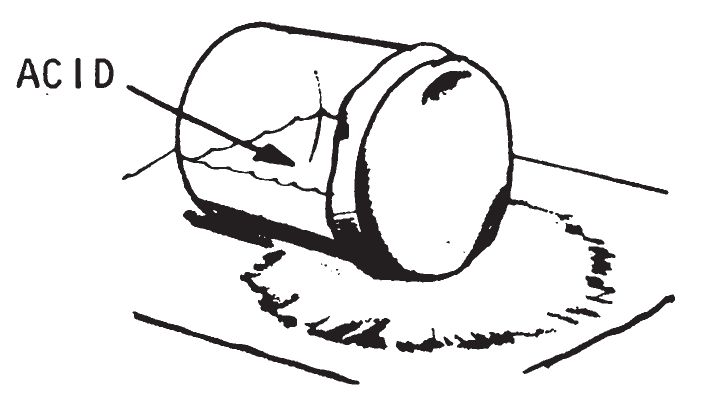
- This device is not reliable at temperatures below 40° F. The time delay depends on the thickness of the paper. A similar device should be tested to determine the delay provided by various thicknesses of paper. It should be tested at the temperature at which it will be used, to be sure of positive ignition. Ignition should occur in about 2 minutes at 68° F. when using writing paper. Higher ambient temperatures shorten delay times, and lower temperatures lengthen delay times.
0405. PAPER DIAPHRAGM (GLYCERIN)
a. Description.
- This device consists of potassium permanganate crystals wrapped in layers of absorbent paper. Glycerin is brought into contact with the wrapped potassium permanganate crystals by slowly soaking through the paper. This wets the wrapped crystals causing combustion. This delay can be used for directly initiating all igniters listed in chapter 3 except White Phosphorus (0209). The igniting ability of this delay is increased when magnesium or aluminum particles are mixed with the potassium permanganate crystals.
- The following incendiaries (ch 4) can be directly ignited using this delay: Napalm (0301), Gelled Gasoline (exotic thickeners) (0302), Gelled Gasoline (Improvised thickeners) (0303), Paraffin-Sawdust (0304), and Incendiary Brick (0309). Other combustible dry materials such as paper, rags, straw, and excelsior can also be directly initiated. This delay is not recommended for use in tempertures below 50° F.
b. Material and Equipment.
Absorbent paper (toilet paper, paper, toweling, newspaper).
Glycerin (commercial grade).
Magnesium or aluminum particles (consistency of granulated sugar).
Rubber bands or string.
Small shallow dish.
Potassium permanganate (consistency of coarse ground coffee).
Small bottle (approximately 1½ fluid ounces).
Spoon (perferably nonmetallic).
c. Preparation.
- Fill the small bottle with glycerin.
- Wrap a quantity of potassium permanganate crystals (a mixture of 85 parts potassium permanganate and 15 parts magnesium or aluminum particles can be substituted to produce a hotter flame) in absorbent paper. Make certain that none of the crystals fall out.
- The bottle and package may be carried by the person without hazard to himself, and will be available for use when needed.
d. Application.
- To use this delay, pour the glycerin into a small shallow dish or pan. Pile incendiary material around the dish so that when the glycerin ignites it will ignite the incendiary material. Place the paper container of potassium permanganate crystals into the pan of glycerin. When the glycerin soaks through the paper and contacts the potassium permanganate, ignition occurs within a few seconds.
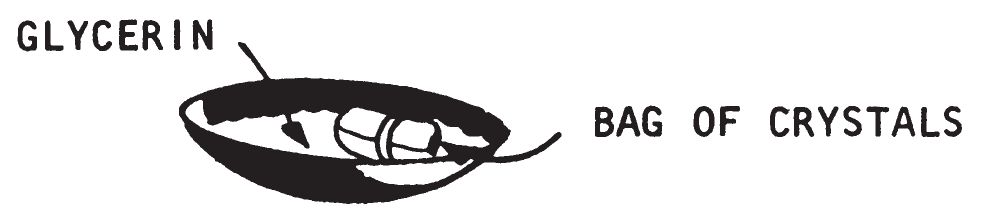
- By using various kinds of paper, different delay times can be obtained. Using more layers of paper for wrapping will increase the delay time. Using this delay at higher temperatures will also decrease the delay time. Delay times from one minute to approximately one hour are possible, depending on the conditions.
- The delay time should be checked under conditions which are similar to those expected at the target.
0406. CANDLE
a. Description.
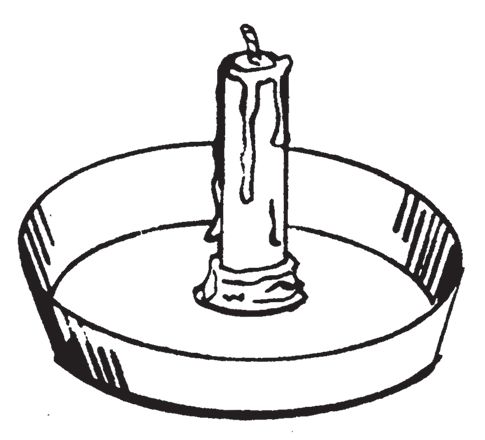
This delay ignites flammable fuels of low volatility such as fuel oil and kerosene. A lighted candle properly inserted in a small container of flammable liquid of low volatility causes ignition of the flammable liquid when the flame burns down to the liquid level. The flame from the burning liquid is used to ignite incendiary material such as paper, straw, rags, and wooden structures. The delay time is reasonably accurate, and may be easily calibrated by determining the burning rate of the candle. No special skills are required to use this delay. Shielding is required for the candle when used in an area of strong winds or drafts. This delay is not recommended for use with highly volatile liquids because premature ignition may take place. This device is useful where a delay of one hour or longer is desired. The candle delay works well in cold or hot weather, and has the advantage of being consumed in the resulting fire, thus reducing evidence of arson.
b. Material and Equipment.
Candle.
Bowl.
Perforated can or carton.
Fuel oil or kerosene.
Matches.
Small piece of cloth.
c. Preparation.
- Make two marks on the side of the candle, 1½ inches and 2 inches from the top. Light the candle and record the times at which the wax melts at the marks on the side.
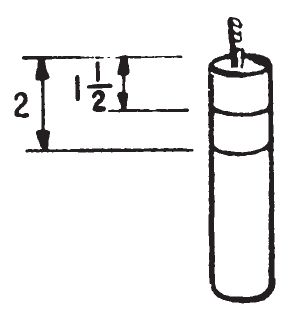
- The distance burned by the candle divided by the elapsed time determines the burning rate of the candle.
d. Application.
- Using a lighted candle of desired length, drip hot wax in the center of the bowl. Melt the base of the candle with a lighted match. Firmly press the softened base of the candle into the hot wax in the center of the bowl. Be sure the candle will stand up securely without toppling over. Extinguish the candle. Wrap a small piece of cloth around the candle and slide it down to the bottom of the bowl. Place a quantity of fuel oil or kerosene in the bowl. Be sure that the level of the fluid reaches the cloth, so it will act as a wick. Pile the incendiary material around the bowl where it can catch fire after the fuel oil or kerosene ignites.
- If this delay must be set in a windy or drafty location, place a shield over it. Notch or punch holes in a metal can or cardboard carton at the bottom and sides for ventilation, and place this cover over the delay.
0407. OVERFLOW
a. Description.
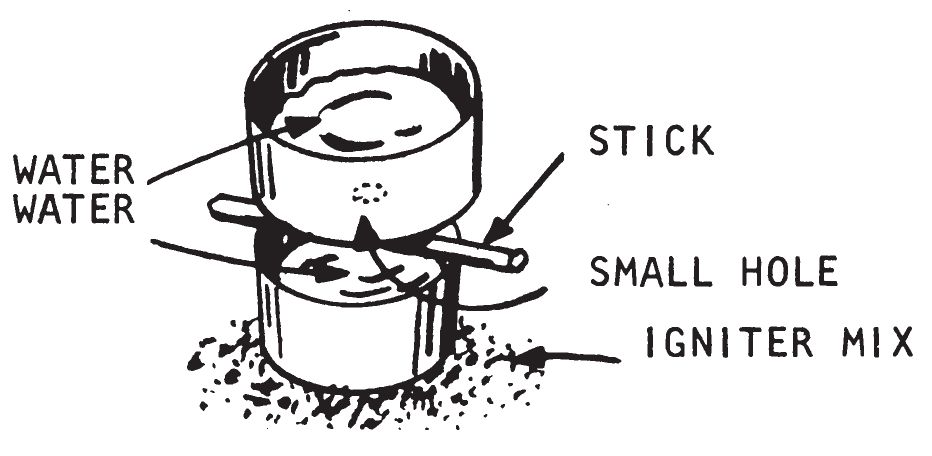
This item provides a time delay in starting a fire. It consists of two tin cans, with tops removed, and uses either water or glycerin to activate the igniter material. A hole is punched in the closed end of one can. This can is placed on top of the other can which is partially filled with the liquid. The top can is completely filled with the liquid. When the bottom can fills and overflows, the overflowed liquid will react with the igniter material placed around the bottom can. This device is used for igniting the following water actuated igniters listed in chapter 3: Sugar—Sodium Peroxide (0203), Aluminum Powder—Sodium Peroxide (0204), and Silver Nitrate—Magnesium Powder (0208). Glycerin is used as the initiating liquid to ignite Potassium Permanganate glycerin (0206).
b. Material and Equipment.
Two tin cans.
Nail or punch.
Hammer.
Water or glycerin.
Can opener.
c. Preparation.
- Remove the tops from two cans.
- Punch or drill a small hole in the closed end of one of the cans.
- Partially fill the other can with either water or glycerin.
- Place the can with the hole in the bottom on top of the can partially filled with igniting fluid. Insert a twig or small stick between the two cans to allow the liquid to overflow from the bottom can.
- Fill the upper can with the same igniting fluid as that previously placed in the bottom can and determine the time required for the fluid to overflow from the bottom can. If two cans of the same size are used, either one may be used for the top. If different size cans are used, place the larger can on top. The delay is variable and adjustable depending on the sizes of the cans, the quantity of liquid used, or the diameter of the hole in the top can.
d. Application.
- Always test the glycerin delay at the temperature at which it will be used. Glycerin flows slowly when cold. Do not use water in this delay near or below its freezing point, 32° F.
- Place the delay in the target area and fill both upper and lower cans to the desired level with the appropriate liquid.
- Pile igniter material around the bottom of the overflow can so the activating liquid can easily make contact with the igniter material as it flows down the side of the can.
0408. TIPPING DELAY-FILLED TUBE
a. Description.
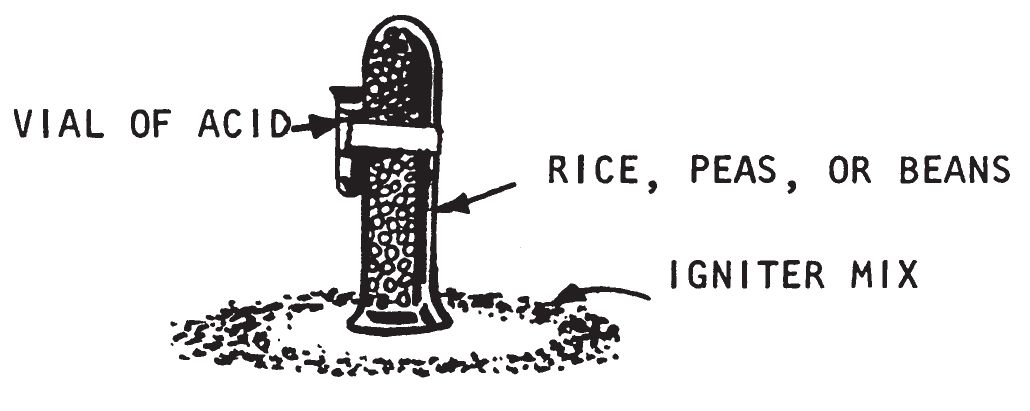
- This delay is composed of a hollow metal rod or bamboo filled with wet beans, rice or peas. The tube is inverted and placed in the center of a ring of igniter material and a small vial of water or acid is tied to the tube. When the wet beans expand, they lift and topple the tube, thereby spilling the acid or water onto the igniter causing combustion.
- This tipping delay may be used with a variety of igniters. They are easily prepared, and give fairly accurate delay times. This delay should not be used at temperatures near or below 32° F. when water is used as the initiator due to freezing. The following water actuated igniters listed in chapter 3 can be used with this mechanism: Sugar—Sodium Peroxide (0203), Aluminum Powder—Sodium Peroxide (0204) and Silver Nitrate—Magnesium Powder (0208). The delay may be used with concentrated sulfuric acid to initiate the above igniters and the following acid activated igniters: Sugar-Chlorate (0201), Fire Fudge (0202), and Match Head (0205). This delay may be used with the Glycerin—Potassium Permanganate Igniter (0206).
b. Material and Equipment.
Metal tube, pipe or piece of bamboo closed at one end, 4 to 6 inches long and 1 inch inside diameter, or glass test tube of similar dimensions.
Small glass vial or bottle with open mouth of 1 fluid ounce capacity.
String or rubber bands.
Rice, peas, or beans.
Water.
Concentrated sulfuric acid.
c. Preparation. The pipe or tube may be made of any material. It must be closed at one end and flat at the other in order to stand vertically. A large glass test tube is ideal for this purpose.
- Using some string or rubber bands, attach the small vial to the larger tube. Attach the vial near the top with the open end of the vial pointing up and the open end of the tube down.
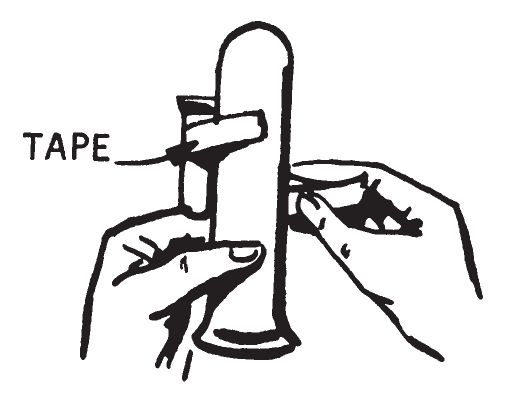
- This assembly should stand up without toppling over. If it appears unsteady, move the vial downward slightly. A final adjustment may be required when the delay is filled with the required materials.
d. Application.
- Rice will usually give delays of about ten to twenty minutes. Peas and beans will usually give delay times up to 4 or 5 hours. Whichever is used it must be first tested to determine the delay time for the tube that will be used.
- To use this device, tightly pack the piece of pipe or bamboo with rice, peas or beans depending on what delay time is required. Add enough water to completely moisten the beans and quickly pour off the excess water. Place the pipe open end down, and immediately fill the small vial with water or concentrated sulfuric acid, depending on which igniter is being used.

- Place a quantity of the igniter mixture in a ring around the delay assembly. Make the ring of such diameter that when the tube falls over, the acid or water from the vial will spill onto the igniter mixture.
- Place incendiary material where the flame from the igniter will start it burning.
- Another way in which the tipping delay can be used is to fill the small vial with glycerin instead of water or acid and then spread potassium permanganate crystals in a ring around the delay. When the glycerin is spilled onto the crystals, combustion will occur and ignite the incendiary material. The glycerin igniter will not work in temperatures below 50° F.
- It is recommended that this device be tested at the same temperature at which it is to be used.
0409. TIPPING DELAY-CORROSIVE OR DISSOLVING ACTION
a. Description.
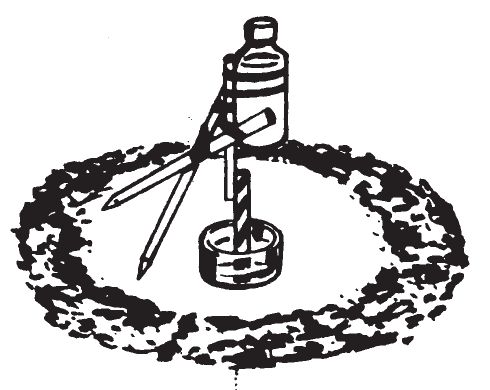
DISSOLVING
TIPPING DELAY
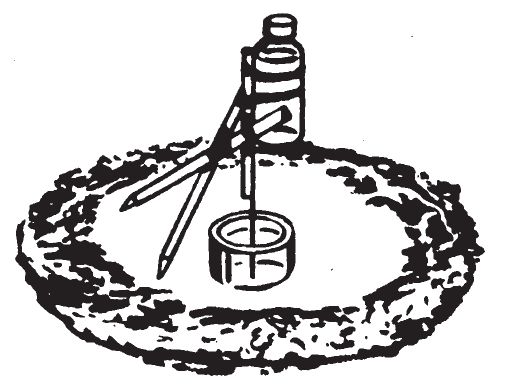
CORROSIVE
TIPPING DELAY
- This device consists of a vial of initiating liquid supported by a tripod. One of the legs which supports the vial of liquid is dissolved by a fluid. The center of gravity of the structure changes and the structure topples over. The contents of the vial spill onto an appropriate igniter mixture and combustion occurs.
- This corrosive or dissolving tipping delay may be used with a variety of igniters. However, it should not be used at temperatures near or below 32° F. when water is used as the initiator due to freezing of the water.
- The following water actuated igniters listed in chapter 3 can be used with this mechanism:
Sugar—Sodium Peroxide (0203), Aluminum Powder—Sodium Peroxide (0204) and Silver Nitrate—Magnesium Powder (0208). The delay may be used with concentrated sulfuric acid to initiate the above igniters and the following acid activated igniters: Sugar-Chlorate (0201), Fire Fudge (0202), and Match Head (0205). This delay may be used with the Glycerin—Potassium Permanganate Igniter (0206)‘
b. Material and Equipment.
Three wooden sticks or wooden pencils (approximately 6 inches long by ¼ inch diameter).
Glass vial (1 fluid ounce capacity).
String, tape or rubber bands.
Any one of the igniter mixtures mentioned above. One of the following combination of items:
- Long sticks of hard candy and water.
- Lengths of bare copper wire and concentrated nitric acid.
- Iron nails or wire approximately
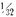 inch diameter by 4 inches long and concentrated hydrochloric acid.
inch diameter by 4 inches long and concentrated hydrochloric acid. - Iron nails or wire and saturated cupric chloride solution.
2 glass containers with glass stoppers for carrying acid.
Shallow glass or porcelain bowl such as soup bowl or ink bottle.
c. Preparation.
- Make a tripod out of three sticks, taping them together at the top. Two legs should be the same length; the third should be about 2—3 inches shorter.
- Tape to the short leg, either a stick of hard candy, piece of heavy bare copper wire, steel nail, or steel wire, adjusting the length so that the wire leg stands almost vertically.

- The finished tripod should have a distance of about 4—5 inches between any two legs.
- To the top of the tripod, on the short leg, firmly tape or tie the small 1-fluide ounce capacity vial, open end up. Make certain that the tripod still stands upright after attaching the vial. The distance between legs may have to be varied to keep the tripod barely standing upright.
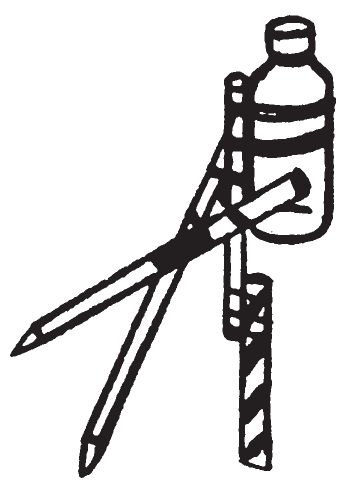
d. Application.
- To use the delay device, insert the leg of the tripod which has the candy, wire, or nails into a glass or porcelain bowl. Fill the vial at the top of the tripod with either water, concentrated sulfuric acid, or glycerin, depending on which igniter is being used. Spread a quantity of the proper igniter material in a ring around the tripod, placing it where the spilled initiating liquid is certain to contact it. Fill the glass or porcelain bowl with the prescribed liquid for dissolving the leg of the tripod in the bowl. For hard candy the liquid is water; for copper wire the liquid is concentrated nitric acid; for steel nails the liquid may be either concentrated hydrochloric acid, or a saturated solution of cupric chloride.
- No definite delay times can be established for these delays because of factors such as temperature, solution concentration, and imperfections in the leg of the tripod. Prior to use, test the device under conditions expected at the target. The following table should be used merely as a guideline of expected delay times for the various materials.
Delay material Delay time Hard candy plus water 5—10 minutes Copper wire plus concentrated nitric acid 2—5 minutes Copper wire plus nitric acid diluted with an equal volume of water. 45—60 minutes Steel wire or nails plus concentrated hydrochloric acid. 24 hours to 7 days Steel wire or nails plus cupric chloride 10 minutes to solution. 5—6 hours. - The delay time will vary greatly with only moderate changes in temperature. Do not use this type of delay mechanism where accurate delay times are required.
0410. BALANCING STICK
a. Description.
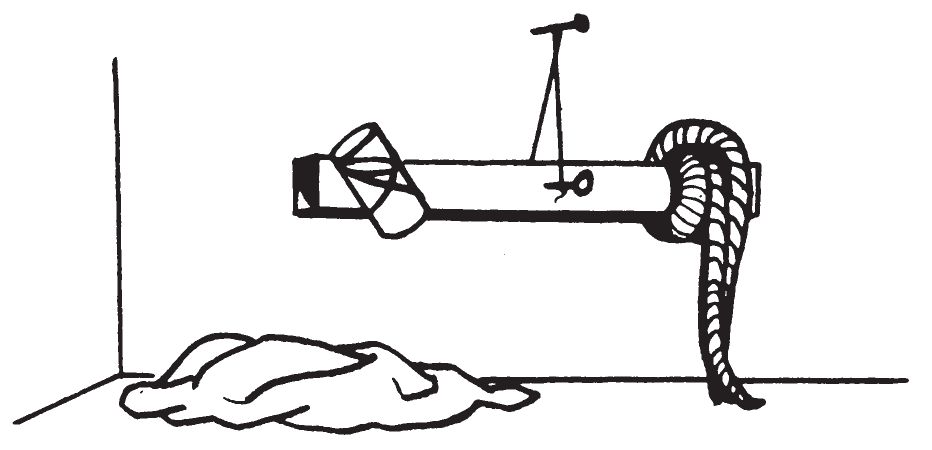
- This delay device consists of a piece of wood or stick, a small vial, a nail, a piece of string, and a long strip of cloth. A hole is drilled through the middle of the stick. The vial is fastened to one end, and the strip of cloth to the other. The length of the cloth is adjusted so that the rod just balances on a nail passing through the hole when the vial is ¾ full. The cloth is wetted with solvent to make it heavy and the vial is filled with initiating liquid to maintain balance. As the solvent evaporates, the end of the stick which supports the vial of initiating liquid becomes heavier than the end supporting the cloth. The unbalanced stick rotates about the nail until the initiating liquid spills onto the igniter mixture and combustion occurs. Fire then spreads to and ignites incendiary material.
- This device may be used with a variety of igniters. However, it should not be used at temperatures near or below 32° F. when water is used as the initiator due to freezing of the water. The following water actuated igniters listed in chapter 3 can be used with this mechanism: Sugar—Sodium Peroxide (0203), Aluminum Powder—Sodium Peroxide (0204) and Silver Nitrate—Magnesium Powder (0208). The device may be used with concentrated sulfuric acid to initiate the above igniters and the following acid activated igniters: Sugar-Chlorate (0201), Fire Fudge (0202), and Match Head (0205). It may also be used with the Glycerin—Potassium Permanganate Igniter (0206).
b. Material and Equipment.
Piece of wood ⅞ by ⅞ by 16 inches).
2 Nails.
String.
Strip of cloth.
2 glass vials (1 fluid ounce) with stoppers.
c. Preparation.
- Drill a hole through the middle of the stick as shown below.
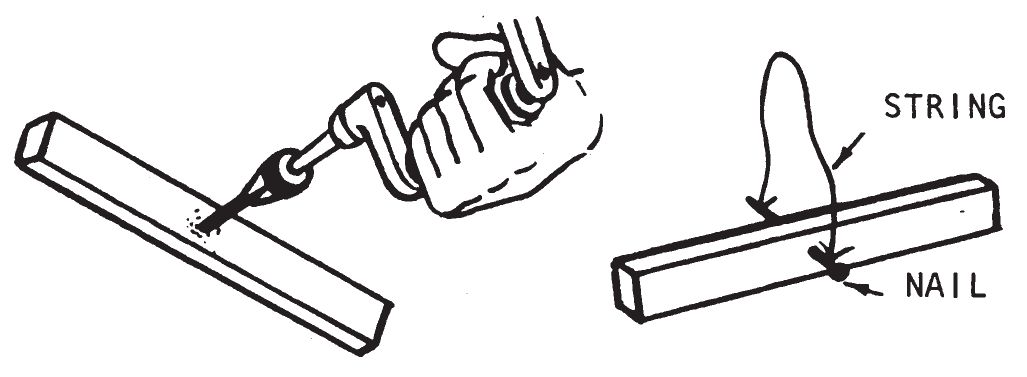
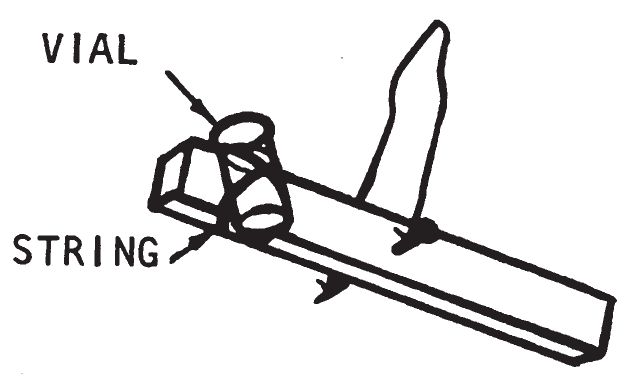
- Insert a nail through the hole. The nail should permit the stick to turn freely. Tie a piece of string (4-6 inches in length) to both ends of the nail, forming a loop. It is not important that the stick balance exactly.
- To one end of the stick tape a small glass vial. Tilt the vial when attaching it so that when this end of the stick is about 8 inches above the other end, the vial will be vertically upright. On the other end of the stick tie a strip of cloth, rag, or rope. This strip should be heavy enough so that the stick is balanced when the vial is about ¾ full of initiating fluid.
d. Application.
- To use this delay, drive a nail (approximately 4 inches long) into a wall or wooden box about 8 inches above the floor, leaving at least 2 inches of the nail projecting. Place the loop of string on the nail near the head of the nail. The stick should not touch the box or wall, but must swing freely. The rag should touch the floor. Pour enough solvent on the rag to soak it thoroughly (approximately 1 fluid ounce). Working quickly, fill the vial with initiating liquid and balance the rod by shifting the cloth. Spread a quantity of appropriate igniter mixture on the floor where the initiating liquid will spill when the solvent on the cloth evaporates. In a few minutes the solvent will evaporate, causing the stick to become unbalanced. The vial will tilt with the stick and, the liquid in the vial will pour out and initiate the igniter mixture.
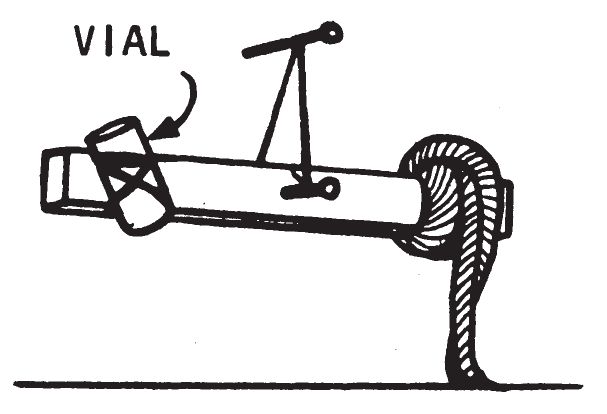
- Where no solvent is available or where the odor of solvent may make the device easy to detect, do not use cloth soaked with solvent. Use a wire basket containing ice as shown below.

- When ice is used, the delay time will be a matter of minutes, depending on the ambient temperature. Ice cannot be used at temperatures near 32° F. Be sure that the drippings from the melting ice does not wet the igniter or interfere with initial combustion of flammable material.
0411. STRETCHED RUBBER BAND
a. Description.
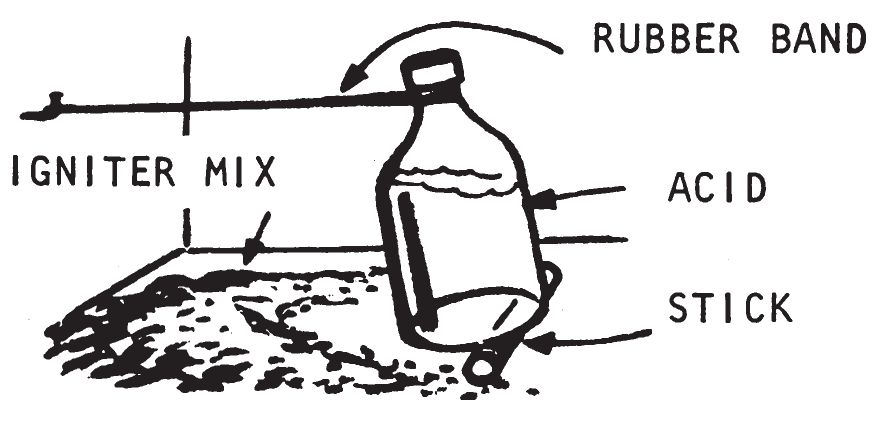
This item utilizes a rubber band, which has been soaked in gasoline or carbon disulfide until it has considerably expanded. After removal of the rubber band from the solvent, the rubber band is attached to a wall and to a bottle containing igniter fluid. As the rubber band contracts due to solvent evaporation, the bottle is tipped and initiator liquid comes in contact with an appropriate igniter material. This stretched rubber band delay may be used with a variety of igniters. However, it should not be used at temperatures near or below 32° F. when water is used as the initiator because the water freezes. The following water actuated igniters listed in chapter 3 can be used with this mechanism: Sugar—Sodium Peroxide (0203), Aluminum Powder—Sodium Peroxide (0204), and Silver Nitrate—Magnesium Powder (0208). The delay may be used with concentrated sulfuric acid to initiate the above igniters and the following acid activated igniters: Sugar-Chlorate (0201), Fire Fudge (0202), and Match Head (0205). This delay may be used with Glycerin—Potassium Permanganate Igniter (0206).
b. Material and Equipment.
Bottle or jar (1 to 2 fluid ounce capacity).
Rubber bands.
Gasoline or carbon disulfide.
Air tight container for carrying the gasoline or carbon disulfide.
Nails.
Igniter.
c. Preparation.
- Fill a bottle (1 to 2 fluid ounce capacity) with water, acid, or glycerin, depending on which igniter is to be used.
- Soak the rubber bands in gasoline or carbon disulfide for about one hour. Do not soak too long or they will become excessively weakened.
d. Application.
- At the place where the delay is to be used, drive a large headed nail into the wall, leaving about 2 to 2½ inches exposed. Loop the rubber bands over the head of the nail. Place the bottle two bottle heights away from the nail. Quickly loop the free end of the rubber bands over the neck of the bottle. Move the bottle back and forth until there is just enough tension in the rubber bands to hold the bottle without it toppling when a pencil or twig is placed under the far end. The stick under the end of the bottle is used as a tilt device to make sure that the bottle topples over when the rubber band contracts.
- Place some incendiary material close to the bottle. Sprinkle a quantity of igniter mixture about the area in which the liquid will be spilled. As the solvent evaporates, the rubber bands will shrink, tip the bottle, spill the liquid, and initiate the igniter material.
Note. Always set up the bottle before spreading the igniter mixture.
0412. ALARM CLOCK
a. Description.
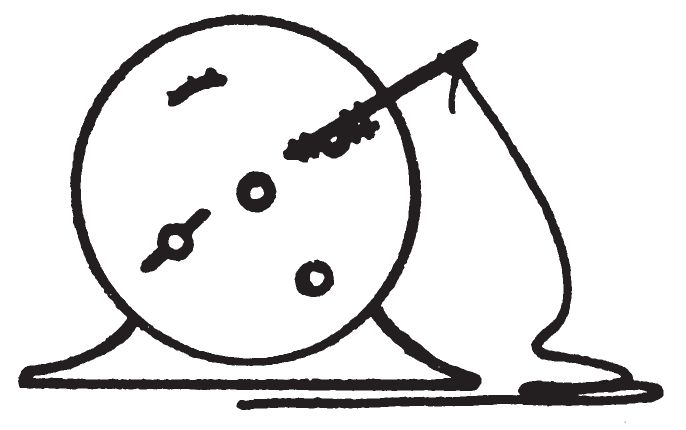
- This device is used for igniting materials after a definite delay time. The device employs a manually-wound alarm clock, with the alarm bell removed, as the timing mechanism. A piece of string is fastened to the key used to wind the alarm. The other end of the string is fastened to a bottle of appropriate initiating liquid. When the modified alarm mechanism is tripped, the winding key will reel in the string and overturn the bottle of initiating liquid and start a fire.
- This alarm clock delay may be used with a variety of igniters. However, it should not be used at temperatures near or below 32° F. when water is used as the initiator because the water freezes. The following water actuated igniters listed in chapter 3 can be used with this mechanism: Sugar—Sodium Peroxide (0203), Aluminum Powder—Sodium Peroxide (0204), and Silver Nitrate—Magnesium Powder (0208). The delay may be used with concentrated sulfuric acid to initiate the above igniters and the following acid activated igniters: Sugar-Chlorate (0201), Fire Fudge (0202), and Match Head (0205). This delay may be used with Glycerin—Potassium Permanganate (0206).
- This device will produce fairly accurate delay times between one and eleven hours.
Caution: The ticking sound of the clock may reveal the presence of the device.
b. Material and Equipment.
Alarm clock, manually wound (without bell, if possible).
Bottle.
String.
Initiator liquid.
Cloth or absorbent paper.
c. Preparation.
- Remove the bell or striker from the clock.
- Fully wind time and alarm springs.
- Set desired time on alarm.
- Tie the string to the alarm key so that it will be pulled when the alarm mechanism is tripped. If necessary, tie a twig or stick to the alarm key to obtain a longer level.
d. Application.
- Tie the string to the alarm key or stick. Set the clock in place and anchor it if necessary. Muffle the clock with rags, making sure that the rags do not interfere with the reeling action of the alarm mechanism. Tie the free end of the string to the bottle of activating liquid. The bottle should be tilted in the direction of the fall by a pencil or twig. When this device is placed on a smooth surface, the clock should be taped, tied, or weighted down to prevent it from sliding when the tension in the string is taken up by the revolving key.
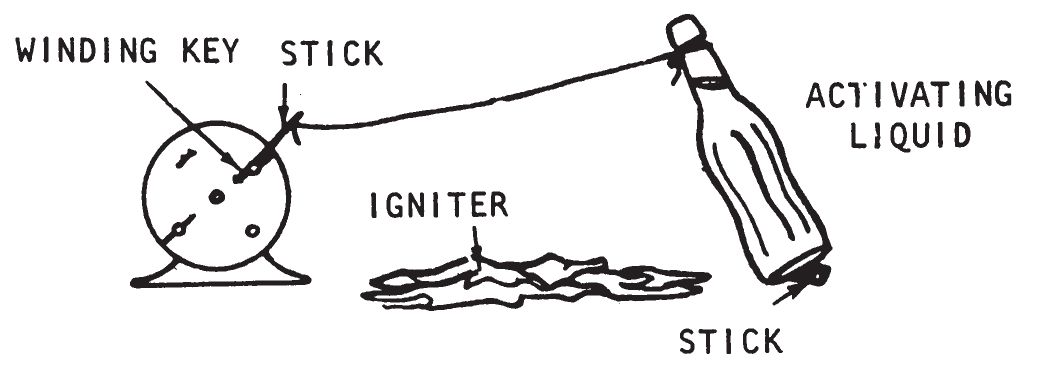
- Adjust the spacing so that the string is taut. Place a cloth or an absorbent paper towel where the contents of the bottle will be spilled. Place a quantity of igniter mixture on the cloth or paper towel. Partially overlap the igniter mixture with a flammable material so as to assist combustion.