CHAPTER 2
The Fetus as Battleground: Early Exposure and Psychiatric Fate
Our mothers’ wombs the tiring-houses be, where we are dressed for this short comedy.
—SIR WALTER RALEIGH
In the early autumn of 1957, Edwin Fuller Torrey, a premedical student at Princeton, got a phone call from his worried mother. His sister, Rhoda, seventeen, an excellent student and popular cheerleader who was heading to Elmira College herself in a week, had begun behaving bizarrely. Just then, she was hallucinating. Lying on the lawn of their family’s home in Clinton, New York, her eyes fixed on a spectacle that only she could see, Rhoda repeatedly shouted, “The British are coming! The British are coming!”1
“We knew nothing about what was going on because people don’t grow up knowing about these diseases, especially in the 1950s,” recalls Torrey. To find answers and treatment, Torrey, Rhoda, and their mother made a pilgrimage to the revered Massachusetts General Hospital, or MGH, a Harvard-affiliated institution dubbed “Man’s Greatest Hospital” by Boston wags. There, doctors told Torrey’s mother, a young widow raising her children alone, that Rhoda had schizophrenia caused by “dysfunctional” family relationships. “It’s hard to believe now, but the Freudian ideas about schizophrenia were prominent,” says Torrey. “My mother was told that my sister got sick because my father had died and because of problems within the family.”
Rhoda suffered from schizophrenia for the rest of her life, spending long periods in Marcy State Hospital and Mohawk Valley Psychiatric Hospital before her 2010 death in Utica, New York, at age seventy.
What causes schizophrenia? While its origins are much debated, its ravages are devastatingly clear. Rhoda’s story is all too common. Schizophrenia tends to seize the young, as early as the late teens or twenties, just as adolescence begins to yield to the promise of education, career, love, marriage, and a family of one’s own. As their peers embark on college, careers, and marriage, the afflicted find themselves suddenly struggling to complete their thoughts, communicate logically, discern the difference between actual events and hallucinations, and perform simple tasks of self-care.
It’s hard to say which aspect of schizophrenia is most terrible. The disease brings disorganized thinking; the invention of meaningless words, called neologisms; and difficulties in sustaining activities and in speaking to and connecting with others.2 Plagued by failing executive functioning, some people with schizophrenia find it hard to understand information and use it rationally to make decisions—for example, to prioritize tasks. Others find that they can no longer retain memories well or focus their attention. To rise in the morning, bathe, dress, prepare a meal, or carry on a conversation while hearing voices, hallucinating, and continually losing one’s equanimity and train of thought can prove impossible. So can accepting the confusion, delusions, loss of friends, and the feeling of one’s very personality slipping away as psychosis and bizarre behaviors take over.
The murky origins of this tragic psychosis escalate the fear it engenders. Many theories seek to explain the alarming symptoms that can accompany schizophrenia, such as the break from reality and aural and visual hallucinations. Dr. Miriam Spering, codirector of the University of British Columbia’s NOVA Lab, thinks that the efference copy produced by a normal nervous system is key. An efference copy is an internal copy of an outgoing signal that’s sent to the motor system. Efference copies let the brain predict what an action’s effects will be. When a brain cannot generate or interpret efference copies, the person cannot correct incomplete perceptions. To fill in the blanks, the brain may resort to prior experience. But combining old images with experiences in present time, Spering’s theory suggests, can present as hallucinations and other symptoms of psychosis.3
In the United States and most of the developed world, medications help many people with schizophrenia, and some of them lead near-normal lives. But few schizophrenics are cured, and only half are treated effectively. There are several reasons for this, but one factor is anosognosia—a lack of awareness of their limitations. Because many people with schizophrenia don’t accept that they have an illness or don’t understand how serious it is, they fail to take their medications or get treatment. This ensures that they will remain ill.
Schizophrenics are damaged not only by the reality of their symptoms but also by the mythology of the disease. According to the National Alliance on Mental Illness (NAMI), 64 percent of people in the United States think that schizophrenia is characterized by a split personality that makes its sufferers careen between normal and outrageous behavior. This false belief feeds the perception that schizophrenics are volatile and prone to unpredictable violence, which in turn causes society to view them as dangerous elements that must be controlled.
Dr. David Crepaz-Keay, chief of social inclusion at the UK’s Mental Health Foundation, agrees: “People with a diagnosis of schizophrenia are feared still and perceived as dangerous.” Crepaz-Keay has personal experience to supplement his expertise: he was diagnosed with schizophrenia in 1979.
Is there a rational basis for the stereotype of the violent schizophrenic? In 2006, Dr. Seena Fazel of Sweden’s Karolinska Institute conducted a study that found that only one in twenty crimes is committed by a person with mental illness, a far smaller number than most people assume. Fazel also found that schizophrenics were four times more likely than those without the disorder to commit a violent offense—if they also engaged in drug or alcohol abuse. However, the odds fell to nearly normal—only 1.2 times more likely than others to engage in violence—when there was no drug or alcohol abuse involved.
Thus, the violent propensities of the mentally ill are both exaggerated and controllable, says Fazel: “There are evidence-based treatment strategies for drug and alcohol abuse, so the risk of violence can be reduced.”
Schizophrenia is just one type of psychosis, a category of mental disease whose hallmark is the inability to distinguish reality from delusions and hallucinations. But it is one of the most common and important psychoses. This chapter’s main focus is the evidence that infectious agents drive schizophrenia, but I’ll also discuss the infectious roots of some other psychoses, such as the mental disorders that followed in the wake of the 1918 influenza pandemic.
Schizophrenia is most often described as a universal disease that affects between 1 and 2 percent of the global population. The World Health Organization (WHO) reports that twenty-four million people worldwide have it, and half of them get no care. In much of the developing world, with its inadequate public-health infrastructure, that is likely to be a gross underestimate. But the disease also presents in a spectrum of variations, so the abilities of people with schizophrenia vary dramatically, and many navigate the world quite capably despite the disease’s hurdles.
The outcomes of schizophrenia also vary dramatically around the globe. Rigorous WHO studies conducted over nearly two decades have revealed that people with schizophrenia in developing countries are far more likely than those in the United States to marry, hold a job, and maintain their social status. Americans with schizophrenia are far more likely than schizophrenics in the Global South (Africa, Central and Latin America, and most of Asia) to commit suicide, while the latter are more likely to recover. Anthropologists and psychiatrists ascribe most of these dramatic differences in outcome to culture rather to biology, although some psychiatrists consider the WHO studies flawed and deny that any such “Third World” advantage exists. This basic disagreement highlights how much remains unknown about this common psychosis.
But our ignorance of what causes schizophrenia may be the most dangerous unknown. Medication can tame its symptoms, but it does so very inconsistently. Not until we understand the cause of the disease can we craft better treatments and devise preventives.
In trying to understand the cause of schizophrenia’s mystifying symptoms and patterns, scientists have intensely examined the usual suspects that are perp-walked in the search for the etiology of a mental ailment: psychological experiences, trauma, stress, and—especially—genetics.
The limits of genetics
We often read of genetic studies performed to determine the risk of developing schizophrenia or other mental illnesses, which lends credence to the belief that these diseases are genetic in nature. But the evidence is easily explained by infectious agents as well. In fact, as evolutionary biologist Paul Ewald wrote in Perspectives in Biological Medicine: “Although evidence generally accepted as demonstrating genetic causation can be readily explained by hypotheses of infectious causation, some of the evidence implicating infectious causation cannot be similarly explained by genetic causation.”4
Still, it is easy to see why contemporary psychiatric research focuses largely on genetics. For one thing, schizophrenia does run in families. One of every one hundred U.S. residents has it, but the likelihood of an individual having it leaps tenfold, to one in ten, if an immediate family member is affected. This pattern makes genetics a favored research focus, and to elucidate its role, scientists have conducted genetic research, including twin studies, for decades.
Scientists often investigate the genetic contributions to disease by comparing the medical fates of identical twins—also called monozygotic, or MZ, twins—who have long been assumed to share all their genes. Comparing these twins should rule out genetics as a variable so scientists can concentrate on searching for environmental differences, such as diet, poisoning, trauma, family psychosocial dynamics, or infection. When MZ twins have been subjected to different agents or exposures—because they have been raised in different families or simply because they have had different experiences—comparing them shows how environment might affect risks and offers clues as to why one twin develops schizophrenia when the other does not.
For it is usually the case in schizophrenia that only one twin is affected. If one identical twin develops it, there is only a 40 percent chance that the other will, which means that schizophrenia cannot be wholly genetic. Were genetics the sole determinant, every twin pair would be concordant—that is, either both individuals would have schizophrenia or neither would have it.
However, when identical twins are compared to fraternal, or dizygotic (DZ), twins, who share only half their genes as opposed to all of them, the results suggest the strength of genetics as a contributor to the disease: only 17 percent of fraternal twins are concordant, compared to 40 percent of identical twins.
According to Torrey, now the director of the Stanley Medical Research Institute in Chevy Chase, Maryland, genetics is an important factor in schizophrenia, but a secondary one. “I personally think that the majority of cases of schizophrenia are caused by an infectious agent with a genetic predisposition, and that the initial infection takes place in early childhood,” says Torrey. “From two-thirds to three-quarters of schizophrenia cases (as well as cases of schizoaffective and bipolar disorders) will turn out to have an infectious component, although they may have genetic predisposition as well.”
Moreover, no single-gene mutation that produces schizophrenia has been identified. This could mean that a complex interaction among genes causes schizophrenia, or it could mean that family members of a schizophrenic have a heightened risk of developing the disease because they share another, nongenetic risk factor, such as exposure to the same toxin or microbe.
People with schizophrenia suffer higher rates of rare genetic mutations, genetic differences that involve hundreds of different genes and may disrupt brain development. So, many genes may interact in a complex multifactorial manner with one another or with an environmental insult—including pathogens—to produce schizophrenia. Thus, genes and microbes are not mutually exclusive causes of the disease. Both may be necessary, and there are likely to be more than one set of factors, just as many genes have been implicated so far.
And while twin studies have provided some of the best-regarded evidence for genetic roots of schizophrenia, they have been haunted by several misconceptions and limitations. For one thing, twins are not truly representative of the population; they’re more often born premature and lower in weight than singletons.5 As I’ve said, if schizophrenia were wholly genetic, we would expect a 100 percent concordance. But 52 percent—more than half—of schizophrenic MZ twins are discordant, and so are 40 percent of twin pairs with autism. Yet researchers have tended to see the glass as half full, arguing that a 52 percent discordance rate for schizophrenia means that 48 percent of MZ twins are concordant for the disorder, suggesting a strong role for genetics.
The problem is, identical twins are not genetically identical. Although they have the same DNA sequences, identical twins vary slightly genetically for several reasons, from changes called single nucleotide polymorphisms (SNPs) to copy number variations (CNVs), in which small additions or deletions are made to regions of the DNA.6 DNA methylation, a biochemical process in which a methyl group (CH3) is added to certain DNA building blocks, called nucleotides, is another source of change that affected 6 percent to 20 percent of twins in one study.7
Pairwise Twin Concordance Rates for Schizophrenia and Other Disorders of the Central Nervous System
Disorder: Huntington’s disease
Identical Twins (%): 100 (14/14)
Fraternal Twins (%): 20 (1/5)
Disorder: Down’s syndrome
Identical Twins (%): 95 (18/19)
Fraternal Twins (%): 2 (2/127)
Disorder: Epilepsy
Identical Twins (%): 61 (20/46)
Fraternal Twins (%): 10 (13/126)
Disorder: Mental retardation
Identical Twins (%): 60 (18/30)
Fraternal Twins (%): 9 (7 /77)
Disorder: Bipolar disorder
Identical Twins (%): 56 (44/79)
Fraternal Twins (%): 14 (16/111)
Disorder: Cerebral palsy
Identical Twins (%): 40 (6/15)
Fraternal Twins (%): 0 (0/21)
Disorder: Autism
Identical Twins (%): 36 (4/11)
Fraternal Twins (%): 0 (0/10)
Disorder: Poliomyelitis
Identical Twins (%): 36 (5/14)
Fraternal Twins (%): 6 (2/31)
Disorder: Congenital anomalies of the CNS
Identical Twins (%): 33 (2/6)
Fraternal Twins (%): 0 (0/5}
Disorder: Schizophrenia
Identical Twins (%): 28 (97 /341)
Fraternal Twins (%): 6 (36/587)
Disorder: Multiple sclerosis
Identical Twins (%): 27 (17 /62)
Fraternal Twins (%): 2 (2/88)
Disorder: Parkinson’s disease
Identical Twins (%): 0 (0/18)
Fraternal Twins (%): 7 (1/14)
Source: E. Fuller Torrey, Ann E. Bowler, Edward H. Taylor, and Irving I. Gottesman. Schizophrenia and Manic-Depressive Disorder. New York: Basic Books, 1994.
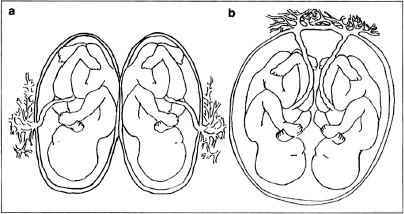
Quite aside from these small but possibly significant genetic differences, identical twins can look very different and enjoy vastly different states of health. Twin-to-twin transfusion syndrome is a perfect example. In this syndrome, identical twins differ in weight, color, and overall health. In nearly one of three cases, these identical twins differ so significantly that they don’t resemble each other. One twin might weigh as much as two pounds more than the other; this twin owes his size to the fact that the twins shared the same placenta (not all identical twins do) and he received the lion’s share of the circulating nutrients and oxygen from their mother during fetal development. In fact, these oversize twins are often born so packed with red cells that they look reddish, while the deprived twins are small, pale, with low blood sugar and poorly nourished. The large, ruddy twin is often jaundiced and suffers from cardiac hypertrophy. As you can see from the photograph of twins who have twin-to-twin transfusion syndrome, there is little identical about them aside from their DNA sequences.

These newborn twins suffer from twin transfusion syndrome (TTS), which causes a marked difference in their size, coloring, and medical status.
The significance for infection and mental illness lies in why this dramatic difference defines the twins. Most identical twins—three of every five sets—share a placental circulation, and this circulation carries not only nutrients but also antibodies and pathogens like those suspected of causing schizophrenia—including Toxoplasma gondii, influenza, herpes simplex virus 2, cytomegalovirus, and others. This means that identical twins who share a placental circulation8 risk acquiring these infections and antibodies from their mother. If infection (or damage caused by overzealous antibodies) is the cause of schizophrenia, then twins who share circulations should have a higher percentage of concordance than those who do not.
And this is precisely the case. The late developmental neurobiologist Paul H. Patterson, professor of biological sciences at the California Institute of Technology, performed in-depth studies and determined that “the concordance of schizophrenia in monozygotic twins who share a placenta is much higher (60 percent) than in the minority of monozygotic twins who do not share a placenta (11 percent).”9 Another 1995 study in the Schizophrenia Bulletin analyzed the possible permutations and concluded that “a shared prenatal viral infection may account for much of the high concordance for schizophrenia in identical twins.”10
It was long thought that a mother’s infection could not trigger schizophrenia or other psychoses in the fetus because the placenta acts as a barrier. But, says Torrey, this is a very imperfect barrier and it is frequently breached.11
The genetic interplay may be complex, but today, evidence from twin studies and other research suggests that sharing something—infection, nutrition, toxins—in the fetal and early childhood environments increases one’s risk of schizophrenia. As this chapter will explain, the evidence for infection’s role is now well established.
We know all this today, but over the past half century, doctors have causally linked schizophrenia to everything from family dynamics to brain-chemistry imbalance.
Schizophrenogenic mothers
Not too long ago, in fact, they knew whom to blame: Mom.
“Schizophrenogenic mothers,” wrote psychiatrist Frieda Fromm-Reichmann in 1960, trigger insanity in their children with their “domineering, cold, rejecting, possessive, guilt-producing” personalities.12 Fromm-Reichmann’s Principles of Intensive Psychotherapy and other textbooks used in psychiatrists’ training endorsed these theories, unquestioningly citing bad mothering as a prime risk factor. Throughout the 1980s, schizophrenia continued to be laid at Mom’s door as she busily inculcated madness in her children with her harsh, shrewish brand of controlling behavior.
Stanford psychological anthropologist Tanya Luhrmann described the theory behind this maternal vector of insanity in a 2012 essay, explaining, “She delivered conflicting messages of hope and rejection, and her ambivalence drove her child, unable to know what was real, into the paralyzed world of madness. It became standard practice in American psychiatry to regard the mother as the cause of the child’s psychosis, and standard practice to treat schizophrenia with psychoanalysis to counteract her grim influence.”13
Psychiatry held that the fathers in these schizophrenia-incubating homes were abjectly submissive to the mothers—when Dad had not died, absconded, or otherwise disappeared. Thus, fathers could not shield their children from the mothers’ poisonous influence. This information was presented unquestioningly in my 1970s psychology classes, and we scribbled away in a Pavlovian response, keeping our heads down and our notebooks filled as we swallowed such “facts” and regurgitated them for tests.
No one understands the corrosive calumny of blaming Mom more than E. F. Torrey, Rhoda’s brother. Their mother did not escape the veiled maternal reproach when, on that autumn day in 1957, she and her family sought answers at Massachusetts General Hospital about why schizophrenia had struck Rhoda. “They said it had been brought on by ‘family problems’ and the shock of my father’s death when my sister was young,” said Torrey. “It made no more sense to me than the man in the moon. Why didn’t I have schizophrenia if that’s what caused it?”
Torrey researched Rhoda’s disease and quickly learned that a belief in maddening mothers failed to explain key facts about the illness. “Because of my sister,” he said, “I had a firsthand contact with schizophrenia, and it looked no more like a psychosocial disease than diabetes does. The idea that it was psychosocial to me was absurd. What my sister had looked very much like a brain disease. It was a brain disease.” In the 1960s and 1970s, as Torrey trained in medicine, he started looking at the epidemiology of the disease, and several things intrigued him.
As Torrey familiarized himself with the medical literature on schizophrenia, he was struck by cases of inpatients who had been admitted with diagnoses of schizophrenia but began limping a week or so after the beginning of their hospital stay, prompting staff to call in a neurologist. When the neurologist performed a lumbar puncture, the person turned out to have encephalitis, cytomegalovirus infection, or another infectious disorder. Acting on his insights, Torrey compiled cases that presented like schizophrenia or bipolar disorder but turned out to have a clear infectious basis, and in the 1990s he published a paper detailing them and their likely significance.
“To me, that was an important precedent,” Torrey concluded. “While a medical student, I had learned that schizophrenia was seasonal, it was urban, and it looked like a lot of infectious neurologic disorders. It looked like an infectious disease.”
Why, then, had not his more experienced professors grasped this? “Psychiatry is just like all parts of medicine; it has its fashions, and at that time the fashion was obviously Freudian or genetic,” Torrey explained. “But it was one or the other, and if you wanted to be successful in your chosen profession, you knew you should follow what the senior people said. That was the way things were at that time.
“But”—he sighed—“how can I say this politely? I did not come out of my training with a strong belief that my senior colleagues necessarily knew what they were talking about. I think that’s putting it as politely as I can.”
But despite such insights, his attempts to reassure his mother that Rhoda’s illness was not her fault were in vain. He was, after all, a recent medical-school graduate arguing against fervently held articles of psychiatric faith.
One could say that Torrey has sought to exonerate his mother and help his sister ever since. He decided to become a psychiatrist, and he chose schizophrenia as his focus. As his clinical experience mounted, he learned that schizophrenics suffer from puzzling physical deficits as well as mental symptoms; for instance, when a schizophrenic’s gait was tested, she often staggered slightly and deviated from the straight and narrow, like a drinker over the limit. The presence of large numbers of lymphocytes, a type of white blood cell, indicated that their bodies were fighting inflammation. CT scans revealed enlarged ventricles in the brain, fluid-filled spaces within each hemisphere that expanded alarmingly as brain tissue was lost. But lost to what?
He received his psychiatric training during a period when Freud’s psychoanalytic theories and Fromm-Reichmann’s schizophrenogenic mothers were universally embraced, but Torrey pursued a very different vision of the illness that diverted his research attentions from psychology to infectious disease.14
As a psychiatrist, researcher, NIMH official, and, later, founding director of the private Stanley Medical Research Institute, Torrey has done more than spend his career unraveling the physical origins of schizophrenia. Probing, identifying, and documenting the infection at its core, he has also fostered a research climate that enables other investigators the world over to do the same; since 1989, SMRI has awarded more than $550 million in research grants to researchers in over thirty countries.
In 1980, a decade after Torrey’s arrival in the Washington, DC, area, he partnered with infectious-disease expert and kindred spirit Robert Yolken, MD, of Johns Hopkins University. Among other research goals, the pair sought to discover which infectious agents could produce the physical deficits of schizophrenia. They considered Epstein-Barr virus, or EBV, which causes mononucleosis, nasopharyngeal carcinoma, and, rarely, Burkitt’s lymphoma, an aggressive but curable cancer of the lymphatic system. Cytomegalovirus, or CMV, was another candidate, as it is easily passed from a pregnant woman to her unborn child, and so was Toxoplasma gondii, a unicellular parasite carried by house cats that is spread by undercooked foods and by cat litter and that at the time infected 20 percent of the people living in the United States. The duo also scrutinized influenza and herpes simplex virus type 2.
The schizophrenic subjects of Torrey and Yolken tended to harbor antibodies for these microbes, which provided evidence of an infection. But the researchers could find no actual microbes; the pathogens themselves eluded them, and they concluded that the infections must have occurred years earlier, leaving only antibody “footprints” to show the microbes had passed through.15 Might infection have left its mark as early as childhood, infancy, or even in the womb? they wondered.
Back to the future
In their seemingly novel search for infectious causes of schizophrenia, Torrey and Yolken were in fact not crafting a new theory but updating an old, all-but-forgotten one.
How so? As chapter 1 disclosed, Sigmund Freud began his career as a neuroanatomist, but when he ceased scrutinizing brain structure to plumb the unconscious mind and develop the talking cure, he took the field of psychiatry with him.
Well, not all of it. For the first three decades after its discovery and labeling, schizophrenia, or dementia praecox, as it was then known, was regarded by many psychiatrists as a disease caused chiefly by infection.
The term schizophrenia was coined by Eugen Bleuler, a fin de siècle Swiss contemporary of influential psychiatrist Emil Kraepelin, who developed a categorization, or nosology, of psychiatric disorders in the late 1890s.16
Some turn-of-the-century doctors, including Kraepelin, associated schizophrenia with infection because psychosis was an occasional symptom of bacterial diseases like typhoid fever, tuberculosis, and diphtheria. In 1904, a review article observed that “insanity following infection is generally of short duration,” but in some cases it was known to linger.17
Richard Noll, a clinical psychologist and historian of medicine, assures us that in the late nineteenth and early twentieth centuries, the dominant theories attributed schizophrenia to heredity and “intoxication,” or poisoning, caused by focal infections of the sex glands, the intestines, and the mouth.18
Although Kraepelin’s devotion to the infection model is often downplayed or ignored altogether, he might have been the theory’s most famous proponent.19 His 1895 text Lehrbuch der Psychiatrie speculated that dementia praecox was not a psychological issue but rather a “tangible” morbidity of the brain caused by autointoxication from pathological substances that were produced “somewhere in the body.” It could be cured, he averred, by locating and removing the sources of this infection. Accordingly, Kraepelin recommended major abdominal surgeries,20 such as the excision of (presumably infected) colons, as well as ovaries or testes and other organs associated with reproduction.
Although we identify Freud with the talking cure, even he indulged in a flirtation with surgery as a cure for psychiatric symptoms. As psychoanalyst Jeffrey Masson,21 former director of the Freud archives, revealed, it proved disastrous. Having ascribed his Viennese patient Emma Eckstein’s masturbation to a “nasal reflex neurosis,” Freud and surgeon William Fliess performed a series of operations on the twenty-seven-year-old, removing portions of her nose to correct the putative nose malformation behind her sexual compulsion. Infections and other complications ensued, some due to errors such as failing to remove a gauze pack in her nose after surgery.22 These complications nearly killed her and left her permanently disfigured “with the left side of her face caved in.”23
Such gruesome outcomes did not quell psychiatrists’ interest in the focal-infection theory. Two decades later, Chicago surgeon Bayard Taylor Holmes’s research on schizophrenia led him to undertake corrective surgeries. In a once common, if ethically dubious, tradition,24 in May of 1916, he performed abdominal surgery on his own child, his schizophrenic son, Ralph, twenty-six.
Four days later, Ralph died.
This did not stop Holmes, who continued to excise suspect organs and downplayed the tragedy of his son’s death as he sought to popularize the surgical removal of organs to cure mental disease.25
In 1917, Holmes established the Psychiatric Research Laboratory of the Psychopathic Hospital at Cook County Hospital, where surgeries were performed on twenty-two patients with schizophrenia. Within ten months, two of them died.26 Meanwhile, at Trenton State Hospital, Dr. Henry Cotton performed 645 major surgeries on patients with schizophrenia, manic-depression (bipolar disorder), and other psychiatric diseases. Thirty percent of them died—nearly one in three, a truly deplorable mortality rate.
The tragic deaths from surgeries meant to cure mental illnesses cast a pall over infection theories. Freud, who had distanced himself from such surgery after Emma Eckstein’s mutilation, focused on developing a structural theory of mind, writing the Interpretation of Dreams and becoming the father of psychoanalysis. Infection theories were dismissed by his many acolytes, even after the discovery, detailed in chapter 1, that general paresis was caused by the syphilis bacterium.
But soon, a global crisis, the 1918–1920 influenza pandemic, made one infectious cause of psychosis impossible to ignore. The pandemic left in its wake a raft of psychoses and other mental disorders among survivors that dramatized the role of infection in madness. Influential Kansas psychiatrist Karl Menninger, who popularized psychiatry for the everyman, also declared his support for the infection theory.
Pandemic madness
The global influenza pandemic that began in 1918 supported theories that infection lay at the root of schizophrenia by providing dramatic evidence that infection—in this case, influenza infection—could result in psychosis.
The Spanish flu pandemic, as it was known, eclipsed the Black Death by killing fifty to a hundred million27 people,28 more than all the deaths in World War I. This dread global superbug began like a garden-variety flu, but within hours, the usual symptoms were compounded by dizziness, weakness, and pain.29 The victim’s inflamed mucous membranes and sneezing rapidly progressed to hemorrhaging, vomiting, and constipation.30
Cytokines, those signaling molecules that figure extensively in cellular communication, are known to respond to infection by recruiting immune cells to attack the invader, and some epidemiologists now speculate that the damage was due to an ill-fated cytokine storm as the bodies of the infected mounted a vigorous but ineffective assault against the virus.
Mental symptoms also abounded, according to frequent newspaper and physicians’ reports as well as respected journals such as the Journal of the American Medical Association, or JAMA, which noted, “The frequency of mental disturbances accompanying the acute illness in the epidemic has been the subject of frequent comment.”31 As early as the 1890s,32 the medical and popular conception of influenza had been changing to include a mental-illness component,33 as physicians reported the “hypochondria, melancholia, mania, and depression that characterized general paralysis,” or paresis. They also described anhedonia (a lack of enthusiasm for activities a person once enjoyed), loss of energy, apathy, and sadness.34
Moreover, a psychiatric symptom called nervous exhaustion, or neurasthenia, became the definitive symptom of influenza. Specialists35 like London’s Julius Althaus, senior physician at the Hospital for Epilepsy and Paralysis, began to compare influenza to paresis, opining that in both cases, the infectious agent attacked the nervous system to produce insanity.36 Sir Benjamin Ward Richardson, a physician and the author of Hygeia: A City of Health, described influenza as an “epidemic neuroparesis” that generated “intense depression” in patients.37 Even murders and suicides were attributed to the lingering legacy of influenza infection.38
The theory that infection might cause schizophrenia and other psychoses is bolstered by the fact that some of the 1918 flu survivors never recovered but instead developed a spectrum of lingering mental disorders, most notably postencephalitic Parkinson’s syndrome.39 Between 1915 and 1926,40 an epidemic of encephalitis lethargica, also known as von Economo’s encephalitis, attacked their brains, generating tremors, a slowing of physical and mental responses, profound personality changes, and even psychosis. Their psychiatric ailments included emotional disorders such as depression, anxiety, obsessive-compulsive disorder, and apathy.
These survivors of the 1918 flu pandemic sank irretrievably into psychosis and catatonia, and they were institutionalized, untreated, and forgotten as the century wore on.41 “Interest in infectious theories of psychiatric disorders waned in the United States and Europe in the 1930s as Freudian theories became prominent,”42 write Yolken and Torrey. Thus it was that fifty years after the pandemic, when Torrey proposed brain infection as a cause for schizophrenia in the 1970s, the precedent had been forgotten and the idea struck his psychiatric colleagues as absurd.
Undeterred, Torrey and Yolken compared normal and schizophrenic brains, probing them for evidence of inflammation and structural abnormalities (like enlarged ventricles), and, seeking the pathogens responsible, they focused on another signature of infection: seasonality.
A hibernal plague?
People born in the late winter and early spring, the peak flu season, are more likely than others to develop schizophrenia. The children born during cold-weather months in the Northern Hemisphere are also more likely to be exposed at an early age, either in utero or soon after birth, to microbes that are common during those months. This seasonal rise in schizophrenia risk is modest—only from 5 to 8 percent—but it has proven remarkably consistent across more than 250 studies. “How many psychosocial factors are going to give you a seasonality of birth?” asked Torrey. “The only seasonal birth patterns I knew were German measles, influenza, and other infectious agents, especially when the peak is in late winter and early spring: It just cried out ‘infection!’ The birth-month effect is one of the most clearly established facts about schizophrenia,” continued Torrey. “It’s difficult to explain by genes, and it’s certainly difficult to explain by bad mothering.”43
Thinking of cold-weather viruses puts people in mind of the flu, and indeed, influenza epidemics have been followed a generation later by waves of schizophrenia in England, Wales, Denmark, Finland, and other countries. The seasonal pattern also emerged when scientists studied people suffering from bipolar disorder, which is characterized by extreme mood swings, from euphoria to profound sadness, and seasonality emerged as a feature of multiple sclerosis as well. If these illnesses also turned out to be associated with an infectious disease that strikes in winter and early spring, the infection might explain the diseases’ seasonality, wrote Torrey.
Torrey dedicated himself to identifying which pathogens triggered diseases like schizophrenia and bipolar disorder. He knew that fingering the microbial culprits was necessary if scientists were to prevent or even cure schizophrenia with a vaccine or a specialized antibiotic. Preventive strategies such as intensive prenatal care that stressed pathogen avoidance might also help protect future generations from schizophrenia. He knew that schizophrenia was a progressive disease and there was evidence that treatment mitigated its severity. Determined to unmask the offending viruses or bacteria, he embarked on what would be a decades-long microbial quest.
The laboratory advocate
Torrey cares about the fate of schizophrenics outside the laboratory as well. His research into schizophrenia’s shrouded infectious origins is just one aspect of his zeal on behalf of the mentally ill. He became the prime catalyst in building the National Alliance for the Mentally Ill into a politically powerful advocacy organization, contributing the hundreds of thousands of dollars he earned from his successful 1983 handbook Surviving Schizophrenia: A Family Manual.44 He has always given of himself, too, volunteering his services in the South Bronx, on a remote Alaskan island, and at Washington, DC, homeless clinics for several decades.
In his 1974 book The Death of Psychiatry, Torrey took his fellow psychiatrists to task for devoting themselves to the relatively minor concerns of the privileged “worried well,” to the detriment of poor psychotics.
In his view, misguided policies such as deinstitutionalization, or “community psychiatry,” which emptied mental institutions in the 1970s, contributed to homelessness, as it exacerbated the plight of the seriously ill, flooding the streets with people who were too sick to care for themselves. It’s been suggested that “untreated psychiatric illnesses constitute one-third, or between 150,000 and 200,000 people, of the estimated 744,000 homeless population.”45 Torrey also denounced the profession for embracing theories that blamed devastated parents (like his mother) for their children’s ailments. Families were grateful to Torrey, but this stance evoked bitter resentment from many members of his profession.
Torrey was careful to temper his criticisms with expressions of respect for his fellow practitioners, noting that, although Freudian models had not worked well, this negated neither the benevolence nor the good intentions of the healers who embraced them.
This was in contrast to the approach of his better-known contemporary Dr. Thomas Szasz, author of The Myth of Mental Illness. Szasz, who died in 2012, dismissed mental illness as a malicious invention of psychiatry that was used as an agent of control. A strong advocate for human freedom and individual liberty, he shocked the profession by arguing that mental illness was in no way a real disease akin to the physical disorders caused by bacteria or viruses. Szasz admitted the reality of only those few mental disorders with a clear biological pathology, like Alzheimer’s. The others, he averred, were convenient fictions.
In his testimony to a U.S. Senate committee, Szasz not only decried psychiatry as an unalloyed agent of control but also compared doctors who incarcerated the mentally ill to prison wardens. “Since theocracy is the rule of God or its priests, and democracy rule of the people or of the majority, pharmacracy is therefore the rule of medicine or of doctors.”46
Some lump Torrey in with Szasz, seeing in the latter’s views a wholesale rejection of psychiatry and a reductio ad absurdum of Torrey’s theories. But in every important way, they are diametric opposites.
Torrey consistently expressed respect for both the reality of mental illness and the aims, if not the methods, of the profession. For him, mental illness was all too real, while Szasz denied the biological reality of schizophrenia and other serious mental diseases. Szasz’s staunch defender Kentucky psychologist Robert A. Baker called Szasz “Psychiatry’s Gentleman Abolitionist” in an article that dismissed the search for microbes that trigger schizophrenia, Torrey’s holy grail, as “a category error analogous to attempting to photograph a dream.”
For Szasz, the primacy of individual liberties led him to unilaterally oppose the forced treatment that Torrey regarded as essential to the health and recovery of many schizophrenics whose anosognosia caused them to reject the very medication they needed to remain well. Torrey definitively distanced himself from Szasz in the pages of the New York Times, calling him “a man who has produced more erudite nonsense on the subject of serious mental illness than any man alive.”
Despite the more measured language with which Torrey criticized psychiatric theories and methods, his writings brought painful political consequences, forcing him to rely on his considerable resourcefulness. When the NIMH dismissed him in a political dispute over his writings, Torrey obtained the support of a wealthy couple whose son suffered from schizophrenia. With their funds, he established the Stanley Medical Research Institute, with himself as director and his patrons’ son as its lawyer. He now commands a budget so massive that its disbursements rival the government’s own, and he also controls the largest library of human brains in the world, which allows him to influence the direction of schizophrenia research by supporting selected researchers with these funds and neural tissues.
The enemy within
As Torrey and Yolken continued to ponder theories that might explain the seasonality of schizophrenia, French scientist Hervé Perron came into their orbit. Although still a doctoral candidate at France’s Grenoble University, Perron jettisoned his PhD research topic when he became intrigued by the possible role of unusual agents called retroviruses in disease. Unlike DNA viruses, which take over cells by translating their DNA into RNA, retroviruses do the opposite, converting their RNA into DNA. Today, the term retrovirus is familiar largely because HIV is one, but in 1987, retroviruses were truly novel. So was Perron’s theory that they were implicated in multiple sclerosis,47 a disease that shows a seasonality similar to that of schizophrenia. Torrey and Yolken took interest.
Perron procured spinal fluid from many people with MS and tested it for reverse transcriptase, an enzyme used by all retroviruses. He found the enzyme and took electron-microscope photographs of the retrovirus that harbored it. Perron sought to identify the retrovirus.48
It was 1987 and the scientific world was catching up to Torrey. New research technologies, including neuroimaging, expanded the physical and metaphorical vision of scientists and helped to supplant the psychosocial view of schizophrenia with a focus on identifying distorted brain structures and function. Just as microscopes enabled sixteenth-century Dutch scientists to see various creatures that caused disease, powerful tools like magnetic resonance imaging, or MRI, machines visualize the brain with crystal clarity. Positron-emission tomography, or PET, scans showcase dynamic video images of the living brain. Scientists now agreed that they were looking at a brain disease.
Torrey and Yolken were in their second decade of seeking the microbes that cause schizophrenia, and Perron was still on a parallel track, seeking the retrovirus that seemed to be involved in multiple sclerosis. In 1996, eight years of sixteen-hour workdays paid off for Perron when he finally identified the responsible retrovirus. It was marvelous. Not only was Perron’s discovery a previously unidentified viral factor in all MS, but it was also a previously unidentified type of virus. Unlike viruses that are normally borne on the breath, sneezes, blood, sweat, saliva, or semen of others, this virus is endogenous—it lives within us.
Because the virus Perron discovered lurks within the DNA of each of us, he called it human endogenous retrovirus W, or HERV-W. In the 1970s, scientists had been mystified to see viruses emerging from the cells of healthy baboon placentas in electron-microscope images.49
Unlike typical viruses, such as influenza, which kill the cells they infect, retroviruses allow the cells to live; they insinuate themselves inside, slipping their own genes into the cell’s DNA so that future cell divisions will incorporate the retroviral genome. Perron estimates that perhaps sixty million years ago, a few million years after the dinosaurs disappeared, HERV-W slipped into the genome of one of our pre-simian ancestors, where it met with an uncommon piece of good luck: it landed in a germ-cell line that produced reproductive material—either sperm or eggs—and thereby assured itself of an entrée into all future generations; “a rare, random event,” as Robert Belshaw, an evolutionary biologist at the University of Oxford, put it in Discover magazine.50 Today, the human genome contains 100,000 of these viral squatters, which account for more than 40 percent of all human DNA.51
“Endogenous retroviruses are a very interesting set of agents,” says Yolken. “To some extent, they’re genes, part of the genome; to some extent they’re viruses, because they’re actually derived from viruses that infected our ancestors.”
“This makes a disease they encourage look like a genetic disease,” adds Torrey.
If HERV-W lives within us all, why doesn’t everyone develop MS or the other diseases that endogenous retroviruses code for? Because our bodies are vigilant. We suppress endogenous retroviruses, preventing their translation into proteins and subsequent expression into disease by straitjacketing them, binding them tightly to coils of macromolecules. But occasionally a HERV-W virion escapes to crank out dangerous proteins and cause disease. When HERV-W does this, more than a dozen studies have shown, that person develops MS. Torrey and Yolken speculated that although our bodies normally restrain HERV-W, an infectious illness that strikes during the neonatal period can weaken the bonds that confine it.
As Perron triumphantly unveiled the mystery of MS and endogenous retroviruses, Torrey and Yolken considered whether retroviruses might also be culprits in some mental disorders. They knew by then that AIDS was caused by HIV, a retrovirus, so the duo investigated whether this retrovirus might trigger the psychosis experienced by many AIDS patients, who suffer symptoms that are also found in schizophrenia.
They found that the retrovirus they sought was HERV-W.52 Several studies verified the presence of HERV-W in the brains and body fluids of people with HIV, and Perron’s own follow-up study in 2008 revealed that 49 percent—nearly half—of people with schizophrenia harbor HERV-W, while only 4 percent of people without schizophrenia do.
Finally, after decades of searching, Torrey and Yolken had unimpugnable evidence that an infectious agent—the retrovirus HERV-W—played a key role in schizophrenia. This eureka moment was further heightened by the discovery that the retroviral concentration was proportional to the degree of brain injury, says Perron: “The more HERV-W they had, the more inflammation they had.”53
A question remained: How does the same retrovirus produce MS and psychosis and raise the risk of schizophrenia, bipolar disorder, and severe depression? Torrey and Yolken theorized that the mechanism of the immune response, not the specific infectious agent, was key and that a number of pathogens might trigger schizophrenia. Accordingly, they determined to probe the roles of influenza, Toxoplasma gondii, and other microbes.
Poisoned wombs?
And yet, in a perverse manner of speaking, is Mother to blame after all? Do pathogens sow the seeds of schizophrenia in the womb, and if so, how? In one scenario outlined earlier, the fetus or newborn falls victim to friendly fire from its own immune-system defenses or from those of its mother. HERV-W may trigger not only schizophrenia but bipolar disorder and other illnesses.
Infection with herpes, toxoplasma, cytomegalovirus, influenza, and half a dozen other common pathogens release the HERV-W viruses, which flood the newborn’s cerebral fluid and brain, ferrying proteins that can trigger an inflammatory response from the infant’s fledgling immune system. White blood cells, which engulf or otherwise neutralize immune-system threats, emit cytokines, which are molecules that summon other immune-system cells in an attempt to vanquish dangerous intruders. But these immune cells can also attack healthy brain tissue, especially in infants and young children whose untutored or naive immune systems are less able to recognize and selectively assault invading threats.54
To test this theory, Perron injected HERV-W from people with MS into mice. The animals quickly lost their motor coordination, just as those humans with the disease do. After stumbling about for a while, the mice became paralyzed and then died of brain hemorrhages.
But if Perron first removed the immune cells known as T cells from the mice, they survived receiving HERV-W. This murine model illustrated an important variable: immune vigor. The immune system’s purpose is to protect us from infectious threats by disabling pathogens. But in this case of friendly fire, the immune cells cause diseases, including schizophrenia, by attacking the person’s own brain cells instead of the pathogenic invaders.
When Perron removed the animals’ T cells, he prevented such injury, and so prevented the MS. This means that whether a person who is exposed to HERV-W will develop MS or schizophrenia may depend on his or her immune system’s responses.
Like people with MS, people with schizophrenia seem to suffer profound but indirect damage from the inflammation created by their own immune systems. However, in schizophrenia, the neurons are overstimulated, not killed, which is why the symptoms of schizophrenia are more subtle. “The neuron is discharging neurotransmitters, being excited by these inflammatory signals,” Perron explained. “This is when you develop hallucinations, delusions, paranoia, and hyper-suicidal tendencies.”
In people diagnosed with schizophrenia, the cortical surface of the brain, the thalamus, the limbic system, and the basal ganglia shrink, while crevices called sulci and normally fluid-filled spaces, ventricles, enlarge by as much as 50 percent. Such changes may be the terrible legacy of a prenatal virus, although as Ian Lipkin, MD, director of the Center for Infection and Immunity at Columbia University, points out, people, including fetuses, may react very differently to infections. “A gunshot to the stomach is bad news for anyone, but microbial assaults yield more varied responses partly based on phenotype.”55 Factors such as genetic phenotype, age, general health, stress, inflammation, and the presence of environmental cofactors all affect an individual’s susceptibility.
Crazy cats
“Schizophrenia was first seen in the late 1700s and first described clearly between 1808 and 1810 independently in both London and Paris. The bulk of people who described it said ‘We haven’t seen this kind of thing before,’” says Torrey. “I became completely convinced that schizophrenia was a relatively recent disease. I still am.”
In his book The Invisible Plague: The Rise of Mental Illness from 1750 to the Present, Torrey reveals that around 1871, schizophrenia swiftly transformed from a rare to a relatively common disease. That same year, as U.S. schizophrenia rates rose sharply,56 cat ownership became popular in England and America, as chapter 1 noted.
This is no coincidence, Torrey explains; cats transmit the one-celled parasite called Toxoplasma gondii to humans. T. gondii causes the disease toxoplasmosis, and it is already implicated in congenital illness when it infects a fetus.57 Torrey and scientists on two continents think it does more; they think that a T. gondii infection causes schizophrenia.
In 1938, a newborn girl at New York City’s Babies’ Hospital became the first person to be definitively diagnosed with a Toxoplasma gondii infection, which she had acquired in her mother’s womb. The parasite killed her within days, and doctors soon realized how dangerous T. gondii is for the unborn. It can not only kill outright but also cause children who are infected in the womb to be plagued by a congenital syndrome that includes deafness, retinal damage, seizures, mental retardation, and microcephaly (an abnormally small head).58 They may also develop the disease toxoplasmosis, in which flu-like symptoms are followed by an inflammation of the brain, referred to as encephalitis, and various neurological deficits. Toxoplasmosis can harm the heart, liver, ears, and eyes as well. Because T. gondii is transmitted by cats, obstetricians warn pregnant women who have cats not to touch litter boxes and to cook food thoroughly in order to kill any errant parasites.
Indoor cats can shed the parasite, so if they walk on surfaces that later hold food, the food can become contaminated. Playing with cats can lead to contamination if you don’t wash your hands carefully before eating or placing your hands in your mouth. Outdoor cats that leave their feces on the ground also sometimes excrete into domestic animals’ feed. This adulteration results in T. gondii tissue cysts within the animals’ muscles. If humans eat this meat without cooking it thoroughly, they may become infected.59
So it is not surprising that one in every four U.S. residents is infected with T. gondii. In France the infection rate is about 50 percent,60 thanks in large part to Gallic gustatory habits like a penchant for steak tartare and other forms of uncooked meat. In regions such as West Africa, the infection rate can soar as high as 80 percent.
Conventional wisdom long held that healthy adults who became infected weren’t harmed, only those who were immunocompromised in some way due to HIV infection or some other illness. But Torrey and Yolken found that symptoms from headache to fever to anorexia were common even in adults with healthy immune systems.
Moreover, decades of human studies in countries such as the Czech Republic, Turkey, and Mexico have revealed that T. gondii dramatically changes the mental status of adults. Infected adults suffer behavioral changes that include increased recklessness, sexual attractiveness, sexual aggression, and receptivity. They also engage in risk-taking behavior that can be hazardous. For example, it makes the infected dangerous behind the wheel. In one Czech study, infected men were twice as likely as others to be involved in a traffic accident, while infected women seemed more than usually receptive to the opposite sex, a tendency that would certainly serve the parasite’s purposes by increasing its spread to their sexual partners. A study of new mothers even revealed that infected women are more likely to commit suicide.
It’s fortunate that we have human studies, because trying to evaluate subtle changes in cat behavior when they are infected with T. gondii reveals the limits of animal models, says Yolken. “How can you tell if a cat is crazy? Well, my daughter thinks that if a cat is nice and comes up to you when called and doesn’t scratch the furniture, that would be a cat with schizophrenia.
“On the other hand we can ask questions about whether T. gondii changes behavior by looking at rat and mouse behavior: That can be done in animal models. Primates are more similar, so we might use chimpanzees or monkeys to investigate, but their changes are not the same ones we have, so we’re limited in what we can do.”
For decades, Torrey, Yolken, and their colleagues abroad, including Czech parasitologist Jaroslav Flegr, author of Frozen Evolution: Or, That’s Not the Way It Is, Mr. Darwin, suspected that T. gondii caused subtle changes in an infected fetus that could lead to schizophrenia twenty years later. In 2008 Yolken and Torrey published a study indicating that the peak age for becoming infected by T. gondii, between eighteen and thirty-five, coincides with the peak age of the first signs of schizophrenia. They also noted that in areas where felines are rare, the prevalence rates of both toxoplasmosis and schizophrenia are low.61 In 2005, studies in journals like the American Journal of Psychiatry62 found that children of mothers who contracted T. gondii while pregnant did suffer higher rates of schizophrenia than other children. This is a highly suggestive association, especially because the parasite is known to be neurotropic—to target brain cells. Collectively, these studies strongly suggest that infection with toxoplasma is a significant risk factor for the development of schizophrenia.
So, fetal infection with T. gondii does correlate with higher schizophrenia rates. However, studies that followed people who moved from one region of the world to another found that their rates of both toxoplasma infection and schizophrenia reflected the rates in the part of the world where they’d spent their childhoods. In reviewing thirty other studies, researchers found that individuals who developed schizophrenia or bipolar disorder were significantly more likely to have grown up in a family that owned a cat, but not a dog, between that person’s birth and age thirteen.63
Torrey found that the most strongly positive schizophrenia correlations were not with T. gondii infections acquired in the womb but with infections that struck children and teenagers.64
Why? What could possibly explain why childhood, not gestation or infancy, is when the young are most likely to acquire the toxoplasma that raises their risks of schizophrenia?
Torrey and Yolken blame sandboxes.
“A likely mechanism for exposure to T. gondii in childhood is playing in the dirt of sandboxes contaminated with T. gondii oocysts,” they write, explaining that every uncovered public sandbox studied was used as a litter box by from four to twenty-four cats.65 The cats shed T. gondii eggs and cysts that found their way onto the hands of children, who, being children, eventually put their unwashed hands into their mouths, ensuring T. gondii’s transit into their bodies.
The risk isn’t limited to sandboxes, of course, because the same oocysts are found in the dirt and on outdoor surfaces on which children play. But the sandboxes provided convenient sites for research that showed how urban areas where cats had a high rate of infection became areas where later schizophrenia rates were similarly elevated.66
According to Flegr’s studies, toxoplasma causes schizophrenia by affecting neurotransmitters in the brain, especially dopamine, glutamate, and GABA. For example, T. gondii increases the brain’s dopamine levels by 34 percent, probably through the actions of cytokines, leading Flegr to describe dopamine as the “missing link between schizophrenia and toxoplasmosis.”67 The work of Flegr and others has implicated T. gondii in attention deficit disorder, hyperactivity disorder, and obsessive-compulsive disorder as well as schizophrenia.
The connection between toxoplasmosis and schizophrenia has positive implications for treatment because some of the antipsychotics used to treat schizophrenia are active against T. gondii. People whose schizophrenia is caused by T. gondii could be effectively treated by anti-infectives such as azithromycin, trimethoprim-sulfamethoxazole, and pyrimethamine-sulfadiazine.68
Torrey and Yolken think that both the influenza virus and T. gondii are likely triggers of schizophrenia and bipolar disorder. Perhaps they work via HERV-W as subsequent infections trigger the release of HERV-W, adding to the brain inflammation and causing schizophrenics to lose brain matter over time, as their enlarged ventricles—the spaces or “holes” in their brains—testify. “Enlarged ventricles mean that the brain is shrinking,”69 declares a University of Toronto neurowiki site, and other parts of the schizophrenic’s brain, including the thalamus and the insular cortex, shrink too.70 If influenza and T. gondii infections trigger HERV-W release, this would explain why some schizophrenics are first diagnosed after an infectious illness71 and why the disease, like MS, often waxes and wanes, with other infections causing an exacerbation of symptoms.
“Historically, rubella virus (which causes rubella, or German measles) would be on that list of agents that cause schizophrenia as well,” says Yolken, but the vaccine has all but eliminated it in the West. Different agents cause psychoses, including schizophrenia, in different parts of the world. Malaria and rubella are likely culprits in the developing world, but Yolken also points out that “there are many infectious agents in the Third World that are unknown to us because studies have not been done.”
“Typically, in animal models if you cure the toxoplasma, which we can do, the symptoms get better,” says Yolken. “Preventing or treating T. gondii infection with a vaccine, on the other hand, is a great idea but it is a little further down the line.”
Fetal infection
Children old enough to play in the pathogen-rich dirt are most vulnerable to acquiring mental disease, but earlier exposure—even before birth—can madden as well. The idea that the womb environment may exert lifelong effects on the fetus is certainly not new. In 1992, English epidemiologist D. J. Barker first argued that an undernourished fetus faces an increased adult risk of future heart disease. He also speculated that the same malnourishment somehow inculcates a tendency toward diabetes. Barker didn’t offer much in the way of supporting data, but subsequent research has validated his observations, especially an analysis of pregnant women whose fetuses survived the famine of the Nazi-engineered72 Dutch Hunger Winter of 1944–1945.73
A mother’s high blood pressure, diabetes, and behaviors such as smoking and drinking are all implicated in fetal harm. Each may carry consequences, like mental retardation, diabetes, low or high birth weight, an increased heart-disease risk, or schizophrenia.74 Of course, the father’s medical conditions and behaviors may contribute heavily as well, but fewer studies have been done to document this.
In a joint study by Sweden’s Malmo University Hospital and the National Institute of Mental Health, Thomas F. McNeil learned that trauma at the time of delivery, especially prolonged labor, appeared to affect an infant’s brain structure, resulting in anomalies associated with schizophrenia.75
Infectious triggers do not exclude genetics as a risk factor, because genetics can be closely bound to immune response. For example, Nature published several studies implicating genes for human leukocyte antigens, or HLAs, in schizophrenia, positing that HLAs, not genes, control the production of neurotransmitters. Genes may also require a cofactor in order to be expressed, and a genetic response may determine who gets schizophrenia and who doesn’t when an individual’s immune system is presented with an assault by HERV-W.
Flu season
In the 1980s, Yolken and Torrey turned their attention to the common influenza virus. If neurasthenia was once accepted as a symptom of the flu and if the uncommon flu pandemic of 1918 resulted in psychosis, including schizophrenia-like symptoms, might the common flu trigger schizophrenia in infants and children?
Torrey has conducted carefully designed twin studies of his own to look for associations between influenza, schizophrenia, and bipolar disorder, and he also joined with Yolken to undertake several exhaustive reviews of decades of data76 describing the offspring of women who had contracted influenza during flu epidemics. Dr. Alan S. Brown, a professor of psychiatry and epidemiology at Columbia, conducted large sophisticated analyses of blood assays from pregnant women who had actually contracted influenza, not simply those who’d been pregnant during epidemics.77 This allowed him to more precisely associate infection with later schizophrenia among the offspring. A child whose mother contracts the flu in the first trimester of pregnancy, a period when the fetal immune system is relatively inactive, has a 700 percent higher risk of eventually developing schizophrenia; if the flu hits later, during the mother’s third trimester, the child’s risk of schizophrenia risk is “only” three times greater than that of the general population. Brown concluded that 14 percent of schizophrenia cases were the result of a woman’s contracting the disease while pregnant. All these studies and more, Torrey says, tie schizophrenia to the common flu.78
Torrey hastens to note that not every researcher agrees with him, but he thinks it’s most likely that only the mother is attacked by the virus, but her fetus falls prey to the antibodies she deploys against the infection. The antibodies secrete neurotoxic molecules that can damage the brain, and, in theory, the extent of this damage becomes apparent only as the brain fully develops years later and schizophrenia is diagnosed. In 2007, Paul Patterson and his colleagues at Caltech demonstrated the validity of this theory when they injected pregnant mice with a chemical that stimulated a strong immune response like that caused by influenza. It led to the birth of pups with behavioral symptoms associated with autism and schizophrenia.79
Yolken, however, issues a caveat: “I’m older, so as I always say, ‘That’s the $64,000 question. The big question.’ We know they were infected, but we don’t know when they were infected. So we can’t know if the risk is only fetal infection or infection early in life.”
Moreover, Torrey reminds us that the mechanism, rather than the specific infectious agent, determines the child’s medical fate. The studies that implicate influenza in schizophrenia indicate that the virus plays a similar role in bipolar disorder and autism, and these studies have been replicated widely. Evidence strongly suggests that an assortment of biological villains, including herpes simplex virus type 2 (HSV-2), cytomegalovirus, and, perhaps, bornavirus, carried by horses in Europe, are all capable of causing the same slow devastation. Investigations of HSV-2 have yielded indeterminate findings, but Torrey and other researchers have widened their gaze to scrutinize rubella, which is already known to cause mental retardation and childhood psychoses.
Even in those cases where infection may be shown to cause mental disease, a role remains for genetics, stress, inflammation, trauma, and other risk factors that can render the brain more vulnerable to damage by infection. These actors combine in a synergy of risk to devastate the infected brain.
Germ theory as applied to mental illness is compatible with a role for genetics in schizophrenia, because genes may determine which brains are most vulnerable. Several infectious agents can cause the same disease, analogous to the situation where meningitis can be either bacterial or viral: Evolutionary biologist Paul Ewald suggests that we may one day speak of influenza schizophrenia or T. gondii schizophrenia and treat the disease accordingly.
Today, the evidence for a wide variety of pathogen-spread mental illnesses is copious and rigorous. That evidence is culled by advanced tools that Pasteur and Koch could only dream of. Functional MRI, or fMRI, allows dynamic imaging of the brain; antibody titers quantify infection indirectly by measuring the immune system’s response to a pathogen; and high-throughput sequencing, an assortment of fast and cheap methods to sequence and analyze large genomes, enables the analysis of four hundred million base pairs of DNA in just ten hours.80 Respected scholars at premier institutions have published clues to connections between various infections and autism, schizophrenia, obsessive-compulsive disorder, major depression, and more in peer-reviewed scientific journals. The evidence is mounting rapidly.
As bleak as this may sound, it’s actually good news, because identifying which pathogens cause specific strains of mental illnesses will enable precise treatment. Determining the microbe’s specific nature will allow researchers to target it with the most effective medication, just as discovering that HPV causes cervical cancer allowed scientists to devise a vaccine against this global killer. Antiviral medications and vaccines may quell influenza-induced schizophrenia, for example, while antibiotics can eliminate those cases of schizophrenia that are caused by bacteria.
Unfortunately, vulnerability doesn’t end in early childhood. As the next chapter relates, adolescence brings its own array of infection-borne psychiatric disasters, from anorexia to OCD.