
Normally, the cockroach, that repugnant six-legged creature, that pariah of refined persons everywhere, moves himself about in a rather peculiar manner. He balances on three legs (the front and back on one side, the middle one on the other). Then he pushes off and switches to the other three. The cockroach wanders around your kitchen floor at night, as it were, like an alternating tripod.
But put him in a race, and suddenly everything changes. He shifts his weight backward and scurries about with incredible alacrity on just the two back legs, a bipedal locomotion that almost mimics upright runners like you and me. If you have a desire to watch this, you can fly any January 26th to the Story Bridge Hotel in Brisbane, where they celebrate the national holiday (Australia Day) by hosting the World Championship Cockroach Races. This event, which draws many hundreds of rowdies and is accompanied by live bands, TV coverage, and heavy betting (not to mention a little beer), has been going on annually since 1982. It’s where you stack your animal, who scurries to the finish in a boxing-style ring, against the best in the country. It’s the Super Bowl, the Final Four, the World Series of cockroach racing.
How does the cockroach accomplish this magical feat of locomotion? What, physiologically speaking, wins the race? Well, within that minuscule nervous system of his, the victor possesses an altogether highly talented control center. And, with the aid of what must be a very tiny timekeeping clock, it signals his legs to move in a coordinated, alternative fashion. Add to this another master clock in the brain that sets the tempo of the whole motor machine. It’s an extraordinarily complex mechanism, to be sure.
Now this may come as a bit of abhorrent news, but you and this lowly pest actually share these same masterful neurological devices, the controllers that command how we run, bike, and swim. That’s right. The same ones. Read on.

Consider for a moment the dragon boat. In this narrow craft, 20 paddlers sit facing forward, side by side, competing at distances of 200 to 2,000 meters. Although dragon boat racing is currently experiencing a burst of international popularity, in fact, it has a very ancient history, dating to Chinese water festivals more than 2,500 years ago. The boats are a great deal of fun to watch, with their decorative Chinese dragon heads and tails (figure 2.1). If you head off to Queens, New York, in August, you can witness one of the biggest dragon boat festivals in the United States, with more than 145 teams competing.
At the front of the boat, facing backward, sits the drummer, who signals the stroke cadence to the paddlers by the rhythmic beating of a large drum as the boat surges through the water. Bam! Bam! Bam! The sound echoes down the river as the drummer beats out the indicated pace. Given environmental conditions, the boat’s proximity to the finish line and position relative to other boats, the drummer alters the tempo, thereby affecting the work of the paddlers, all the while maintaining a beautifully coordinated synchronization of effort.
The drummer, I feel confident in saying, would never consider himself an intrinsic motor oscillator, but that’s exactly what he is. With his rhythmic beating, he directs the tempo of a complex motor system (the 20 paddlers) that drives the boat forward. In each of us, too, there exists a sort of dragon boat drummer, an automatic motor controller residing somewhere in our central nervous system that coordinates the many parts of the motor’s machinery and regulates the tempo at which they should turn over.
Just as dragon boat drummers rely on their sense of rhythm to maintain a regular cadence, so our intrinsic controllers of muscular activity listen to an internal clock that, like a metronome, keeps a steady tempo of neuromuscular activation.
Our central motor oscillator is highly intelligent, able to select for the distance runner, for example, an optimal combination of stride frequency and stride length that is most energy efficient for optimizing performance. It’s a controller that, left unperturbed, is amazingly stable. And, best of all, it does this all without causing us to think about it.
Caution! Don’t confuse this central motor oscillator with the alleged central governor we encountered in the previous chapter. Their names are similar, but they’re altogether different directors. They work out of different cubicles, even on separate floors. We all possess the former, which controls the pattern and rate of muscle contractions that let us walk, run, swim, and cycle. The other is a proposed strategizer (with a brake) that might determine our pace during racing and could keep us from dangerous levels of overexertion.
In this chapter, we’re going to explore the characteristics of this automatic motor oscillator. We’ll examine the means by which it decides the best pattern of muscular activity per unit time. To use runners as an example, it deduces which is the right combination of stride frequency and stride length to attain a desired velocity. And, we’ll look at some recent research that has tried to decipher the location and nature of this central oscillator, both in humans and animals. We’ll start out using a model of distance running, since most research has involved this type of locomotion. Then we’ll add on what is known about selecting best cadences for other events, such as swimming, cycling, and rowing.
The overriding practical question here is can athletes manipulate their stride frequency, pedaling rate, or stroke rate as they strategize for best performance? If so, should they? Or, should they rely on the wisdom of the body’s subconscious, timekeeping clocks, which have developed through hundreds of thousands of centuries of biological evolution?
Bang! The 10K starting gun fires and the rhythmic cycle of neuromuscular events begins. The hip joint flexes (during the swing phase when the foot is in the air), then extends (with the foot in contact with the ground during the support phase). Similar alternations occur at the knee and ankle. The muscular activity generating these joint displacements is highly stereotyped. At the initial foot touchdown, the hip and knee joint extensors are activated (gluteus maximus, gastrocnemius). The vastus lateralis and vastus medialis muscles of the thigh become active at the beginning of the support phase. The semitendinosus and semimembranosus, hip extensors and knee flexors, contract at the end of the swing phase and continue during the support phase. Meanwhile, the ankle is acted on by the gastrocnemius (plantar flexion) and the tibialis anterior (dorsiflexion).
So far, so good. You’ve just taken one step! Now the motor oscillator orchestrating all this begins to fire with unrelenting automatism in clocklike fashion, repeating this succession of finely coordinated muscular contractions the many thousands of times required to deliver you to the finish line, all the while keeping you from landing on your nose. Again, you can be grateful this all occurs at an unconscious level.
According to chapter 1, the motor oscillator has received information about the amount of muscular work per time (velocity) that will predict optimal performance for the distance and conditions of that particular race, either from you (by cognitive pacing strategies), a central governor, or both. Now, besides simply coordinating the synchronization of all these muscle groups, the oscillator has an additional task—figuring out the best means of producing the desired velocity. In doing so, it has two choices. It can elect to manipulate the firing rate of the automatic controller (increase the stride frequency—the drummer in the dragon boat would bang faster to increase the cadence of the paddlers and the speed of the dragon boat). Or (this is something the dragon boat drummer can’t do), it can alter the length of each stride by adjusting the mechanics and force of muscular contraction. How does it decide which to do? More strides or longer strides per time? At first glance, it would seem hypothetically possible, at least within physical constraints, to utilize either mechanism to adjust race velocity. But, as the next section shows, that isn’t true. But why should one mechanism be preferable to the other?
If you were sitting next to Jack Daniels high in the stands at the 1984 Olympic Games, you certainly would have wondered what on earth this man was doing. Well, he was counting. In fact, so was his wife Nancy. They were recording the number of times that runners turned over their legs—their stride frequency—during events ranging from 800 meters to the marathon. Daniels was—and still is—a scientist and coach driven by an insatiable curiosity. (He and fellow physiologist Gary Krahenbuhl once designed a pair of Styrofoam shoes that enabled them to walk on water—successfully, it turned out—across the width of Dr. Krahenbuhl’s backyard swimming pool). In the Los Angeles Games, he was trying to figure out how successful athletes varied their stride length and frequency during competitions. What he found was very interesting. For events ranging in length from 3,000 meters to the marathon, almost all the runners had the same stride frequency (a cadence of around 180 steps per minute, or 90 steps with each foot). When they picked up the pace in each of these events, they did so by increasing their stride length. The stride frequency stayed the same. (In the shorter races, the stride frequency was somewhat faster.)
In his coaching experience, Daniels has found that inexperienced runners often have a slower cadence. He thinks this practice wastes energy and can lead to injuries, since slow stride frequencies launch the body into the air longer, causing greater impact on landing. He tells his runners to go lightly while getting into a rhythm of 180 steps per minute. “Imagine that you’re running over a field of raw eggs,” he says, “and you don’t want to break any of them.”
The findings are much the same when subjects are taken into the exercise testing laboratory. Peter Cavanagh and Rodger Kram at Penn State University measured stride frequency and length in a group of 18- to 40-year-old distance runners at treadmill speeds between 3 and 4 meters per second. These speeds are equivalent to a range of race speeds between 8:58 and 6:44 minutes per mile, which they considered typical of recreational runners. Interestingly, between these speeds, increases in treadmill velocity (33%) were paralleled by a similar increase in stride length (28%), but little change was observed in stride frequency (up 6%). That is, as running velocity rose through this range of real-world distance racing speeds, stride frequency remained relatively constant. As the subjects ran faster, they relied more on extending stride length.1
In another study, patterns of stride frequency and stride length for the top finishers in the 1,500-meter, 5,000-meter, and 10,000-meter events were observed in an elite-level international meet. Race velocities for these events averaged approximately 6.3, 5.7, and 5.5 meters per second, respectively. Top finishers in the 1,500-meter race had a stride length 12% longer and a stride frequency 2% greater than those in the 10K race. So, again we see evidence that within the velocity range of distance running competition, changes in speed were achieved predominantly by altering stride length, rather than stride frequency.
So, you’ve just passed the 4-mile (6 km) mark of a 10K road race, and, surprisingly, you really feel pretty darn good. Time to turn up the pace! You tell your hard-working motor controller to go faster. It responds by increasing your stride length, leaving the tempo (or the firing rate) of the oscillator pretty much unchanged.
The alert reader should now be ready with the next question: For distance runners, why is stride frequency—how fast the legs turn over—so relatively constant at different speeds? What keeps the motor oscillator firing at pretty much the same tempo as they move up their running speeds? What are the biological advantages? And is there a price to pay if runners try to manipulate stride frequency and stride length on their own? Let’s consider some possible answers to these questions.
Experience has taught exercise scientists that when confronted with questions of why body systems function the way they do, that the answer usually lies in the most efficient expenditure of energy. We are constantly reminded that biological systems are devised so as to perform most economically, with minimal expenditure of metabolic energy. From a Darwinian perspective, this makes sense. The less energy (read food stores) required to perform life’s activities, the greater the survival value. It would be reasonable to assume that the best stride frequency (SF), stride length (SL), or ratio of SF to SL, might have been selected in the process of evolution, with the goal of minimizing energy cost during locomotion or optimizing work efficiency. Experimental evidence bears this out.
If you ask subjects to run on a treadmill at a constant speed, say 5 miles (8 km) per hour, you can determine their energy expenditure by measuring how much oxygen their bodies use (oxygen uptake, or 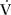 O2). Now, keep the treadmill speed the same, but have them run at several different combinations of stride length and frequency. If you plot a graph of
O2). Now, keep the treadmill speed the same, but have them run at several different combinations of stride length and frequency. If you plot a graph of 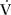 O2 versus either of these, you will see a U-shaped curve, as indicated in figure 2.2. Obviously, a particular tempo of the motor controller (a certain stride frequency that matches the lowest energy demand) is most economical for these particular runners. And if the subjects deviate from this rate or stride length, the energy cost goes up.
O2 versus either of these, you will see a U-shaped curve, as indicated in figure 2.2. Obviously, a particular tempo of the motor controller (a certain stride frequency that matches the lowest energy demand) is most economical for these particular runners. And if the subjects deviate from this rate or stride length, the energy cost goes up.
The fascinating part of this is that now, if you simply ask the subjects to run at a speed that they prefer, it almost always nearly coincides with that most economical velocity. That central motor oscillator has keen insight. It knows what speed will cost runners the least metabolic work, dictating this without even consulting them.2
The next question, of course, is why does metabolic demand change as you alter the speed and stride length? What is so special about the selected cadence that makes it the most economical in terms of energy? The answer must lie in the complex tangle of biomechanical factors that accompany locomotion. Such a discussion clearly lies beyond the scope of this chapter, but here are a few of the likely suspects.
1. As runners turn up the stride frequency, their muscles are called upon to contract at a faster velocity. The energy required for a muscle to contract is directly related to the speed of its contraction. Thus, increasing running cadence does not economize energy. And it costs more to increase the frequency than to increase the stride length as the subjects run faster.
2. In this equation, you must consider that propulsion with running is not simply the consequence of leg-muscle contraction. The act of running can be considered a series of forward jumps, with the elasticity of nonmuscular structures, such as tendons and ligaments, contributing significantly to the work. And that rebound helps minimize energy costs. Running has been likened to a mass bouncing along on springs. This propulsive effect is more than minor. The stride length, not the frequency, benefits from the energy savings. It has been suggested that while running at middle-distance speeds, the work required to propel the body forward is achieved half by muscular forces and half by elastic recoil.
3. The motor oscillator remains quite stable during steady-state locomotion, but a certain small stride-to-stride change over time still exists in the cadence. If you plot the amount of this variability against stride frequency, you see that U-shaped curve where a certain cadence is associated with the lowest variability. Interestingly, in studies of subjects walking, this frequency of minimizing variability matches up with that of the greatest energy economy.3
4. When stride frequency is increased, of course, the number of times the foot strikes the ground in a minute increases. In contrast, the time the foot actually makes contact with ground during each stride is shortened. The force necessary to move the body weight against gravity for each stride, however, remains constant. That means that the rate of force the foot must apply to the ground for each stride goes up as stride frequency is increased. The implication might be, then, that the risk of stress injury to the leg is increased by greater stride rates.
So, what does all this tell us about selecting stride frequency during distance-running competitions? Let’s go back to the runners who want to pick up the pace at the 4-mile mark. What would happen if they increase their race speed at this point by turning over their legs faster, purposefully overriding the natural tempo of the motor oscillator and increasing stride frequency? If you are impressed with the research data described in the preceding section, you might expect that they might, at least temporarily, increase their race speed. However, the price of this choice is exaggerated energy demand and musculoskeletal stress. So, in addition to increasing risk of overuse injury, performance might well be compromised.
On the other hand, notes the skeptic, you do not hear nightmare stories of running catastrophes resulting from pushing stride frequency during distance racing. How these findings actually might (or might not) translate into performance outcomes has never, to my knowledge, been tested. Too, the question has been raised that the magnitude of the negative effects of self-generated cadence might not be appreciable. From their studies, Peter Cavanagh and Keith Williams contended that selection of an optimal ratio between stride length and frequency is not a major determinant of running economy for trained distance runners. Instead, they note, factors such as biomechanical parameters (smoothness of gait, contributions of elastic recoil, and footwear) are much more likely to play important roles in energy economy during running.4
For my money, I’ll stick to the opinion of most running coaches that you had best leave the selection of stride frequency and length to the wisdom of the central motor generator, whose thousands of years of experience probably outweigh that of a 25-year-old upstart. To paraphrase the old margarine advertisement, it’s not a good idea to mess with Mother Nature. There is actually some solid science behind the idea that stride frequency and length adopted during competition by an individual runner in a given race are automatically selected for a good reason. Energy demands are minimized and conscious efforts to perturb either stride frequency or stride length are probably counterproductive for optimal performance. Here’s the bottom line: Distance runners should not be too quick to cognitively manipulate stride frequency and length during competition. Instead, they should stick to those that come naturally.
One way to avoid deviating from instinct is to mentally dissociate during running—that is, think about anything other than the immediacy of the race. Divert your thoughts to what you’re having for dinner that night or your plans for the summer. (If the race is a long one, you could review your entire senior year in high school.) In other words, go on autopilot and let your unconscious regulators in the brain do their job. You might shut out some of the discomfort of the running competition. You also won’t interfere with the optimal stride frequency and stride length settings at a given velocity that are prescribed by the controller in your central nervous system.
It’s interesting that when Bill Morgan studied this phenomenon, he found that average middle-of-the-pack marathon runners tended to do just that. They kept their minds on something other than the race itself. Runners at a more elite level were actually more likely to control their behavior during a race. They closely monitored how they felt, the mechanics of their stride, the distance covered, and so on. Just what this means in terms of allowing the brain to keep the best running mechanics is not altogether clear.5
So much for running. Let’s see what we know about optimal stroke rates in other sports, most particularly those that are not weight bearing in nature.
We now move from bipedal locomotion against the forces of gravity to arm motion versus the resistance of water—very different forms of exercise. But there are many parallels in the story of optimization of stroke rates in distance swimmers and stride frequency of runners, with a few twists in the plot.
Mathematically, it’s the same issue. The number of arm strokes in the water multiplied by the distance traveled per stroke equals the velocity. The former is determined by the tempo of the motor oscillator and is characteristically an energy-expensive means of generating speed. The latter, more energy efficient, is created by factors such as the power of the stroke, drag reduced by body position, leg force while kicking, and hand size. The swimmer will move through the water at the highest velocity for any particular distance with a particular combination of stroke rate and stroke distance. Finding that optimal ratio for a particular athlete in a given event is a critical component of swim training. The extent to which this best ratio of stroke frequency to distance reflects an intrinsic pacemaker, with knowledge of factors like work efficiency and minimizing energy demands, is unclear. As a corollary, then, it is controversial whether swimmers should be taught to manipulate stroke rates or to leave them to their intuition.
That’s the summary. Let’s back up now and examine the details. If you have swimmers perform a series of maximal distance swims and then plot their velocity versus different stroke rates, you’ll see the same inverted U-shaped curve we saw for runners. There’s a best stroke rate, and it corresponds to the one that the swimmer prefers. This sounds a lot like a central controller subconsciously dictating an individual swimmer’s optimal ratio of stroke rate to distance, much like we considered for distance running.
In general, differences in velocity in the performance of swimmers at the elite level have been attributed to variations in distance per stroke, not stroke rate. In fact, the stroke rate that allows swimmers to achieve their maximal velocity is often similar. A comparison of the times in the U.S. Olympic swimming trials between 1976 and 1984 found that the average velocity was greater in 1984 in 9 out of 10 women’s events and in 3 of the 10 men’s events. Of these 12 improvements, the increased velocity was accounted for by a 4 to 16% increase in stroke distance. In 8 of these, there was a concomitant fall in stroke rate (a loss of 3 to 13%). The increased race speed was due exclusively to faster stroke rates in only two short events (women’s 100-meter butterfly and 100-meter backstroke).
When fatigue and slowing of velocity occur near the end of a distance swim race, it is almost always due to a decrease in distance per stroke. Presumably, this reflects a diminishing ability to generate force to overcome water resistance (that is, a decline in production of muscle power), but other issues might be involved, such as increased drag from poor body alignment as the swimmer tires. Interestingly, a number of observers have found that swimmers often increase their stroke rate near the end of a race in an attempt to compensate for this decline in stroke distance as fatigue sets in.
The best way for a swimmer to increase race velocity, then, would be to train to increase muscle power to propel the body, to learn to carve out an effective stroke pattern, and to assume and maintain a proper, hydrodynamic body position in the water. For most swimmers, training to increase velocity over distance by increasing stroke rate is not expected to be beneficial, since it only shortens the stroke length. Most people find that the tempo of the central motor oscillator does not like to be perturbed.
The preceding information describes trends of stroke rate and distance seen in competitive distance swimmers. But it is important to realize that if you sit by the pool and watch top competitors, you will, in fact, see all sorts of combinations of stroke length and distance for achieving race speed. Some elite swimmers have found success with high stroke rates, others with low. Some increase speed by turning over the arms faster, others by a more powerful stroke. Some show a falloff in both stroke frequency and length as fatigue sets in. In others, stroke rate progressively declines as the race progresses.6
In this mélange of styles and preferences, it is difficult to sort out the role that an intrinsic motor oscillator might have in selecting, below the level of consciousness, the ratio of stroke frequency to length that would be the most energy efficient. For coaches and athletes, this boils down to a long-standing controversy: Should swimmers be taught to train and compete at a certain stroke frequency and length? Or, should they go with what feels intuitively right (which would be saying that the message on proper stroke frequency comes from a subconscious, intrinsic timekeeper)? There is no agreement on the question.
David Pendergast is an exercise physiologist at the State University of New York at Buffalo who has devoted his career to understanding the factors that go into successful swimming. He notes that “it is known that swimmers left to self-select a frequency: velocity ratio will swim at a stroke frequency that results in less than their maximal velocity. However, whether this frequency can be sustained for given distances remains an issue. One must consider ‘what feels right’ may also be influenced by the swimmer’s training. That is, training at lower velocity and frequency may make swimming at higher velocities and stroke rates uncomfortable.”
But, all this uncertainty notwithstanding, there is one concrete strategy here. Don’t fret over whether a subconscious central governor is in charge or not, just experiment! Do a few time trials while varying stroke frequency, and see what happens to the finish time. The goal would be to find the slowest stroke cadence that still maintains a top velocity.
The veteran coach E.W. Maglischo summed it up this way. “One job of the coach is to help athletes find the optimum combination of stroke rate and length that will allow them to swim at some desired speed with the least energy expenditure. That combination will undoubtedly be different for each swimmer and each event. There is no guarantee that swimmers self-select the best combination of stroke rate and stroke length.”6
Important, too, is the idea that the best combination of stroke frequency and length at the beginning of the race, in a rested condition, is probably going to be very different from that near the end of the event, when the swimmer faces increased levels of fatigue. So, experimenting to find how a particular swimmer should strategize an optimal ratio of rate to length during competition should be done in both conditions.
Knowing your best stroke frequency may come in handy. Suppose you dive into the pool trying for a gold medal in the 200-meter butterfly. Suddenly, you’re blinded—your goggles have filled with water. That’s what happened to Michael Phelps during the Beijing Olympic Games. No problem! Unable to see his opponents or the oncoming wall, he simply counted his well-tuned strokes to estimate when to make his turns. The result? A world record time of 1:52.03. You should be so lucky.
On the wall of my study hangs a print of Pierre and Marie Curie. They’re standing in front of their Paris home in 1894, ready to take a ride on the new bicycles they gave each other as wedding gifts. The cost of about 200 francs each must have made the bikes quite an extravagance (Pierre’s annual salary was 3,600 francs). Marie looks happy, but she’s wearing a look of quiet determination. This is perhaps just some leftover fatigue from all those hours of mixing pitchblende in the laboratory. I find this photo captivating. In one image, we see the joy of living, of devoted human relationships, of exercise, of the rewards of committed scientific work. And it reminds us that scientists and bicycles have never been too far apart.
During that latter part of the 19th century, the popularity of the bicycle was spreading rapidly. Inevitably, so was bicycle racing. In 1903, to promote the circulation of his newspaper, Henri Desgrange devised a six-day event he called “le Tour de France,” which drew 60 entrants for a 2,397-km race throughout the French countryside. (It worked. The circulation of L’Auto doubled by the race date.) It was apparent to scientists watching these competitors struggle through this grueling competition that cyclists were like machines—you fed them fuel, and they converted energy to mechanical work, like unconscious automatons. You could, evidently, readily study this human motor in the laboratory and gain an understanding of which factors determined cycling performance.
Furthermore, it was expected that such scientific research should be able to provide practical information that would be useful for cyclists in learning the best means of training and competing. Thus began a long marriage between science and cycling, one that today has brought us lightweight machines, ways of reducing wind resistance, proper diets, and heart rate monitors.
As information from such research began to unfold, one observation noted early on that still sticks with us today has proven perplexing. It is not at all well explained. By now, you are quite familiar with the supposition that from many different perspectives, conservation of energy is a key element to success in distance sports. If energy supply is a central determinant of athletic performance in terms of aerobic endurance, we would expect that the minimum energy expenditure for any level of athletic work would be advantageous. That is, the greatest amount of work (power output in running, swimming, rowing, or cycling) that you can do with the least amount of energy (higher work efficiency), the longer you should be able to exercise at a given velocity, or the higher the speed you should be able to achieve for a given distance.
We’ve already seen much evidence for this in these pages. In walking, running, and probably swimming, the preferred cadence (selected by the athlete) is the one that is usually the most economical energetically, and is associated with the greatest velocity.
It’s a neat conceptual package, and we like it because it makes sense.
But then we come to cycling. If you take different pedaling rates while the subject is performing the same amount of work, and then plot these values against metabolic demand (oxygen uptake), you see, like with running, a particular pedaling cadence that is best, that is the most energy efficient. And that cadence has been repeatedly shown to be around 50 to 60 revolutions per minute (rpm). Moreover, the same rate of pedaling has been linked to optimal levels of other markers of metabolic stress, such as heart rate, muscle production of lactic acid, and lung ventilation. Fair enough, but what is difficult to understand is that competitive cyclists normally pedal at frequencies of 90 to 105 rpm, almost twice as fast. According to what we know, this should be terribly energy inefficient, a real detriment to successful performance.
Alejandro Lucia and his fellow Spanish investigators nicely demonstrated this when they recorded pedaling rates of elite cyclists competing in the Tour de France, Giro d’Italia, and the Vuelta a España. The average cadence was 71 rpm during uphill cycling (through the high mountain passes), 92 rpm for individual time trials, and 89 rpm during flat, long stages. Pedaling rates rose to about 110 rpm for brief periods of accelerations or breakaways. Studies indicate that if you increase the pedaling cadence by 60 to 100% (from around 50 to 90 rpm), while keeping the work output constant, you’ll increase energy cost by 14 to 21%. That’s a very appreciable amount of energy. Think about that when you power your way up Mt. Ventoux during your next Tour de France.
It’s not just elite cyclists who pedal at these fast, energy-efficient cadences. Anthony Marsh and Philip Martin compared the effect of changing cadence on metabolic demand and the preferred cadence of eight trained cyclists and eight untrained subjects. They all cycled at the same work load of 200 watts.
The most energy-efficient cadence (lowest 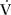 O2) was 56 rpm for the cyclists and 63 rpm for the untrained subjects. But the preferred cadence was 85 rpm and 92 rpm for the two groups, respectively. In this particular study, the difference between oxygen demand at the most efficient and preferred cadence was not very great, about 5%.
O2) was 56 rpm for the cyclists and 63 rpm for the untrained subjects. But the preferred cadence was 85 rpm and 92 rpm for the two groups, respectively. In this particular study, the difference between oxygen demand at the most efficient and preferred cadence was not very great, about 5%.
Why does the motor oscillator of the world’s best cyclists (and even you and me) fire at this extraordinarily high tempo that is apparently energy inefficient? Many think it has something to do with minimizing muscle strain. At a fixed work load, the faster you pedal, the less the force on the pedals per revolution. That would seem to make sense. You would suppose, then, that the rating of perceived exertion (RPE)—how the cyclist feels—would be lowest at those 80-to 100-rpm cadences, Alas, not so. In fact, investigations of RPE at various pedaling rates have provided very inconsistent results. Some have verified a minimum RPE at a cadence of 80 to 100 rpm, others have found a nadir of RPE at 60 to 70 rpm. In the preceding study by Marsh and Martin, RPE was pretty flat between 65 and 80 rpm, but then increased at faster pedaling rates. Their conclusion? “RPE may not be a critical variable in cadenced selection during submaximal power output cycling.”7
So, just why an intrinsic pacer insists on pedaling at a high cadence during cycling remains a mystery. Is it a trade-off between minimizing muscle strain (best at high cadences) and maximizing energy efficiency of muscles (best at low cadences)? Sometimes, perhaps when more than one factor creates a best cadence, even the intrinsic motor oscillator has to make compromises. Perhaps, too, the controller of tempo is listening to physiological or mechanical messages that, to cite the Bard, are more than are dreamt of in our philosophy.
Maybe that philosophy has something to do with the function of the skeletal muscle as an auxiliary circulatory pump. This is an idea that been around for a long time in scientific circles, although it has been difficult to study and confirm. But many are in agreement that as the skeletal muscles contract around veins, they create a pumping action that propels blood flow, much as the heart does. The downside of this is that as the muscles contract at high levels of force, they actually close off the blood vessels and impede, rather than promote, blood flow.
During cycling, pedal cadence may have something to do with this. Researchers at Colorado State University showed that as cadence was increased from 70 to 110 rpm (keeping the work load constant), the output of the heart increased. That rise was in excess of increases in metabolic rate. These authors concluded that the skeletal muscle pumped blood more effectively at high cadences. This fits, since such rapid pedaling rates are associated with less force per revolution. Consequently, the veins in the muscle would be less occluded and blood flow would be more free.8
And, finally, a couple of other ideas spring to mind as to why cycling may be an anomaly in this question of cadence and economy. First, because the bicycle is a machine, maybe it operates outside of evolutionary forces that have influenced human muscle function. And, second, perhaps the explanation is somewhere in the fact that a bicycle does not permit you to increase stride length, only cadence.
In this chapter, we’ve encountered a pacemaker that orders the sequence of innervation of our limb muscles so we can run, swim, bike, and row. This oscillator originated as deeply in our evolutionary past as the process of animal locomotion. And then there’s a separate subconscious motor oscillator that governs the ratio of frequency, tempo, or muscle activation to its force production while listening carefully to an intrinsic clock. It controls how frequently (at what rate) we contract our limb muscles. It controls our stride rate in running, our stroke rate during swimming and rowing, and our pedaling cadence when we cycle.
We can override the decisions of this second motor pacer by our conscious decisions. But there is evidence, at least for runners, that if left unperturbed, this controller will pick the tempo that is energetically best, the one that will optimize performance. If so, it will be counterproductive for the athlete to purposefully alter cadence in these sports beyond what feels natural. And so, going with what intuitively feels right generally seems to be the best advice.

Thus far, this chapter has discussed how a central motor oscillator might act, but it has ignored the more difficult and challenging question of what it is. How does it work? Where is it located? Can it be manipulated? So, it’s time now to explore the nature of this metronomic central controller that coordinates our exercising machine, sets the tempo, and regulates muscular force, all below the level of our consciousness. Indeed, the oscillator doesn’t even consult us in these matters, freeing us to dream of athletic glory.
In terms of any clear understanding of just how this central controller works, we’re still pretty much in the dark. But the story of the timekeepers of motor performance—the internal clocks that tick away, governing locomotion and athletic success—is an intriguing one. One important point of this excursion into some basic science is that these timing mechanisms that operate beneath our awareness are coded in our genetic material and are the end product of hundreds of thousands of years of evolutionary time. We need to appreciate their extraordinary wisdom.
But first, some terminology. Let’s be correct about this. Throughout the first portion of this chapter, I have taken liberties with the vocabulary, using all sorts of descriptive names for this central machine, such as oscillator, controller, or regulator. The official term utilized by card-carrying neurobiologists is central pattern generator, or CPG. So, that’s what we’ll use from now on.
If you think about it, all muscular activity in the body is characterized by rhythmicity. Peristaltic motion of the gut, beating of the heart, breathing, chewing, swallowing—they all rely on a central neurologic clock that controls rate (or tempo) and synchronizes sequence of muscular activity. Vital body functions depend on it, not just locomotion.
The basis of this idea of a CPG is that certain nerve cells located in the central nervous system (brain and spinal cord) can spontaneously and independently generate regular, clocklike electrical impulses. The timing and rate of these rhythmic electrical discharges is by some means signaled to those cells responsible for commanding the coordinated and repetitive contraction of skeletal muscle that permits locomotion. Indeed, such neurons within and outside the central nervous system with spontaneous oscillatory electrical properties are well known (the heart’s pacemaker—the sinus node—is an obvious example). Understanding just how such single-cell electrical generators can be combined into systems that regulate the muscular complexities of running, swimming, and cycling remains, quite clearly, a large challenge.
This CPG is not just one central controller. A good analogy might be that it is a system of franchises, or a number of separate timepieces with different functions that coordinate and synchronize the exact sequence of muscular innervation over time that permits locomotion. If you run faster, that sequence of muscle contraction must necessarily be maintained by some timekeeper. Another clock precisely regulates the tempo of the entire system. If you want to kick to the finish, that clock speeds up with metronomic regularity. A host of additional controllers adjust coordination and balance in space, receive information from the periphery, and listen to your cognitive commands. Given the multitude of the functional components, it is not difficult to recognize, then, that the CPG is not a single wizard sitting at the controls in Oz, but rather several control centers that are dispersed throughout the central nervous system. Indeed, we can predict that the CPG is, in reality, a functional network. We’ll keep that idea in mind as we examine the nature of the CPG in animals and humans a bit later.
A good deal of research interest currently exists about CPGs and how they work. Admittedly, how they relate to athletic performance has not been at the top of the priority list. Insights into the basic mechanisms of rhythmic motor movements have been of more interest. This research activity has been recently energized by more practical issues—the potential for rehabilitation of stroke patients and those with spinal cord injuries, for instance, or how to design propulsion mechanisms for robots.
Almost all insights into the existence, function, and location of CPGs have come from work with animals, particularly in studies looking at effects of surgical interruption of different portions of the nervous system. (Human subjects are traditionally resistant to dissection of the spinal cord.) Indeed, with a quick run through the literature, you can become enlightened on matters ranging from CPG control of intestinal activity in lobsters, to regulation of the heartbeat in leeches, to the initiation of vomiting of dogs. New information regarding CPGs is becoming available for humans, too. We will review this in the following section. All would suggest that the basic control of rhythmic motor activity in you and me is similar to that observed throughout the animal kingdom. This observation has supported an assumption that development of CPGs originates from the obscure evolutionary past, and that they have been critical to the growth of the evolutionary animal tree. More particularly, it also supports the likelihood that animal models can serve as appropriate surrogates for humans in investigations of CPGs.
One other point. It is obvious that CPGs, wherever they are located, must be influenced by brain structures that accept sensory information from the outside (for example, cats dash out of the way as they see a car approaching) and, in humans, cognitive decision making (I want to run faster). However, CPGs should not be confused with brain motor centers, which signal muscle contraction and control purposeful motor activity. Theirs is a different task. CPGs are assigned the specific role of subconsciously controlling the rhythmicity and tempo of that activity. They are distinct from motor control areas in the cerebral cortex that we can willfully command.

The marching bands for Michigan State University and the University of Michigan are both superb musical organizations, capable of stirring the passions of 100,000 fans on Saturday football afternoons, regardless of how well or poorly the team is doing at the moment. There is a difference between the two. Do you know what it is? That’s right. (Good guess!) It comes down to their respective central pattern generators.
At the command, “Band, take the field!” the Michigan State band (figure 2.3) emerges from the tunnel at a frenzied rate of 250 steps per minute. (That’s right—more than four steps per second.) Their CPGs are really humming. (It is not recommended that you try this at home, particularly if you’re wearing a full band uniform and carrying a tuba.) Meanwhile, 70 miles down the road in Ann Arbor, the University of Michigan band marches in at a more laid-back pace of 208 steps per minute.
Why is this important? I guess it’s because a close analysis might indicate that MSU trumpet players are likely to be about 5% leaner than their counterparts at the U of M. This will please their parents, who are footing the bill.
The volume and level of sophistication of research regarding CPGs has grown dramatically in the last decade. What follows is simply a quick overview of where this information has taken us. We’ll start with findings in more simple animals and move up the evolutionary ladder to what is known about humans. Those wishing a more detailed examination of current investigations into the nature of CPGs can consult a number of in-depth reviews.9 (Also, a warning: lovers of cats—and, for that matter, eels—may wish to skip the following sections.)
The lamprey is a repugnant, eel-like creature that makes its living by sucking the blood and body fluids out of other fishes. Perhaps to compensate for its lack of social deportment, this primitive vertebrate has provided researchers some intriguing insights into the evolutionary origins of CPGs.
The lamprey swims by producing waves of muscle activity, contracting sequentially from head to tail. Contraction of each of the segmental muscles is triggered by nerve cells located in the spinal cord. While each segment has to be coordinated with the next one down the line, there must be a time lag to create a wave. (If not, obviously, the entire set of muscles would contract simultaneously, rather than producing a wave. That would be like everyone in the football stadium trying to do the wave by standing up at the same time.) It turns out that the delay from segment to segment is about 1% of the total wave duration, and about 100 segments exist. Cycle duration depends on the swimming speed. If the lamprey is swimming at top speed, the cycle duration is about 100 milliseconds, meaning that the firing rate sequence of spinal motor neurons involves a lag of 1 millisecond between each segment and its downstream neighbor.
Control of this sequence of muscle contractions lies in the spinal cord itself. We know this because if you remove the lamprey’s brain and then electrically stimulate the spinal cord, you still witness coordinated swimming motion. And, if you go so far as to chop the spinal cord into pieces containing only two or three muscle segments, electrical stimulation still causes swimlike activity in what muscle still remains. (You can try this in your spare time at home, best after your spouse has gone to bed.) On the other hand, the control of the rate of the entire system of locomotion resides higher up in the lower part of the brain stem (something called the rhombencephalic reticular nuclei). Artificial stimulation of these structures puts the whole tail into action.
This information in this most primitive of vertebrates serves as the foundation for studies of higher mammals. The lamprey is telling us that there are really two neurologic controllers of automatic locomotor activity that function in respect to time:
1. Coupled segmental oscillators in the spinal cord dictate a finely timed sequence of motor neuron firing to provide locomotion.
2. A central controller in the brain stem dictates the generation of tempo and force for the entire system.
Too, this controller must act in response to sensory input. (The lamprey has to locate and chase down its prey, for example.) And, needless to say, the lamprey out searching for unsuspecting fishes to suck dry gives no conscious thought to the matter.
So, this is the basic model of two-tiered CPGs, with an autonomous oscillator that fires at a constant frequency (that’s the spinal cord CPG) and a calibration unit (in this case, brain stem centers) that adjusts this basic frequency in response to information from the environment. Now, let’s see what we find when we move up the evolutionary tree.
Experiments performed almost 100 years ago indicate that a cat whose spinal cord has been transected still demonstrates alternating, rhythmic contractions in the muscles moving the ankle that mimic locomotor activity. They also found (remember my warning, cat lovers) that if you sever the cat’s brain stem and then electrically stimulate certain centers below the cut, the cat demonstrates complete quadrupedal stepping. In some studies, animals are even capable of spontaneous walking. This confirms the dominant effect of spinal cord CPG in feline gait.
Alterations in speed of walking, trotting, or galloping can be produced by changing the strength of the stimulus descending from motor centers in the brain. This central modulator, located at the level of the brain stem, regulates the turnover velocity during locomotion through descending nerve fibers that influence the oscillator in the spinal cord. There seems to be little control from higher brain centers, however, since cats do not struggle with locomotion after these areas are damaged.
The basic model of the CPG (or CPGs), we see, is the same as in the lamprey. A rhythmic generator exists in the spinal cord that controls the stereotyped, oscillatory pattern of muscle activation for locomotion in respect to time. And a higher, more central regulator at the base of the brain sends signals down the spinal cord to initiate and dictate the power output of the CPG (and presumably the proper relationship of stride frequency and duration) to achieve a certain velocity of locomotion in response to sensory stimuli.
As one examines ascendancy of the evolutionary tree, animals progressively exhibit extensive development of higher brain structures, particularly the cerebral cortex. It might be expected, then, that higher controlling sites within the brain would begin to play a role as CPGs. Monkeys and other nonhuman primates provide some information on this point.
In monkey spinal cords, the evidence for an intrinsic CPG that controls the sequence and timing of muscle innervation for locomotion is much less clear than in the cat. The few studies of spinal transection that have been performed in monkeys have not shown convincing persistence of locomotor patterns. Some reports reveal no hind-limb stepping after the spinal cord has been cut. It has been suggested that as compared to cats, the influence of the primate brain dominates over spinal cord circuitry for locomotion. The purpose of this shift might be to free the movements of the upper extremities, since the hands and arms are more engaged in nonlocomotor activities than the legs are.
Not much is certain about CPGs in human beings. Their existence, location, and function have generally been inferred from studies in animals. What data are available come from investigations of those who have suffered traumatic injuries to the spinal cord or cervix or vascular strokes. Patients who experienced complete transection of the spinal cord very rarely showed rhythmic locomotor activity. This has led some to doubt the influence of a spinal-cord CPG in humans.
Sandy Stevens, who teaches at Tennessee State University, doesn’t agree. She has had a good deal of experience dealing with rehabilitation of spinal cord injuries. Her take is that previous investigations did not provide the proper sensory input in their research models. “Typically, in these studies, a harnessing apparatus is used that supports the body through the use of a groin strap,” she says. (One of her patients actually called this system a demasculinator.) “This certainly does not replicate the sensory experience of normal weight bearing during ambulation.”
Her answer to this? Underwater treadmill walking.
We find that patients with partial cord injury who have virtually no ambulatory ability can demonstrate normal walking patterns when supported by the buoyancy of water. I can’t help but conclude that this approach facilitates spinal cord CPGs in stimulating normal stepping patterns. The CPG is there. It just needs the right sensory stimulus.
Supporting that conclusion, electrical stimulation of the spinal cord in patients with complete spinal interruption at the thoracic level has been shown to elicit some stepping. Steplike activity is observed in human newborns, even in those rare babies with anencephaly (they lack higher brain centers). Rhythmic muscular activity is evident in human fetuses within 10 weeks of intrauterine life. From the studies in nonhuman primates, however, it is expected that higher brain centers might play a more dominant role in timing rhythmic muscle innervation during locomotion than they do in lower vertebrates. This evolutionary progression of the central nervous system has been described as a progressive encephalization of motor rhythms.
Humans thus appear to possess the basic CPG blueprint (see figure 2.4)—a spinal cord generator that creates the rhythm and sequence of muscular innervation to coordinate locomotion in clocklike fashion, and a brain stem locus that controls the tempo of the spinal cord generator. Both of these structures are magnificently intelligent. The former knows just the right firing sequence of motor neurons that will allow a 6-foot (2 m) human to run upright on two skinny legs without falling over. The latter has inherited the wisdom of ages past that select the proper stride rate and length that are optimal for any given running velocity. In humans, of course, we must consider the potential added influence from higher brain centers that provide cognitive input—that is, what we consciously command of our arms and legs. The difference between humans and lower animals lies in the limited autonomy of the isolated spinal cord to generate muscular activity. That is, as we ascend the evolutionary tree, the driving factor for the spinal CPG increasingly involves higher brain centers. Evidence suggests a decrease in the independent nature of spinal cord CPGs in human beings as compared to, say, the cat or the lamprey.
All of the neural structures and systems previously discussed operate within close time frames. Neurobiologists talk about internal clocks and specialized neural structures for timing that coordinate the automatic functions that let us run, bike, and swim. Whether such clocks are actually a part of CPGs or are separate neurological constructs is uncertain. Certainly, other structures within the human brain appear to participate in this CPG network that is so linked to the passage of time. The cerebellum, for example, appears to influence movement timing, since patients with disease in this structure have difficulty timing muscle activation during rapid limb movements. Patients with Parkinson’s disease, who have abnormal function of basal ganglia, may tend to underestimate time intervals. This could be a sign that their internal timekeepers are slowing down. (Chapter 5 deals with the issue of time perception.)
It seems unlikely that a single master clock exists in the body, relaying temporal information to the different components of locomotor activity (the spine, brain stem, higher brain centers). After all (to cite an example that laypeople can understand), you can talk to a companion at the same rate while walking whether you are moving fast or slow. It’s much more likely that numerous separate pacemakers exist within the nervous system that run at a similar rate. The intrinsic rates of such clocks could then be modified individually to adapt to local system demands. (A good pianist can play a 4/4 rhythm with one hand and a 3/4 rhythm with the other.) If you change the tempo of an action, the sequence of the action is not changed, but is simply condensed or expanded in time. This fits nicely with the idea that the spinal CPG of an animal is an intrinsic automatism, or clock, whose rate can be influenced by higher CNS structures, but whose basic firing pattern remains unchanged.
One particularly intriguing observation that may have bearing on the organization of intrinsic clocks is that stride rhythm is often coupled with breathing during locomotion. That is, for a certain number of strides, there is a breath. This is called entrainment of ventilation and locomotion, and it has been observed in cats, dogs, horses, jackrabbits, gerbils, rhinoceroses, wallabies, turtles, guinea fowl, alligators—pretty much the whole ark.
Given this evidence for the evolutionary persistence of entrainment in the animal kingdom, it is no surprise that the phenomenon is also observed in humans who are running, walking, cycling, or rowing. In human studies, the reported frequency of the link between breathing and striding has varied widely. (In fact, some have found no evidence of entrainment at all.) Perhaps insightful, though, are the observations of Dennis Bramble and David Carrier at the University of Utah that the frequency of entrainment depends on the performance level of the runner. Experienced runners commonly coupled stride and breathing patterns very tightly. The most common ratio was a 2:1 ratio of strides and breaths, but at slow running speeds, this was frequently 4:1. However, in less talented runners, no synchronization of breathing and striding was observed at all.10 (So, here’s another laboratory exercise to try. Check yourself for breathing-striding entrainment during your next run and see which category you’re in.)
The explanations for the link between breathing and striding during locomotion have generally involved mechanical issues. Gait, for instance, may physically constrain breathing, thus requiring the two to be synchronized. However, such mechanisms would seem to be less likely linked during the upright bipedal locomotion of human beings. Alternatively, a combination of effects of a single internal clock that links the two different forms of rhythmic activity is an intriguing possibility.
Does entrainment help performance? It depends, it seems, on which expert you talk to. Jack Daniels, who was introduced earlier in this chapter, thinks so. Given his extensive experience as an athlete (U.S. national titlist in the modern pentathlon), coach (University of Texas, SUNY at Cortland), and exercise physiologist (University of Wisconsin), he’s somebody who should know. He notes that the best runners use a 2-2 rhythm, taking two steps (one with each foot) during inhalation and two steps during exhalation. If you’re striding at Daniel’s proffered rate (90 steps with each foot per minute), you’ll then be taking around 45 breaths per minute. He believes this provides the most efficient ventilation of the lungs.
So, according to his renowned book Daniels’ Running Formula, a 2-2 rhythm is preferred for the majority of a distance race. However, near the finish, you will probably want to breath faster, around 60 breaths per minute. Then you can switch to a 1-2 rhythm (take one step while breathing in and two while breathing out), or 2-1. Slower rhythms, such as 3-3 and 4-4, might be okay for easy training runs. Daniels says to avoid a 1-1 pattern because the shallow breaths decrease actual lung air exchange.11
Jerry Dempsey is to respiratory exercise physiology as Jack Daniels is to distance running coaching. And Dr. Dempsey disagrees. “My argument, purely theoretical, is as follows. We have shown that the oxygen cost of breathing at maximal exercise in highly trained athletes can comprise as much as 15% of the total oxygen demand. This means a fair bit of blood flow is being required by respiratory muscles that is potentially stolen from locomotor muscles. So we need our breathing to be as mechanically efficient as possible. To me, the mechanisms in the brain stem are best designed to receive all of the input from the lung, chest wall, cerebellum, and hypothalamus in order to sculpt the optimal depth and rate of each breath to minimize the work of respiratory muscles. Higher centers, including the cortex, willing our breathing pattern to coordinate with limb movement would not provide the same level of optimization. So, in essence, I believe the brain stem of the runner is better equipped than the cerebral cortex of a coach to determine breathing pattern.”
Distance runners Paul Norton and Jack Mahurin, whom you met in the previous chapter, line up with Professor Dempsey on this one. They both told me that anything that departs from just doing what comes naturally with your breathing is counterproductive. They think that concentrating on creating a certain pattern of breathing and striding wastes mental effort.
So, it comes right back to chapter 1’s argument of the subconscious central governor versus our own conscious dictates, doesn’t it? Should we let Mother Nature, with centuries of evolutionary practice, have her way? Or can we consciously adopt certain running (or in this case, running and breathing) strategies that will improve performance? I guess if people like Professors Dempsey and Daniels can’t agree, we can safely call the answer inconclusive. But there may be a takeaway suggestion, too: Runners can try out active entrainment to see if it works.

Before leaving this chapter, we should perhaps step back and consider a more basic question: Why have all these CPG systems and intrinsic clocks that help us run or bike or swim for distance developed in the first place? More to the point, why are humans, compared to our mammalian brethren, so good at aerobic endurance exercise?
Certainly other animals have extraordinary abilities to sustain physical effort. Like the white-rumped sandpiper, Calidris fuscicollis, who flies the 2,900 miles between the Arctic Circle and the northern shores of South America nonstop every year. Or the pronghorn antelope, which can sustain high speeds for 20 to 30 minutes, and whose ability to utilize oxygen at maximal exercise is almost four times that of the best human marathon runners. But these are isolated examples. In fact, out on the primate branch of the evolutionary tree, only human beings are capable of endurance running. Apes, chimpanzees, and monkeys can sprint, but only for very short distances.
How did this human ability to sustain exercise come about? What evolutionary forces have permitted 20,000 scantily clothed humans to cram themselves onto Route 135 in Hopkinton, awaiting the gun to signal their 26.2-mile run into downtown Boston each April?
Dennis Bramble and Daniel Lieberman are two people who have thought a lot about this. Writing in the journal Nature, they describe the anatomic and metabolic features that enable sustained exercise that evolved for Homo sapiens.12 The list includes fossil evidence for musculoskeletal adaptations that improve energy economy derived from enhanced elastic recoil of the legs (long leg tendons, high longitudinal foot arches), factors allowing trunk stabilization (widened sacrum, enlarged gluteus maximus muscle), and optimized thermoregulation for hot African plains (effective sweat glands, lack of body hair, erect posture).
So much for the how. But what about the why? What selective advantage did long, slow distance running carry for our distant ancestors that now permits you to pin on that Boston marathon number? The fossil evidence cited previously suggests that our human ancestors first became equipped for distance running between 1.5 and 3.0 million years ago. Now, the hominid evolutionary branch diverged from that of other primates 5 to 8 million ago. The point is, then, that whatever Darwinian forces acted to make modern-day humans into distance running animals must have acted specifically on our closest ancestors (relatively speaking). When thinking about other aerobic endurance animals, this is evident, too. Clearly, distance running has developed independently in different evolutionary lines.
You might suppose that the survival benefit of being able to sustain exercise would come from an ability to scavenge or track down prey, particularly of animals who were faster—who could escape by sprinting away—but who could not maintain exercise for long periods of time. There are, in fact, primitive peoples today who chase down animals until their prey becomes exhausted. However, most contemporary hunter-gatherers rely on weapons—bows and arrows, spears—to kill animals they can’t immediately outrun. These arms first appeared on the scene about 40,000 years ago, so we would have to surmise that selective pressures that favor distance running ability would have been applied before that time. But we really just don’t know.
So, despite some good guesses, the full answers to our questions are not forthcoming. Our athletic endeavors today represent the heritage of millions of years of biological experimentation, of trial and error. We’ve inherited a magnificent clock-driven motor machine, one whose complex inner workings we can only faintly discern. Just where our capabilities for aerobic endurance exercise fit into this grand schema is not altogether clear, but you can bet that nature does things for a reason. And there may be broader forces and compromises to consider. I like the way Bernd Heinrich thought about this in his book Why We Run:
A race is like a chase. Finishing a marathon, setting a record, making a scientific discovery, creating a great work of art—all, I believe, are substitute chases we submit to that require, and exhibit, the psychological tools of an aerobic endurance predator, both to do and to evaluate. When fifty thousand people line up to race a marathon, or two dozen high schoolers toe the line for a cross country race, they are enacting a symbolic communal hunt, to be first at the kill, or at least to take part in it.12
1. For additional information on the relationships between stride frequency and length in humans, see the following articles: Cavanagh, P.R., and R. Kram. 1989. “Stride frequency in distance running: Velocity, body dimensions, and added mass effects.” Medicine and Science in Sports and Exercise 21: 467-479. Dillman, C.J. 1975. “Kinematic analysis of running.” Exercise and Sports Science Reviews 3: 193-218. Grillner, S. et al. 1979. “The adaptation to speed in human locomotion.” Brain Research 165: 177-182.
2. For those of you who were wondering, the human pattern of muscular activation and stride frequency or length with changes in velocity is similar to that observed in other mammals. This observation speaks to an evolutionary origin of our central motor oscillator (certainly not an unreasonable assumption given its subcortical, subconscious nature). However, differences do exist. In contrast to other mammals, for instance, primates (monkey, gorillas, chimpanzees, humans) have significantly longer stride lengths at a given velocity of locomotion. Animals such as cats and dogs, on the other hand, rely on increases in stride frequency to augment speed. Why should this be so? First of all, primates have unusually long limb bones compared to other mammals. Some have noted that primates generally possess a more arboreal lifestyle (Except for you and me, they swing from limb to limb in trees). Maybe they are thus more adapted to leaping and consequently have a longer stride length for overground running. (Ponder this the next time you dash to the fridge for a cold one during a TV commercial.)
3. This is not, however, the entire story. Although minimal, variability in interval of striding time (stride frequency) during locomotion does exist. When these fluctuations between individual steps are plotted over time, we see what at first glance appears to be a total random variability. If you plug these into a computer that knows a lot about Fourier and fractal analyses, however, certain patterns begin to emerge. J.M. Hausdorff and his colleagues in Boston have done just that in a pair of studies that described step-to-step fluctuations as subjects performed sustained walking at a self-selected pace. Among the apparent random variability, their computers were able to identify temporal fractal patterns. That means that patterns of fluctuation in one time segment were similar to patterns in longer time durations. They found that variations in the stride interval could be statistically correlated to fluctuations in the stride interval several thousands of steps before. This self-similar rhythmicity in respect to time suggests that the variations in stride frequency during locomotion are not simply random events, but instead are under some form of biological control (from a central pattern generator). The biological meaning of such rhythms remains a mystery. What is their purpose? In this particular case, what evolutionary benefit would be gained by a certain regular pattern of changes in stride frequency during human locomotion? It is not clear if these regular fluctuations are a fundamental property of biological systems or if they represent alterations in response to environmental changes. We can’t help feeling that some basic underlying principle that governs living matter looms here. If you figure it out, call me.
4. Serving as a backdrop to these considerations of firing rates of the central motor controller lie some intriguing notions of natural rhythms, or timing cycles, in humans. Indeed, what has been termed a central resonant frequency of human movement may actually be an expression of a more generalized, intrinsic biologic phenomenon. Velocity and stride rate during walking have been compared to the physics of pendulum movement. A pendulum requires external force to keep it swinging because of the dampening influences of factors like gravity and stiffness forces. In such physical oscillations, there exists a certain frequency at which such external forces are minimized. When subjects are allowed to self-select a walking pace, the stride frequency (1 cycle per second, or 1 Hz) has been found to be similar to that of a force-driven harmonic oscillator, such as a pendulum (0.92 Hz). These principles of physics may thus underlie rhythmic locomotor movements in humans, which could be indicative of the body’s goal to minimize energy requirements.
5. Read more on dissociation by runners in this article: Morgan, W. 1978. “The mind of the marathoner.” Psychology Today 11: 38-49.
6. This book provides a concise discussion of stroke rates during swimming competition: Maglischo, R.M. 2003. Swimming fastest. Champaign, IL: Human Kinetics.
7. Some good information on pedaling cadences during cycling can be found in the following articles: Marsh, A.P., and P.E. Martin.1993. “The association between cycling experience and most economical cadences.” Medicine and Science in Sports and Exercise 25: 1269-1274. Padilla, S. et al. 1999. “Level ground and uphill cycling ability in professional road cycling.” Medicine and Science in Sports and Exercise 31: 878-885.
8. Gotshall, R.W. et al. 1996. “Cycling cadence alters exercise hemodynamics.” International Journal of Sports Medicine 17:17-21.
9. Comprehensive discussions of central pattern generators can be found in the following articles: Dietz, V. 2003. “Spinal cord pattern generators for locomotion.” Clinical Neurophysiology 114: 1379-1389. Ivory, R.B. 1996. “The representation of temporal information in perception and motor control.” Current Opinion in Neurobiology 6:851-857. Marder, E., and R.L. Calabrese. 1996. “Principles of rhythmic motor pattern generation.” Physiology Reviews 76: 687-717. Shik, M.L., and G.N. Orlovsky. 1976. “Neurophysiology of locomotor automatism.” Physiology Reviews 56: 465-501. Zehr, E.P. 2005. “Neural control of rhythmic human movement: The common core hypothesis.” Exercise and Sports Science Reviews 33: 54-60.
10. Bramble, D.M., and D.R. Carrier. 1983. “Running and breathing in mammals.” Science 219: 251-256.
11. Daniels, J. 2005. Daniels’ running formula. 2nd ed. Champaign, IL: Human Kinetics.
12. The evolution of endurance exercise skill in human beings and how it is related to that of other animals is treated in the following sources. The latter is particularly readable. Bramble, Dennis, and Daniel Lieberman. 2004. “Endurance running and evolution of Homo.” Nature 432: 345-52. Heinrich, Bernd. 2001. Why we run. New York: Harper Collins.
1. The central nervous system includes a system of subconscious central pattern generators with the following:
a. An oscillator within the spinal cord that coordinates the rhythmic sequence of muscle innervation for locomotion.
b. Higher brain motor centers that dictate the tempo of the oscillator and the force of muscle contraction that define stride frequency and length.
2. This system is under the control of intrinsic biological clocks that maintain a strictly timed sequence of muscle action and control pace of locomotion.
3. During running, the stride frequency and length selected by these systems for achieving a particular velocity is in some way most efficient, either metabolically or mechanically. Efforts by a runner to purposefully alter spontaneous SF-SL relationships may be counterproductive. The lesson here, as per the traditional coach’s dictum, is “Pace, don’t race.”
4. In other sports, going with what intuitively feels best in respect to stroke or pedaling rates has not proven to be wise. Although coaches will argue, the best approach appears to be a process of individual experimentation to determine the best rate for velocity. Whether this corresponds to that which might be dictated by an intrinsic motor pacemaker remains to be seen.