FOUR
9q34: The ABO Gene
In 1990, an international consortium of governments launched the Human Genome Project to determine the 3-billion-nucleotide sequence of human DNA. The first completed draft of the genome was reported in February 2001. The implications of these genetic investigations are astounding. It is estimated that mutations in human genes predispose or cause at least 1,500 diseases, from diabetes to asthma to cancer. Scientists believe that the anatomy of the genome is a significant step in the process of identifying and fixing those mutations. In the process, the ABO blood groups have emerged as important genetic markers that play a prominent role in cellular function and dysfunction.
The DNA Code
Each of us shares the same qualities that categorize us as the species Homo sapiens. We have the same organ size and structure, the same number of appendages, and many of the same responses to physical stimulation. In fact, genetically we are about 99.9% identical. It’s the one-tenth of a percent that makes all the difference.
Our genetic heritage is our biologic hard drive. Embedded within it are the recordings of past “writings” that were saved for use later—along with, in some cases, a few “disk errors.”
Traits are inherited because discrete units called genes are passed from parent to offspring when a new organism is conceived. These genes are a unique blueprint for an individual organism, providing all the information needed for the development and life of that species, as well as for the characteristics that make each individual unique.
In general, each gene determines one function, such as the production of an enzyme, which may then go on to produce a protein, hormone, and so on. Complicated processes within the cell are contributed to by the functions of numerous genes. Just as in a machine, where the removal of one part can disrupt the ability of the entire engine to run, so the removal of the influence of one gene can have a severe effect on the life of an organism.
How do we know genes exist? In the late 1800s, an Austrian monk named Gregor Mendel inbred varieties of pea plants and kept careful track of the traits displayed by their offspring. He discovered that the traits of the parent plants were passed on to the progeny plants in strictly reproducible patterns. Mendel postulated that discrete “units of inheritance” determined the traits he examined. He observed that two units of inheritance existed for each trait—one from each parent plant. We now call these units genes.
Genetics is elegant and powerful, but essentially indirect. Inference plays a critical role. Mendel’s experiments led him to infer the existence of genes. He never actually saw them. Similarly, the analysis of defects, called mutations, in genes help scientists to infer normal gene function. By seeing what happens to the organism when the function of a gene is missing, scientists can make educated guesses regarding what the normal job of the gene is in the cell.
The Human Genome
The human body contains between 50 and 75 billion cells. The nucleus of each cell contains 23 pairs of chromosomes; 22 are inherited from the mother, 22 are inherited from the father, and two are sex chromosomes—XX for a female, and XY for a male. Within each chromosome there are about 30,000 paired genes composed of DNA (deoxyribonucleic acid), which is the genetic hard drive where information is stored. (Before coding the genome, scientists had long believed there were at least 100,000 genes and were astonished to find less than one-third that number.)
The simplest way to visualize DNA is to picture a spiral staircase. The handrails and balustrade of the staircase are composed of repeated sequences of phosphate and deoxyribose sugar. The steps of the staircase are made of pairs molecules called bases, which repeat themselves again and again. Each of the four pairs are identified by their initial letter—A for adenine, G for guanine, C for cytosine, and T for thymine.
Each of the pairs are connected by hydrogen bonds. Because of the chemical nature of these bonds, A (adenine) and T (thymine) can bind only with each other; and G (guanine) and C (cytosine) can bind only with each other. So four combinations are possible:
These four combinations are referred to as base pairs. The arrangement and order of these base pairs determines the actual information that DNA carries—much like the arrangement of the 1s and 0s on a computer program assume meaning by virtue of the order they are arranged in. The sequence of bases is read by machinery inside the cell. Various sections of DNA comprise specific genes, which can be read again and again, depending on whether the cell needs to produce what the gene codes for.
A single DNA molecule is composed of approximately 3 billion base pairs, tightly wound into the spiral helix form. Too infinitesimal to be viewed by even the most powerful microscope, the genetic information contained in a single molecule would stretch out uncoiled to about six feet long, and if written down, would fill over 125 massive books the size of the Manhattan telephone directory. The directory would be about 1 million pages long and 210 feet thick, using variations of the four-letter DNA code. DNA is not, however, just a “phone book” of human genes. Most of the genome consists of so-called “junk DNA”—rambling sequences of As, Ts, Gs, and Cs that seem to have been written in gibberish.
The genome has also revealed that the genes composing human DNA are not evenly distributed among the chromosomes. Some chromosomes are densely packed, while others are sparsely populated.
Alleles
What determines a person’s unique DNA? Mendel also learned that a particular trait could be characterized by more than one type of information. For example, a gene for flower color could be of the red or white variety. These alternatives for a gene’s information are called alleles. Alleles differ in their DNA sequence.
Every person’s DNA contains alleles—alternate forms of genes. The alleles determine whether you have blue eyes or brown, are tall or short, have black hair or red, and other distinctions. Alleles are formed by differences in the sequence of base pairs, which can be as long as 10,000 base pairs. Genes with alternate forms are called polymorphisms, meaning “many forms.” Polymorphisms are the presence in a population of two or more genotypes for a given trait.
There are three blood group alleles—A, B, and O. In different combinations they are capable of producing four variations, or alternatives, for your blood group. Since these variations occur within an otherwise highly similar unit (such as a species) they are called polymorphisms.
Think of a gene as a multiple-choice question on an exam, and the gene’s alleles as the possible answers to the question. Some alleles have dominant characteristics over others. For example, if a child has one blue eye color allele and one brown eye color allele, the child’s eyes will be brown—just as if the child had two brown eye color alleles. Brown is the dominant eye color in humans; blue is the recessive eye color. Needless to say, people with blue eyes have two blue eye color alleles. However, the influence of your blood group allele is far greater than that of the gene that gives you eye color.
Genotype versus Phenotype
The basic genetics of the ABO blood groups is really quite simple, very similar to the way eye or hair color is determined. We are the physical result of our genes; this is called our phenotype. Phenotype is defined as all the physiological, behavioral, biochemical, and other characteristics of an organism that develop through the interaction of their genes and the environment. We get these genes from our parents, and the combination of our parents’ genes that we carry is called our genotype. Genotype is defined as the genetic or hereditary information borne by an individual, as distinct from an individual’s visible features. In a nutshell, you are your phenotype, and it was your genotype that got you there.
In multiple-allele genes, such as those for the ABO blood groups, one of the two alleles will usually be dominant, which is the key to your physical differences. In the ABO system the A and B alleles are dominant to the O allele (which is actually a blank, or “null” allele), just as in eye color, where the gene for brown eyes is dominant to the gene for blue eyes.
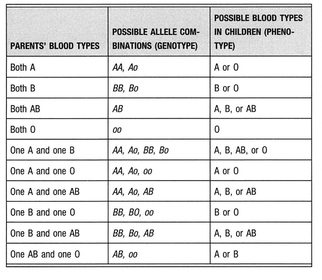
Genes are typically written in italics. Thus if we were referring the A allele, we would express it as A. Dominant genes are usually capitalized and recessive genes are usually written in lower-case. Thus a person of blood group A may have either of two possible genotypes: Ao or AA. All blood group O individuals have the genotype oo, while all blood group AB individuals have the genotype AB.
For example, if you’ve received an A allele from your mother and an O allele from your father, your genotype will be Ao, but your phenotype will be blood group A. However, you carry a latent O, which in turn can be passed on to your offspring. The distinction between phenotype and genotype is what confuses so many people about blood group genetics. It explains why a blood group A mother and a blood group O father can have a blood group O child, even though you can only become O if you receive an o allele from each parent.
The A and B alleles have an interesting relationship with each other: they do not dominate each other, as each does with the null o allele; rather they are co-dominant, meaning that if you receive an A allele from one parent and a B allele from the other, you become blood group AB.

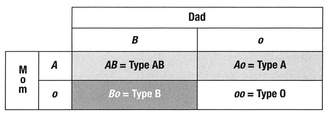
Here’s a simple illustration. Called a Punnett square, this tool is used by geneticists to determine possible combinations of phenotype from genotype. Mom (along the left side) is phenotype O (blood type O) and genotype oo. Dad (at the top) is phenotype A (blood type A) and genotype Ao. As we can see, each offspring has a 50% chance of being either A (Ao) or O (oo). Since neither parent carriesaBallele, it is impossible for the offspring of these two parents to be either B or AB. This is why blood type can be useful in determining paternity. It cannot confirm that a person is the father of a child; however, in certain circumstances it can confirm that a person is not the father. If the offspring of disputed paternity in the preceding case was either blood group B or AB, we would have to look elsewhere for the father of the child; the combination shown cannot give the child a B antigen.
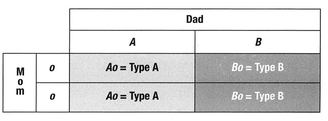
Here’s an interesting combination: Mom is blood group O and Dad is blood group AB. In this circumstance, the offspring will be either A or B, as both A and B alleles are dominant over O. No offspring will possess the blood type of their mother. However, all will possess a recessive o that can be passed on to their own offspring, potentially producing O children. This answers another question—why blood group O doesn’t disappear over time, since group A and group B have the dominant alleles: There is a constant regenerating supply of the o allele in the human gene pool.
In a third scenario, we can see how a group B father and a group A mother can produce offspring of every group. Thus, although it is a long shot, it is possible for each member of a family to have a different blood type.
TABLE V. Blood Type Inheritance
9q34: The ABO Gene
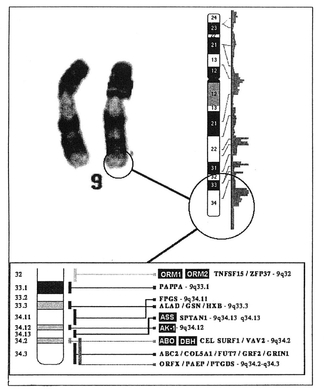
Each chromosome pair is joined at the center at a spot called the centromere. The characteristic banding of chromosomes (shown in the following illustration) is obtained by staining with various dyes. The band width and the order of bands is characteristic of a particular chromosome; a trained geneticist can identify each chromosome (1,2,3...22, X and Y) by observing its banding pattern under the microscope.
Chromosomes are divided into 2 legs; a “p” leg above the constriction point, and a “q” leg below it. By combining the chromosome number, the p or q leg, and a particular band number, it is possible to construct an address for a particular gene.
Each gene has a particular locus where it can be found. Think of the locus as the location or address of a gene. The gene locus for ABO blood group is located on the q leg of chromosome number 9, around band 34. It is here that the three basic alleles of the ABO blood system are found, which in combination determine whether you are group O, A, B, or AB. But the effect of the ABO blood group gene doesn’t just stop there.
If we were to examine the band around 9q34, we would find that the area that determines ABO blood group is densely stained, indicating that there is a lot of thickly packed DNA, which is somewhat odd since the mechanics of ABO selection are fairly simple; the gene codes for the activity of only two enzymes. This brings us to one of the deepest, though least appreciated, aspects of the ABO gene: It can often influence the effect of other, seemingly unrelated genes.
We have already seen an example of this when we looked at the secretor subtype, as the basic mechanics of ABO and secretor status involve genes located on two different chromosomes. Our secretor status, which influences the ability of our bodies to secrete our ABH antigens in free form, is controlled by the secretor gene locus found on chromosome 19 (19q13.3). Interestingly, the gene locus that codes for the manufacture of the H antigen (which determines the O antigen and serves as the building block for A and B antigens) is located on chromosome 19 as well. If you are A, B, or AB, you have active alleles on 9q34; however, if you are blood group O, your DNA has a “null allele” there, so you are left with just the output of chromosome 19 (H) but nothing from 9q34 (A or B).
Gene Linkage
It is often said that great science occurs because good questions are asked. In 1933 T. H. Morgan won the Nobel Prize for work that began in 1910 at Columbia University. In that year he discovered a white-eyed fruit fly mutation. Up to this point it was pretty obvious that genes are on chromosomes. But on which chromosome is a given gene and where on the chromosome is it?
Morgan mated a red-eyed female fly with a white-eyed male and found the offspring to be red-eyed. He concluded that the gene for red eyes must be dominant. Morgan then mated these offspring. Now, according to Mendel’s laws, there should be three red-eyed flies for each white-eyed fly, and when the flies were analyzed over several months, Morgan counted 3,470 red-eyed flies and 782 white-eyed flies—which was close to the expected one-to-three ratio.
However, Morgan noted a curious feature. None of the females had white eyes. Among the males, 1,011 had red eyes and 782 had white eyes. Further work was able to show that
• White eye color was recessive to red eye color.
• The gene for eye color was carried on the X (female) chromosome.
• There is no gene for eye color on the Y chromosome.
Morgan’s work was a vital key to understanding gene linkage, one of the most important concepts in genetics. The concept itself is quite simple: Genes located on the same chromosome tend to be transmitted together. These are referred to as linked genes. The linkage of eye color to sex in the fly is a special case of the general phenomenon of gene linkage, in which two or more genes tend to be inherited together because they are on the same chromosome. In the case of blood groups, several genes are linked to the ABO locus, and tend to transfer in a manner that is dependent on the outcome of one’s ABO group (actually, the combination of the ABO alleles).
Adjacent alleles for genes on the same chromosome tend to be coupled more often than expected. This is most likely a consequence of the “founder effect,” in which a mutation for a disease arose at an early point in history in a particular population on a particular chromosome, and the allele was consequently linked to a particular adjacent gene marker. This is called linkage disequilibrium, since over time we would expect that enough genetic recombination would occur to randomize the relation of the mutant allele with respect to other marker alleles on other chromosomes—that is, to establish linkage equilibrium. Therefore, when linkage disequilibrium is seen, it is likely that inadequate time has passed for equilibrium to be established.
The discovery of a relationship between particular genes and diseases is accomplished through gene linkage studies. Linkage studies examine inherited genetic markers, such as ABO blood group. One of the first examples of gene linkage ever discovered in humans was the coinheritance of ABO blood group with a rare disease called nail-patella syndrome, in which people have abnormal nails and kneecaps. Knowing that the gene causing nail-patella syndrome was linked to blood group told scientists that the gene for nail-patella syndrome is located on chromosome 9—in other words, there is a gene proximity between the gene for blood group and the gene that causes nail-patella syndrome.
By looking at the genetic expression of the ABO blood groups, we can now understand many apparently unrelated physical and mental correlations. Attributes that seem unrelated—such as physiological adaptations to environment, disease, or diet, may have had a survival rationale, and can be seen as linked traits in genetic memory. If these adaptations were successful and persisted, they would be hard-coded into one of the blood group alleles as a variant strategy for use by later generations.
Blood groups are also valuable markers in gene linkage analysis, and their study has contributed enormously to the mapping of the human genome.
Here are some of the initial findings linking the ABO blood group locus at 9q34 with other genes in close proximity:
BREAST CANCER
In 1984, researchers reporting in the journal Genetic Epidemiology presented evidence of a family pedigree in which a major gene for breast cancer susceptibility appeared to link to the ABO gene locus‘. This supports other studies showing a similarity between the A antigen and a common tumor marker for breast cancer2.
DOPAMINE METABOLISM
Dopamine beta-hydroxylase (DBH) is associated with the conversion of dopamine into the catecholamines adrenaline and noradrenaline. The gene for DBH virtually sits atop the gene for the ABO blood groups. Several studies have noted the relationship and its corresponding impact, with particular significance for blood group O.
In 1982, researchers measured DBH and catechol-O-methyltransferase (COMT) levels in 162 patients with major affective disorders (depression) and 1,125 of their relatives. A linkage of a locus for DBH to the ABO locus was indicated3.
In 1988, a report indicated that the previously described activity variation in levels of serum DBH may reflect alterations in either the structure or regulation of the DBH coding. The researchers pointed out that the structural gene for the enzyme is close to the ABO blood group locus, and thus may be influenced by it4.
A 1988 article published in the American Journal of Human Genetics stated that “Previous studies have presented evidence suggesting that levels of dopamine-beta-hydroxylase (DBH) activity are controlled by a gene linked to the ABO blood group locus.” The researchers were able to verify this, showing a direct linkage between the gene regulating DBH activity and the gene for ABO blood group locus5.
Researchers writing in the journal Biological Psychiatry, by combining data from a number of other studies, provided evidence for a “susceptibility allele” for affective disorder (depression) near the ABO region on chromosome 9q34, perhaps related to the gene locus for dopamine beta hydroxylase6.
BLOOD CLOTTING
Lower levels of the blood clotting chemicals factor VIII and von Willebrand factor (vWF) have been reported in individuals with blood group O compared to individuals of other ABO blood groups, which is one reason their rate of heart disease is lower than the other ABO groups. Recent studies have shown that this is probably the result of gene linkage7.
BLADDER CANCER
The area of the ABO genes (9q34) is an area where genes are commonly lost as cells of the bladder turn cancerous. The loss of ABO antigens is a common occurrence as bladder cells move toward metastasis. Evidence suggests that gene deletions in bladder cancer cells are linked to similar deletions on the ABO locus8.
MUSCULOSKELETAL INJURY
There may be a genetic linkage between the ABO blood groups and the molecular structure of the tissue of Achilles tendons, as studies have associated a correlation between group O individuals and ruptures of the Achilles tendon9.
SECRETOR STATUS AND EYE COLOR
A Danish study linked green eye color with being an ABO secretor10.
NITRIC OXIDE METABOLISM
The amino acid arginine in the form of argininosuccinate is a basic building block in the synthesis of nitric oxide, a molecule critically involved in a plethora of body functions, including brain function, cardiovascular health, and proper immune function. The amino acid citrulline is recycled into argininosuccinate by the action of arginosuccinate synthetase (ASS), whose gene location is known to be very close to the ABO locus 11,12. Variations in the function of this enzyme may be linked at the genetic level, since variations to inhaled nitric oxide therapy have been shown to occur in individuals who possess a B antigen (blood groups AB and B)13,14.
ABO Blood Groups: The First Few Hours of Life
Normal cells are differentiated by virtue of having only the genes needed for their particular job activated, and all other genes deactivated, or repressed.
If we turn our thinking for a moment back to the growing embryo, we can get an idea of how this can occur. Embryonic cells (such as one might find in a 2- to 3-week-old fetus) do not yet have the full functionality found in the cells of a mature adult. Initially, unlike the thousands of different highly specific cells found in the normal adult, embryonic cells only come in three varieties:
• Ectoderm cells, which differentiate into the cells of the skin and nerves
• Mesoderm cells, which differentiate into the cells of the muscular system, skeleton, and connective tissues
• Endoderm cells, which become the lining of the digestive system
Since these cells, often called germ cells or germ layers, eventually differentiate into a wide variety of different tissues, they obviously need to repress and de-repress a large number of genes in order to wind up with a highly specialized end product.
The ABO antigens are intimately involved with the process of differentiation. We know that their production is greatly increased in the blood vessel cells of fetal organs, and they are thought to be responsible for specifying the location of future blood vessels in the burgeoning organs, where they serve as differentiation markers. We can almost think of the ABO antigens in the growing fetus as a team of railroad engineers, moving ahead of the construction crew, planning the future location of a railroad track.
One study documented the appearance of ABO blood group antigens in different developing animal embryos. It showed that ABO begins to appear first in endoderm cells, followed by ectoderm cells, and finally by mesoderm cells. Since red blood cells were the last cells to acquire ABO antigens, the authors suggested that ABO antigens should be called tissue antigens rather than blood group antigens15,16.
This critical embryonic function of ABO blood group is not typically known or understood by the medical community. However, this is probably the single most important reason that blood type antigens appear and disappear in tissues that are about to go aggressively malignant and metastasize 17, a role that is increasingly being linked to ABO blood group antigens18.
The genetic importance of ABO blood groups is only now emerging as the key to many of the most elusive biological mysteries. We can use this knowledge to maintain health, guard against disease, and even heal already damaged cells. In this respect, the genetic revolution is truly the blood type revolution.
REFERENCES
1. Skolnick MH, Thompson EA, Bishop DT, Cannon LA. Possible linkage of a breast cancer-susceptibility locus to the ABO locus: sensitivity of LOD scores to a single new recombinant observation. Genet Epidemiol. 1984;1:363—373.
2. Garratty G. Blood group antigens as tumor markers, parasitic /bacterial/viral receptors, and their association with immunologically important proteins. Immunol Invest. 1995;24:213—232.
3. Goldin LR, Gershon ES, Lake CR, et al. Segregation and linkage studies of plasma dopamine-beta-hydroxylase (DBH), erythrocyte catechol-O-methyltransferase(COMT), and platelet monoamine oxidase (MAO): possible linkage between the ABO locus and a gene controlling DBH activity. Am J Hum Genet. 1982;34:250—262.
4. Craig SP, Buckle VJ, Lamouroux A, et al. Localization of the human dopamine beta hydroxylase (DBH) gene to chromosome 9q34. Cytogenet Cell Genet. 1988;48:48—50.
5. Wilson AF, Elston RC, Siervogel RM, Tran LD. Linkage of a gene regulating dopamine-beta-hydroxylase activity and the ABO blood group locus. Am J Hum Genet. 1988; 42:160—166.
6. Sherrington R, Curtis D, Brynjolfsson J, et al. A linkage study of affective disorder with DNA markers for the ABO-AK1-ORM linkage group near the dopamine beta hydroxylase gene. Biol Psychiatry. 1994;36:434-442.
7. Souto JC, Almasy L, Muniz-Diaz E, et al. Functional effects of the ABO locus polymorphism on plasma levels of von Willebrand factor, factor VIII, and activated partial thromboplastin time. Arterioscler Thromb Vasc Biol. 2000; 20:2024—2028.
8. Orlow I, Lacombe L, Pellicer I, et al. Genotypic and phenotypic characterization of the histoblood group ABO(H) in primary bladder tumors. Int J Cancer. 1998;75:819—824.
9. Leppilahti J, Puranen J, Orava S. ABO blood group and Achilles tendon rupture. Ann Chir Gynaecol. 1996;85:369—371.
10. Eiberg H, Mohr J. Major genes of eye color and hair color linked to LU and SE. Clin Genet 1987;31:186—191.
11. Ozelius LJ, Kwiatkowski DJ, Schuback DE, et al. A genetic linkage map of human chromosome 9q. Genomics. 1992; 14:715—720.
12. Northrup H, Lathrop M, Lu SY, et al. Multilocus linkage analysis with the human argininosuccinate synthetase gene. Genomics. 1989;5:442—444.
13. McFadzean J, Tasker RC, Petros AJ. Nitric oxide ABO blood group difference in children. Lancet 1999,555:1414—1415.
14. Weimann J, Bauer H, Bigatello L, Bloch KD, Martin E, Zapol WM. ABO blood group and inhaled nitric oxide in acute respiratory distress syndrome. Lancet. 1998;351: 1786—1787.
15. Oriol R, Mollicone R, Coullin P, Dalix AM, Candelier JJ. Genetic regulation of the expression of ABH and Lewis antigens in tissues. APMIS Suppl. 1992;27:28—38.
16. Szulman AE. Evolution of ABH blood group antigens during embryogenesis. Ann Inst Pasteur Immunol. 1987;138: 845—847.
17. Sarafian V, Dimova P, Georgiev I, Taskov H. ABH blood group antigen significance as markers of endothelial differentiation of mesenchymal cells. Folia Med (Plovdiv). 1997;39:5—9.
18. Le Pendu J, Marionneau S, Cailleau-Thomas A, Rocher J, Le Moullac-Vaidye B, Clement M. ABH and Lewis histoblood group antigens in cancer. APMIS. 2001;109:9—31.