C
CANCER (general)-A general term for a group of more than 100 diseases characterized by the uncontrolled growth and spread of abnormal cells.
About Cancer
As we age, so do the cells of our bodies. All organs of the body are made up of cells capable of normal division; they produce more cells when the body calls upon itself to manufacture them. If cells divide when new ones aren’t needed, these cells form a mass of excess tissue called a tumor. As a tumor grows, nutrients are provided by direct diffusion from the circulation. Local tissue invasion can result in pressure on normal tissues, which can lead to inflammation, or the tumor may produce substances (e.g., collagenase) that lead to enzymatic destruction of tissues. Tumors can be benign (non-cancerous) or malignant (cancerous).
As with many other diseases, both genetic and environmental factors are implicated in the cause and development of human cancers. Cancer-causing biological, chemical, and physical agents are referred to as carcinogens, and the process of a carcinogen-instigating cancer is called carcinogenesis. Recent advances in the molecular biology of cancer, stemming from the study of cancer-causing viruses (oncoviruses) and transforming DNA, are providing new ways of investigating cancer-forming genes (oncogenes) and cellular pathways that are involved in the mechanisms of viral, chemical, and physical carcinogenesis.
Carcinogens are grouped into two categories. Direct-acting carcinogens are carcinogenic on their own. Procarcinogens must be converted metabolically inside the body into carcinogens. Paradoxically, most forms of chemotherapy for cancer are direct-acting carcinogens as well. Procarcinogens include aflatoxin (the toxin from mold), many chemical dyes, nitrosamines from smoked foods in the diet, and some metals such as nickel. Carcinogenesis can take years or decades to finally result in a tumor. This latent period between an exposure to a carcinogen and cancer formation is explained by studies showing that carcinogenesis is divisible into two stages, tumor initiation and tumor promotion.
A living creature is the result of a complex symphony of various specialized cells, each of which has a particular job to do. Like a modern city, which could not function without its specialized members such as police, firefighters, sanitation workers, and shopkeepers, the body can only function properly if there are adequate numbers of very specialized cells, each with its particular part to play.
What makes a cell become one particular type or another is what particular part of its DNA is turned on or off. A grossly oversimplified way of considering this would be to think about a cell currently residing on your fingernail. That type of cell is programmed to be very hard and somewhat waterproof, two specialized functions that would not be very helpful if found in your brain. There, you need specialized cells that can conduct electrical charges and stretch out for very long distances. The fingernail cell’s DNA has only the information needed to be a fingernail cell activated; all other parts of the cell’s DNA are turned off. In essence it is a fingernail cell that has only those genes left active. A nerve cell is a nerve cell for the same reason; the genes that would have made it a fingernail cell, or a hair cell, or a muscle cell have all been turned off—or, as it is termed in biology, the genes have been repressed.
Cell function and specialization results in large part from controlling on the genetic level what a cell can or cannot do. Normal cells are called differentiated because they have the same characteristics as all other cells of that cell type. Hair cells look very much like other hair cells, and when they reproduce, they make other hair cells. In this nicely ordered way of things, life goes on. The body goes to great trouble to keep cells differentiated, since the loss of this control is the first step in a process of cellular anarchy that can eventually lead to malignancy.
Differentiation is also called tumor grade. The evaluation of differentiation of cells shows the degree of malignancy. Cells with a high level of differentiation are normal cells with specific functions. Cells with low levels of differentiation are more dangerous in respect to malignancy.
Characteristics of a Cancer Cell
• Cancer cells have a typically round appearance, instead of the normal flat appearance.
• The cancer cells do not adhere to one another as normal cells do. This is due to the reduction in surface adhesion molecules on the cancer cell’s surface. The major adhesion molecules lost are the ABO antigens.
• There is limited contact inhibition of movement. Normal cells terminate movement when in contact with one another; cancer cells don’t.
• The cells have less anchorage dependence. So they are free to invade other tissues and enter the blood and lymph systems (metastasis).
• Cancer cells are not inhibited by the density of surrounding tissue. Cells are able to pile up on top of one another.
• Extracellular growth factors are not required for proliferation, because cells are able to produce their own growth factors. Certain blood type an-tigens can interact with cancer cell growth factors. The lifespan is indefinite, because apoptosis (programmed cell death) is inhibited.
CELL DIFFERENTATION
The DNA code of every cell contains all of the information needed for that cell to be any other kind of cell. What gives a cell its unique ability to perform the function for which it is intended is that only the information for that cell is turned on. So a gland cell performs only the function of glands, the bone cell performs only the function of bones, and so on. These different cells all have distinct appearances.
In medicine, it is typical to grade tumors according to the degree of differentiation, which is usually determined by a pathologist looking at slides of the tissue. The more differentiated a tumor cell is, the more effectively the body’s immune response to it can be. Thus, a well-differentiated tumor will have cancer cells that more or less still resemble each other; an antibody, or killer cell, can be programmed to look for a tumor antigen (or tumor “marker”) that should be consistently found on every cell in the tumor. As the tumor cells become more undifferentiated, they no longer resemble each other enough to allow for a potent immune response. In essence, the immune system would have to make a new and different antibody for each different tumor cell—an impossible job. For that reason well-differentiated tumor cells (such as MELANOMA cells) tend to respond to immunotherapy better than poorly differentiated ones.
Here are the common stages of differentiation normally used by pathologists.
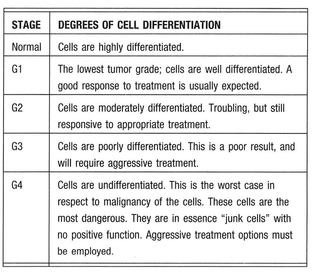
Normal cells are differentiated by virtue of having only the genes needed for their particular job activated, and all other genes deactivated, or repressed. How do tumors lose differentiation? As tumors mutate, the mutations begin to activate latent, repressed genes that are normally turned off. This is called de-repression, and as it occurs, cells very often regress to forms much like those found in embryonic tissue. Embryonic cells (such as one might find in a 2- or 3-week-old fetus) do not yet have the full functionality found in the cells of a mature adult. Initially, unlike the thousands of different, highly specific cells found in the normal adult, there are only three types of embryonic cells:
• Ectoderm cells: which differentiate into the cells of the skin and nerves
• Mesoderm cells: which differentiate into the cells of the muscular system, skeleton, and connective tissues
• Endoderm cells: which differentiate into the cells lining the digestive system
Since these cells (often called germ cells or germ layer) eventually differentiate into a wide variety of different tissues, they need to repress and de-repress a large number of genes to wind up with a highly specialized end product. As a cancer cell mutates and loses control of its normally controlled genetic material, it deteriorates regressively and begins to resemble these early embryonic cell forms. Many cancer cells begin to manufacture glycoprotein antigens that are typically found only in embryonic tissue; many of these are used diagnostically, such as the CEA (carcinogenic embryonic antigen), which can be used to gauge the progression of several types of cancer.
Blood Group Links
ABO blood group antigens are intimately involved with the process of differentiation. We know that their production is regulated in the blood vessel cells of the fetal organs, and they are theorized to be responsible for specifying the location of future blood vessels in the burgeoning organs, where they serve as differentiation markers. This is a very important function of the ABO blood group, and is for the most part not known or understood by the majority of the medical community. However, this critical embryonic function is probably the single most important reason that blood group antigens appear and disappear in tissues that are about to become aggressively malignant and metastasize1.
Studies have shown that blood group A has higher levels of the p53 tumor suppressor gene in certain cancers, especially those of the digestive tract. Although one might think that higher levels of a gene that suppresses tumors might be a good thing, the general consensus holds that it tends to be associated with signs that the tumor is becoming more invasive. Higher levels of the p53 suppressor gene have also been linked to the likelihood that the tumor has begun to develop disturbances in its production of blood group antigens, weakening its resistance2.
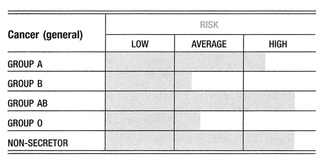
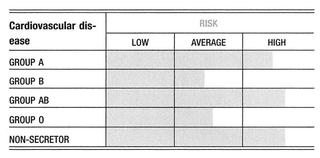
Although there are well over a thousand published findings on the associations between blood types and disease, many are based entirely on statistical analyses. Most of the earlier studies have been controversial, usually because they were small studies that may have had inadequate controls, or been analyzed incorrectly. Nevertheless, it is difficult to argue with the general pattern that emerges from the large body of statistical data on malignancy, coagulation, and infection. Some of the findings on microbe receptors and their association with important immune proteins are most convincing, and suggest that blood type antigens do play an important biological role. Interestingly, it is a role that is often completely unrelated to the red blood cell. Cancers in general tend to be associated with blood group A, and slightly less so with blood group B.
Perhaps the greatest focus of current research on the ABO blood group antigens resides in the field of molecular oncology. Recent findings in membrane chemistry, tumor immunology, and infectious disease have provided a compelling scientific rationale for several blood type associations. Because of this, there is an increase in acceptance of the earlier statistical findings.
The huge interest in blood groups stems from the developing awareness that blood group antigens are important components in the process of cell maturation and control. As an example, the appearance or disappearance of blood group antigens is a hallmark of malignancy in many common cancers.
Several tumor antigens or tumor markers are the known product of certain blood type precursors. Many of these tumor antigens are A-like, which helps in part to explain the striking number of associations with blood group A and AB. On the contrary, autoimmune disorders tend to be associated with blood group O. The contrast with the cancer—group A association is an interesting one in view of the suggestion of earlier immunologists that there is a fundamental antithesis between the two classes of disease. The heightened surveillance and overactive immune activity tend to result in less malignancy, whereas overly tolerant immune activity tends to encourage it. These observations suggest a more general hypothesis that in the tissues of all people, both normal and cancerous, there are A-like antigens present at a biochemical level that are usually inaccessible to the immune system. However, when stimulated by an autoimmune process, or the immune response to a growing cancer, the antigen becomes accessible. At that point, a blood group A person, who cannot make anti-A antibodies, will be more likely than a blood type O person to tolerate the cancer, but the A person’s immune system will be less likely than that of a blood type O person to attack the body’s own tissues.
The cancer—group A link is far from an absolute. There are several tumors that show a consistent association with groups O and B. This implies that cancer is a condition associated with derangement of blood type activity in general, and the expression of A-like antigens on the surface tumors is just simply the most common of these derangements.
Blood Group Antigens and Cancer Cell Adhesion
When cancer spreads away from the primary site it is called metastasis. Metastasis is a complex phenomenon consisting of several sequential steps:
1. Invasion of primary sites
2. Entry into the blood or lymphatic vessels
3. Transport
4. Migration from the blood vessel into the tissues, and growth at the target sites
The results show that certain types of carbohydrate antigen expressions in cancer cells are profoundly related not only to the mode of metastatic spread and the organ distribution pattern of metastasis, but also to the prognosis of cancer patients. Lymphatic metastasis is related to the expression of Tn antigens and Tn-like antigens (see IMMUNITY) where no conspicuous similarities have been found in the hematogenous metastasis-related carbohydrates among the cancers. The expressions of blood group antigens such as ABO, Lewis, and MN, including their precursors, appear to be strong prognostic indicators of these cancers, although the relation of these antigens to each cancer varies. Adhesion molecules and/or carbohydrates may be one of the determinants of cancer metastasis and prognosis, at least in part3.
The reason that deletion or reduction of the A or AB antigens in tumors of A or B individuals correlates with malignancy and metastatic potential may be due to the lack of adhesiveness that a cancer cell achieves when it loses blood group antigens. Findings in human colon cancer patients have indicated that the degree of motility and the proliferation of colon tumor cells are directly associated with the deletion or reduction of the A antigen. As cells lose the A-like antigen, they seem also to lose the ability to express many of the cell adhesion proteins, such as the integrins, which normally express an A-like antigen on their receptors and control cell movement‘.
The loss of blood group antigen results in the tumor cells gaining the ability to move and circulate through the body, because the blood type antigens are needed for the cell’s integrin receptors to work properly as the adhesive glue that holds cells together. This link between blood group and cell adherence is probably as elemental to the development of cancer as it is to life itself. A growing fetus needs the ability to spawn new organs and grow an effective blood supply to feed them; in these instances, the loss of blood group antigens would allow for this migration of embryonic cells to the sites of future organs and blood vessels. In fact, many of the embryonic cell tumor markers (such as the CEA) are expressed almost in parallel with the loss of blood group antigens. The greater the loss of blood group antigens, the greater the production of embryonic tumor antigens. In malignancy, which in many ways is the process of a cell losing function and reverting back to its embryonic state, the loss of blood group antigens means uncontrolled migration, and that indicates metastasis.
Loss of blood group antigens doesn’t occur in all tumors. In fact, it is usually the reverse of what happens under normal conditions. Tissues and organs that in normal circumstances manufacture blood group antigens (like those of the colon) will tend to lose them when undergoing malignant change5. Other organs like the thyroid, which do not make blood group antigens when normal, will tend to gain them when turning cancerous. Sometimes, such as in the case of the thyroid and the colon, changes taking place in the blood group antigen expression in one organ will influence the expression of blood group antigens in another6.
T and Tn: Pan-Carcinogenic Antigens
Many malignant cells (such as those found in breast and stomach cancer) develop a tumor marker called the Thomsen-Friedenreich (T) antigen. This antigen is suppressed in normal healthy cells, much like a rock is covered over by water at high tide. The T antigen only becomes unsuppressed as a cell moves toward malignancy, much like the covered rock in our example becomes exposed as the tide lowers. It is so rare to find the T antigen in healthy tissue that we actually have antibodies against it. It is even more unusual to find a Tn antigen on a healthy cell. Tn is actually a precursor to T antigen, or a less well-developed T antigen.
It has been estimated that in about 90% of all cancers (and in some LEUKEMIAS) T and Tn antigens are expressed and uncovered. As a general rule, an orderly expression of T antigens on a cancer cell usually indicates a cancer with a relatively favorable outlook. However, a prevalence of Tn antigens on a cancer cell usually denotes a highly aggressive, metastatic cancer, irrespective of the organ involved or the form of cancer.
Cancer cells differ radically from healthy cells in the fine architecture of their cell surfaces. A healthy cell surface looks like a well-tended yard, with groomed bushes, shrubs, and flowers. A cancer cell looks like someone has taken a chain saw to the yard, and leveled the bushes, shrubs, and flowers down to their stumps. So, in a very simplistic way, a cancer cell clearly looks like a different yard than a healthy one (your immune system, in this case).
In this example, the T and Tn antigens are the stumps found in cancer, while the well-groomed yard has bushes, shrubs, and flowers that correspond to the ABO markers and other antigens found on healthy cells. The difference between the yards is largely because a cancer cell is unable to completely assemble a normal, healthy cell membrane structure like a blood group ABO antigen.
As a general rule, well-differentiated cancers usually have a preponderance of T antigens and less of the Tn antigens. However, as a cancer cell becomes poorly differentiated, Tn antigen expression predominates. One of the functions of these T and Tn antigens is to promote cancer cell adhesion—the ability of the cancer cell to stick to other cells, including healthy ones. This process of adhesion is a critically important part of cancer cell invasion and metastasis. When it comes to T and Tn antigens, there is good news. Everyone has preexisting anti-T and anti-Tn antibodies—a built-in immune system response against cells with these markers. These anti-T and anti-Tn antibodies are primarily induced by your intestinal flora. Blood group will often influence the amount and activity of these antibodies against T and Tn antigens.
The T and Tn antigens show some structural similarity to the A antigen (even though it is derived from the M blood type antigen)7. Not surprisingly, blood group A individuals have the least aggressive antibody immune response against the T and probably Tn antigens. In fact, the T and Tn antigens, and blood group A antigens are actually immunologically considered to be quite similar because of their shared terminal sugar (N-acetyl-galactosamine), and so might be readily confused by the immune system of blood group A individuals. This finding has led researchers to conclude that the Tn antigen is, in a broad sense, an A-like antigen. The hypothesis: because of the lower level of antibody against T and Tn antigens, and because of this tendency for the immune system of blood group A to be disinclined to attack Tn antigens, blood group A is at an immunological disadvantage in attacking any cell bearing the T and Tn antigenic markers8.
Blood group A cancer patients had the greatest and most uniform suppression of the level of Thomsen-Friedenreich antigen agglutinins, irrespective of age, cancer stage, or tumor morphology, and lower levels of anti-B isohemagglutinins. This is probably at least a part of the explanation for the poorer outcomes in many cancers among blood group A individuals.
Ideally, the immune system is naturally predisposed to fight against cells with incomplete or abnormal structures—just as it would against an invading virus. Blood groups A and AB start with a bit of an immune disadvantage, due to this structural similarity to the T antigen.
Coagulation and Cancer: Another Group A Weakness
The A-like cancer hypothesis is a strong one, well supported in the literature. However, several other aspects of the blood group A antigen, in addition to the T and Tn antibody limitation, appear to convey additional susceptibility to malignancy. Perhaps the second most potent pathway by which blood group A is rendered more prone to severe complications for malignancy is by virtue of their thicker blood, or tendency toward aggregation.
Von Willebrand factor and factor VIII: It has been noted that cancer cells can often hitch a ride on circulating platelets as they begin to spread, or metastasize. This is due to an aberrant platelet glycoprotein receptor expressed by human tumor cells that appears to participate in the adhesive interactions required for the metastatic process. Von Willebrand factor (vWF) and factor VIII are serum proteins that are a sort of molecular glue that platelets use to attach to blood clotting proteins along the lining of the blood vessels. It is also required for this aberrant platelet glycoprotein to bind to cancer cells. Plasma specimens from patients with disseminated metastases showed that their plasma levels of von Willebrand factor and factor VIII were elevated (vWF almost double) above those of normal individuals, apparently because they lack adequate amounts of an enzyme required to cleave vWF and factor VIII into their inactive forms. Because of this, cancer patients with widely disseminated metastasis have levels of platelet activity upward of 150% greater than that of normal individuals.
Fibrinogen: As with STRESS, THYROID DISEASE, HEART DISEASE, and DIABETES, studies have shown that cancer patients who are blood group A have higher levels of blood viscosity than cancer patients who are group O. This appears to be the result of higher levels of the clotting protein fibrinogen. Fibrinogen is an acute phase protein important to the inflammatory response and to wound healing. It is known to be elevated in cancer patients, where its presence is believed to shorten survival and contribute to weight loss. As with vWF and factor VIII, fibrinogen serves as part of the adherence cascade by which cancer cells can attach to platelets and the walls of the blood vessels as a prelude to metastatic spread.
This helps explain why older studies have shown that cancer patients who are on blood thinning medication have a lower incidence of METASTASIS 9. Blood group A is associated with higher levels of vWF and factor VIII over the other blood types, which probably accounts for their “thicker blood.” Similarly, group A has a tendency toward higher levels of fibrinogen, which might also help cancer cells to metastasize. In the case of malignancy, vWF and factor VIII probably help metastasizing cancer cells adhere to platelets, while also having the secondary effect of inducing metastasis as well.
Growth Factors and Group A Risk
One little-known or appreciated effect of blood group A antigen is its ability to attach to the receptor for “growth factors” that are found in much higher concentration on tumor cells than on normal ones. Growth factors are proteins that act on nearby cells in a way not dissimilar to hormones. As a matter of fact, the best known growth factor is insulin itself. Growth factors are very powerful regulatory agents, and their production is normally highly controlled. Growth factors have varied effects, acting not only as regulators of cell proliferation but also as inducers of secretion of chemical attractants and stimulants to cell differentiation. Over the course of our lives, our body changes in a variety of dynamic ways: Our bones elongate, men develop facial hair, women’s breasts grow larger. Growth factors are active in every situation where some sort of tissue remodeling must occur, such as embryonic development, response to injury, puberty, inflammation, and, unfortunately, cancer.
All the above processes require cells to proliferate (hyperplasia), enlarge (hypertrophy), and sometimes die (apoptosis), and a major role of the growth factors is to coordinate the activities of the different cell types in remodeling tissues in an organized fashion.
There is a close relation between growth factor action and oncogenes, genes that were discovered because of their association with the transformation of cells to the malignant state. The idea that growth factors may be related to cancer was given strong support by the finding that the actual products that are the physical result of many oncogenes are related either to growth factors or their receptors. Overproduction of these growth factors as a result of oncogene activity contributes to the loss of the body’s ability to regulate growth, which results in cancerous cell growth.
Epidermal growth factor (EGF): A growth factor normally synthesized to help tissue to repair itself, EGF also has important effects on the growth of prostate, colon, breast, and several other cancer types. These cancers are characterized by cells that have an excessively high concentration of EGF receptors (EGF-R) on their surfaces10. So it appears that EGF-R expression plays an important role in the pathogenesis of human cancer, including but not limited to oral, brain, pancreatic, breast, lung, and colorectal cancers11-14.
Basically, the larger number of EGF-R on the cancer cell means that the cell can bind an excessive number of these molecules. It may be that this excessive dose of growth factor is critical to tumor growth. In fact, it is now clear that the growth of breast cancer is regulated by growth factor receptors (EGFR and Her-2/neu), and that their upregulation is associated with impaired prognosis.
Because of its deregulation in many cancers (BLADDER, BREAST, CERVICAL, COLON, ESOPHAGEAL, HEAD AND NECK, LUNG, and PROSTATE), EGF-R has been selected as a prime target for chemoprevention.
The EGF-R bears an antigenic determinant that is closely related to the human blood group A carbohydrate structure. An increase in the number of high-affinity EGF binding sites was observed in donors with blood group A1-erythrocytes as compared to red cells taken from donors with blood groups O and B15.
It is now very well-documented that the blood group A antigen can bind to EGF-R as well. So it is not unlikely that free A antigen in blood group A and AB individuals (especially if they are secretors) can find their way onto these excess EGF-R and act to simulate cell growth. Like von Willebrand and factor VIII, excessive activation of the EGF-R results in cancer cells that become more mobile and able to develop new and additional blood supplies (angiogenesis)16.
Blood Group and Natural Killer Cells
Natural killer (NK) cells function to destroy cells infected with cancer or a virus. In virtually every type of cancer investigated, NK activity is low. As a matter of fact, if we were to make one blanket statement about cancer patients, irrespective of the form of cancer, or the patient’s blood type, race, gender, or age, it would be that by virtue of having cancer, we can predict that this person will have NK cell activity significantly lower than that found in normal, healthy individuals.
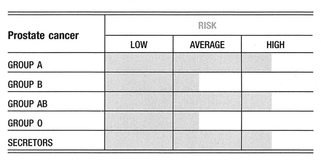
The prognostic significance of NK cell function in cancer, with regards to predicting relapses, poor responses to treatment, and survival time, has been receiving increasing emphasis in medical literature. Compromised NK cell function has been linked to the development and metastasis of cancer, as well as patient survival time. A sampling of cancers known to be associated with low NK cell activity include breast cancer, prostate cancer, RENAL CANCER, STOMACH CANCER, lung cancer, colon cancer, BRAIN CANCER, and leukemia. Let’s examine NK cell activity in two of the most common forms of cancer: breast and prostate cancer.
In breast cancer, a negative correlation is seen between NK cell activity levels and the maximum tumor diameter. NK cell activity is also predictive of advanced disease and the spread of disease in women with breast cancer. Destruction of cancer cells by NK cells is much lower in women with advanced disease (stages II, III, and IV) than in women with limited disease (stage I).
In prostate cancer, changes in NK activity have been reported to be reliably associated with both the likelihood of metastasis and tumor response to therapy, matching in its reliability the tumor markers reflective of prostate cancer (PSA and TPS). In both treated and untreated patients with prostate cancer, a lower NK cell activity level means a greater likelihood of cancer cells escaping containment and finding their way into the circulation (this is not a good thing, since your survival depends on keeping the cancer contained to its original site). The level of NK cell activity also provides a direct reflection of the body’s response to treatment: Normalization of NK cell activity is found in patients in remission following appropriate treatment. As a matter of fact, at least in prostate cancer, the laboratory work done to determine NK activity levels will often provide more useful information with respect to outcomes in advanced disease than the routinely used tumor marker assessments.
The genetics of blood type might influence NK cell activity. And while many factors are involved in the individual variation in NK cell function, again we find a role of blood group antigenic structures. Lowered NK cell activity has been shown to have a familial association in melanoma suggesting a possible genetic contribution. A significant relationship between Lewis antigen expression and resistance to NK cell destruction is found on target cells (meaning that the Lewis antigens being present on a cell make it a less likely target for NK cell destruction. Cell surface A and O antigens also increase resistance to lysis, while the pan-carcinogenic precursor structures (T and Tn antigens, discussed on page 168) increase the sensitivity of tumor cells to be destroyed by NK cells. So blood group antigen expression on target cells certainly influences the ability of NK cells to bind and destroy those cells. Although information is not unequivocal, higher NK activity has been associated with blood group AB when compared either to blood group A alone, or to blood group AB and O taken together as a group. In general, the lowest NK cell activity has been associated with blood group A. Rh blood type might also influence NK cell activity. Although some studies have not found an association, other researchers have observed a higher natural NK cytotoxicity against target cells in individuals with Rh-negative blood type17-26.
Therapies, Cancer
ALL BLOOD GROUPS:
Important therapeutic note:
Without question, the best way to protect yourself from cancer is to follow the Blood Type Diet and reduce your stress levels. Over the years, I’ve developed a number of supplementary strategies. However, I want to emphasize that none of the suggestions in this section are meant to replace the recommendations of your surgeons and oncologists. My experience is that the best approach to take with an adversary like cancer is to place as many independent and mutually supportive strategies at your disposal as possible. My patients are routinely using the best that conventional medicine has to offer. I advise you to do the same. The strategies we will discuss are accessory strategies, which attack cancer from an angle currently ignored or not emphasized within conventional medicine. Some of these angles are actually being explored, and I suspect they will eventually be incorporated into the mainstream. Until that time, view these strategies as additional fences placed around a bad neighbor’s yard.
If you are suffering from a cancerous condition, I do not encourage you to use these strategies by themselves. My experience is that the most successful outcomes are the result of using the best of conventional medicine, supplemented with the best of natural medicine.
BLOOD GROUP A:
1. Cancer Prevention Protocol
2. Chemotheraphy Adjunct Protocol
3. Surgery Recovery Protocol
4. Chronic Illness Recovery Protocol
BLOOD GROUP B:
1. Cancer Prevention Protocol
2. Chemotheraphy Adjunct Protocol
3. Surgery Recovery Protocol
4. Chronic Illness Recovery Protocol
BLOOD GROUP AB:
1. Cancer Prevention Protocol
2. Chemotheraphy Adjunct Protocol
3. Surgery Recovery Protocol
4. Chronic Illness Recovery Protocol
BLOOD GROUP O:
1. Cancer Prevention Protocol
2. Chemotheraphy Adjunct Protocol
3. Surgery Recovery Protocol
4. Chronic Illness Recovery Protocol
Related Topics
Autoimmune disease
Immunity
Also refer to individual cancers by name
REFERENCES
1. Sarafian V, Dimova P, Georgiev I, Taskov H. ABH blood group antigen significance as markers of endothelial differentiation of mesenchymal cells. Folia Med (Plovdiv). 1997;39:5—9.
2. Palli D, Caporaso NE, Shiao YH, et al. Diet, Helicobacter pylori, and p53 mutations in gastric cancer: a molecular epidemiology study in Italy. Cancer Epidemiol Biomarkers Prev. 1997;6:1065—1069.
3. Kawaguchi T. [Adhesion molecules and carbohydrates in cancer metastasis]. Rinsho Byori 1996;44:1138—1146.
4. Ichikawa D, Handa K, Hakomori S. Histo-blood group A/B antigen deletion/reduction vs. continuous expression in human tumor cells as correlated with their malignancy. Int J Cancer. 1998;76:284—289.
5. Sarafian V, Popov A, Taskov H. Expression of A, B, and H blood group antigens and carcinoembryonic antigen in human tumours. Zentralbl Pathol. 1993;139:351—354.
6. Vowden P, Lowe AD, Lennox ES, Bleehen NM. Thyroid blood group isoantigen expression: a parallel with ABH isoantigen expression in the distal colon. Br J Cancer. 1986; 53:721—725.
7. Hirohashi S. Tumor-associated carbohydrate antigens related to blood group carbohydrates. Gan To Kagaku Ryoho. 1986;13(pt 2):1395—1401.
8. Kurtenkov O, Klaamas K, Miljukhina L. The lower level of natural anti-Thomsen-Friedenreich antigen (TFA) agglutinins in sera of patients with gastric cancer related to ABO(H) blood group phenotype. Int J Cancer. 1995;60: 781-785.
9. Oleksowicz L, Bhagwati N, DeLeon-Fernandez M. Deficient activity of von Willebrand’s factor-cleaving protease in patients with disseminated malignancies. Cancer Res. 1999;59:2244—2250.
10. Ciardiello F, Tortora G. Interactions between the epidermal growth factor receptor and type I protein kinase A: biological significance and therapeutic implications. Clin Cancer Res. 1998;4:821-828.
11. Shapiro WR, Shapiro JR. Biology and treatment of malignant glioma [review]. Oncology (Huntingt). 1998;12:233—240.
12. Kurpad SN, Zhao XG, Wikstrand CJ, Batra SK, McLendon RE, Bigner DD. Tumor antigens in astrocytic gliomas. Glia 1995;15:244—256.
13. Neal DE, Mellon K. Epidermal growth factor receptor and bladder cancer: a review. Urol Int. 1992;48:365—371.
14. Defize LH, Arndt-Jovin DJ, Jovin TM, et al. A431 cell variants lacking the blood group A antigen display increased high affinity epidermal growth factor-receptor number, protein-tyrosine kinase activity, and receptor turnover. J Cell Biol. 1988;107:939—949.
15. Engelmann B, Schumacher U, Haen E. Epidermal growth factor binding sites on human erythrocytes in donors with different ABO blood groups. Am J Hematol. 1992;39:239—241.
16. Ueda M, Ueki M, Terai Y, et al. Biological implications of growth factors on the mechanism of invasion in gynecological tumor cells. Gynecol Obstet Invest. 1999;48:221—228.
17. Brenner BG, Margolese RG. The relationship of chemotherapeutic and endocrine intervention on natural killer cell activity in human breast cancer. Cancer. 1991;68:482—488.
18. Garner WL, Minton JP, James AG, Hoffmann C. Human breast cancer and impaired NK cell function. J Surg Oncol. 1983;24:64—66.
19. Parra S, Pinochet R, Vargas R, Sepulveda C, Miranda D, Puente J. Natural killer cytolytic activity in renal and prostatic cancer [in Spanish]. Rev Med Chil. 1994;122:630—637.
20. Aparicio-Pages NM, Verspaget HW, Pena SA, Lamers CB. Impaired local natural killer cell activity in human colorectal carcinomas. Cancer Immunol Immunother. 1989;28: 301—304.
21. Fulton A, Heppner G, Roi L, Howard L, Russo J, Brennan M. Relationship of natural killer cytotoxicity to clinical and biochemical parameters of primary human breast cancer. Breast Cancer Res Treat. 1984;4:109—116.
22. Lasek W, Jakobisiak M, Plodziszewska M, Gorecki D. The influence of ABO blood groups, Rh antigens and cigarette smoking on the level of NK activity in normal population. Arch Immunol Ther Exp (Warsz). 1989;37:287—294.
23. Hersey P, Edwards A, Trilivas C, Shaw H, Milton GW. Relationship of natural killer-cell activity to rhesus antigens in man. Br J Cancer. 1979;39:234—240.
24. Blottiere HM, Burg C, Zennadi R, et al. Involvement of histo-blood-group antigens in the susceptibility of colon carcinoma cells to natural killer-mediated cytotoxicity. Int J Cancer. 1992;52:609—618.
25. Lasek W, Jakobisiak M, Plodziszewska M, Gorecki D. The influence of ABO blood groups, Rh antigens and cigarette smoking on the level of NK activity in normal population. Arch Immunol Ther Exp (Warsz). 1989;37:287—294..
26. Pross HF, Baines MG. Studies of human natural killer cells. I. In vivo parameters affecting normal cytotoxic function. Int J Cancer. 1982;29:383—390.
CANCER, BLADDER-Tumors that occur most often in the elderly, thought to be associated with smoking or chemical exposure.
Symptoms
• Microscopic hematuria
• Blood in the urine
• Frequent urination
• Pain and burning with urination
• Pelvic pain
• A palpable mass
About Bladder Cancer
Bladder cancer is the sixth most common cancer in the United States. About 12,400 people will die every year of this disease. Bladder cancer is more common among men than among women. When found and treated early (as often happens), the chances for survival are very good. The 5-year survival rate for early bladder cancer is 94%. If the cancer has spread to nearby pelvic organs, the rate is 49%, and if distant organs are involved it drops to 6%.
There are three forms of bladder cancer:
• Transitional cell carcinoma (TCC) is by far the most common form of bladder cancer. It accounts for about 90% of these cancers. There are subgroups of TCC. Some of these are more likely to spread than others.
• Squamous cell carcinomas account for about 8% of bladder cancers. Under a microscope, these cancer cells look very much like skin cancer cells. Nearly all cancers of this type are likely to invade deeper layers of the bladder. Squamous cell carcinoma of the bladder has a poor prognosis because it is usually highly infiltrative and is found at a more advanced stage.
• Adenocarcinomas account for only about 1% to 2% of bladder cancers. They are also very likely to involve deeper layers of the bladder.
The causes of bladder cancers are not entirely understood. Risk factors include severe chronic CYSTITIS, ALCOHOLISM, tobacco smoking, and radiation exposure. Chemicals used in a variety of industries—including dye, rubber, leather, paint, and textile manufacturing—may be risk factors for bladder cancer.
With bladder cancer, often there are no symptoms in the early stages. Microscopic hematuria may be the earliest sign. Later, blood in the urine, frequent urination, and pain and burning with urination occur. Pelvic pain occurs with advanced disease. A mass may be palpable with manual examination.
Diagnosis of bladder cancer is made with cytoscopy, the placement of a lens into the bladder that allows a view. If abnormalities are seen, a biopsy is performed. In patients with superficial malignancies, death from bladder cancer is very rare. In patients with deeply invasive lesions of the bladder musculature, survival is poor (about 50% at 5 years), but adjuvant chemotherapy may improve these results.
Early superficial MALIGNANCIES (including superficial invasion of the bladder musculature) can be completely removed by transurethral resection and fulguration. Recurrence at the same or another site in the bladder is relatively common and may be reduced by repeated bladder instillations of chemotherapeutic drugs.
Blood Group Links
Bladder cancer cases for both sexes show a marked frequency of blood groups B and A, as well as an overall preference for non-secretors. One study of transitional cell cancers of the bladder and urethra showed a high rate of reaction when the cancer cells were treated with antibodies to group A blood1. This implies that these types of tumors may be A-like in appearance.
It has also been shown that bladder cancer occurs with a high frequency in people who suffer from recurrent BLADDER and KIDNEY INFECTIONS—conditions that afflict blood group B more often than other blood groups.
Rh-positive individuals have more invasive tumors of the upper urinary tract.
Transitional cell carcinomas of the urinary bladder show variable blood group antigen reactivity even in the noninvasive stage. A correlation exists between the tumor reactivity and subsequent clinical course: when the expected blood group antigen(s) is retained, the clinical course is favorable; absence of the expected antigen (s) denotes an aggressive potential.
Therapies, Bladder Cancer
ALL BLOOD GROUPS:
The most effective preventive measures include the following:
1. Don’t smoke. Smoking has been isolated as a major risk factor for bladder cancer.
2. Avoid chemical exposure. If you work in a chemical environment, wear protective clothing.
3. Moderate your levels of alcohol consumption.
4. Since bladder cancer is a disease of the elderly, maintain general health strategies throughout life, according to your blood type, that will strengthen immunity.
BLOOD GROUP A:
1. Cancer Prevention Protocol
2. Chemotherapy Adjunct Protocol
3. Surgery Recovery Protocol
4. Chronic Illness Recovery Protocol
BLOOD GROUP B:
1. Cancer Prevention Protocol
2. Chemotherapy Adjunct Protocol
3. Antiviral Protocol
4. Surgery Recovery Protocol
5. Chronic Illness Recovery Protocol
BLOOD GROUP AB:
1. Cancer Prevention Protocol
2. Chemotherapy Adjunct Protocol
3. Surgery Recovery Protocol
4. Chronic Illness Recovery Protocol
BLOOD GROUP O:
1. Cancer Prevention Protocol
2. Chemotherapy Adjunct Protocol
3. Surgery Recovery Protocol
4. Chronic Illness Recovery Protocol
Related Topics
Cancer (general)
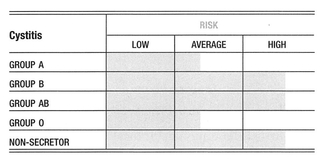
Cystitis
Immunity
REFERENCES
1. Limas C, Lange P. Altered reactivity for A, B, H antigens in transitional cell carcinomas of the urinary bladder. A study of the mechanisms involved. Cancer. 1980;46:1366—1373.
CANCER, BRAIN-See Cancer, glioma and other brain tumors
CANCER, BREAST-Cancerous conditions of the breasts, milk ducts, and lymphatic nodes.
Symptoms
A lump that is painless, hard, and has irregular edges is more likely to be cancer. But some rare cancers are tender, soft, and rounded. Other signs of breast cancer include the following:
• A swelling of part of the breast
• Skin irritation or dimpling
• Nipple pain or the nipple turning inward
• Redness or scaliness of the nipple or breast skin
• A discharge other than breast milk
About Breast Cancer
Breast cancer is the most common cancer among women. And while the mortality rates are falling slightly for some subpopulations of women, it is still a potentially lethal adversary. Standard treatment can vary, but procedures such as lumpectomy surgery (removal of the tumor and some surrounding tissue), mastectomy (removal of the whole breast), chemotherapy, radiation, and hormone-blocking therapy are the norm, with any combination of the above strategies potentially employed.
Five-year breast cancer survival rates vary significantly based on size of tumor and extent of metastasis:
80%—tumor was smaller than 2 cm with no metastases
65%—tumor was larger than 2 cm with no metastases
40%—tumor was greater than 5 cm with no metastases
10%—distant metastases
Blood Group Links
ABO blood group exerts a significant influence on susceptibility and outcomes.
In 1984 scientists discovered a gene for breast cancer susceptibility linked to ABO susceptibility, located on band q34 of chromosome 9. This discovery of a genetic connection confirms an increasing body of statistical evidence connecting blood group to breast cancer‘.
Some researchers have stated that blood groups were shown to possess a predictive value independent of other known prognostic factors for breast cancer.
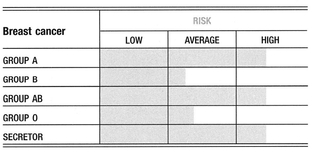
Substantial research shows that blood group A women are over-represented among breast cancer patients, and that this trend occurs even among women thought to be at low risk for cancer. One of the most significant risk factors for a rapidly progressing breast cancer is also blood group A. Blood group A women have been observed to have poorer outcomes once they are diagnosed with breast cancer. On the other hand, blood group O status implies a slight degree of resistance against breast cancer; even among breast cancer patients, group O showed a significantly lower risk of death. Blood group AB has a slight increase in susceptibility over A, and is associated with poorer outcomes and earlier deaths. Blood group B has a reduced risk, which is particularly evident among women who do not have a family history of breast cancer. However, if there is a family member with breast cancer, the protection normally associated with being a blood group B is lost. Group B women who currently have or have had breast cancer have a higher statistical likelihood of a recurrence.
The association between blood type A and breast cancer was evaluated in 648 patients with family histories of the disease, 1,897 general patients, 4,577 institutional blood donor controls, and 14,508 extramural blood donor controls. The familial patients were classified into three pedigree groups in which the lifetime breast cancer risks to first-degree relatives ranged from 11% to 32%. In the pedigree group associated with a relatively low risk to relatives, the authors saw a significant excess of blood type A individuals when compared with either control group. The general patient population, which was considered to refer to a general series of patients, also showed an excess of blood type A individuals2.
Breast cancer shows a weaker association with being a non-secretor3.
Therapies, Breast Cancer
ALL BLOOD GROUPS:
Adhere to breast cancer screening guidelines. The earlier breast cancer is found, the better the chances for successful treatment. The American Cancer Society recommends the following guidelines for finding breast cancer early:
1. Women 40 and over should have a mammogram every year.
2. Between the ages of 20 and 39, women should have a clinical breast exam every 3 years. After age 40, women should have a breast exam by a health professional every year.
3. All women over 20 should do breast self-examination (BSE) every month. The best time for this is 7 days after the beginning of the menstrual cycle.
BLOOD GROUP A:
1. Cancer Prevention Protocol
2. Surgery Recovery Protocol
3. Chronic Illness Recovery Protocol
BLOOD GROUP B:
1. Cancer Prevention Protocol
2. Surgery Recovery Protocol
3. Chronic Illness Recovery Protocol
BLOOD GROUP AB:
1. Cancer Prevention Protocol
2. Surgery Recovery Protocol
3. Chronic Illness Recovery Protocol
BLOOD GROUP O:
1. Cancer Prevention Protocol
2. Surgery Recovery Protocol
3. Chronic Illness Recovery Protocol
Related Topics
Cancer (general)
Immunity
REFERENCES
1. Skolnick MH, Thompson EA, Bishop DT, Cannon LA. Possible linkage of a breast cancer-susceptibility locus to the ABO locus: sensitivity of LOD scores to a single new recombinant observation. Genet Epidemiol. 1984;1:363—373.
2. Anderson DE, Haas C. Blood type A and familial breast cancer. Cancer. 1984;54:1845—1849.
3. Nagata C, Kabuto M, Kurisu Y, Shimizu H. Decreased serum estradiol concentration associated with high dietary intake of soy products in premenopausal Japanese women. Nutr Cancer 1997;29:228—233.
CANCER, CERVICAL-See Cancer, gynecologycal tumors
CANCER, COLON-Tumors of the colon; together with rectal tumors, they’re often referred to as colorectal cancers.
Symptoms
• Rectal bleeding
• Abdominal pain
• Change in bowel habits and/or the size, shape, or color of stool—especially gradually increasing constipation and black stool
• Acute obstruction: colicky (spasmodic) pain, increasing abdominal distention, failure to pass stools or gas
• Weight loss
• Anorexia
About Colon Cancer
Colon cancer will cause about 48,100 deaths and rectal cancer about 8,600 deaths each year. Nine out of 10 people whose colorectal cancer is found and treated at an early stage before it has spread will live at least 5 years. Once the cancer has spread to nearby organs or lymph nodes, the 5-year survival rate goes down to 65%. For people whose colorectal cancer has spread to distant parts of the body such as the liver or lungs, the 5-year survival rate is 8%.
The onset of symptoms is very gradual and depends on the tumor location, size, type, and complications. Often the first sign of colon cancer comes when the tumor irritates an adjacent organ, such as the urinary bladder or the stomach; or if it leads to bowel perforation and peritonitis. Symptoms differ according to the tumor’s location:
Right colon: There may be a palpable mass through the abdominal wall, along with fatigue and weakness from anemia due to a concealed hemorrhage. A change in bowel habits is usually a late sign.
Left colon: A change in bowel habits is more apparent—including alternating constipation with diarrhea, and overtly bloody stools.
Rectum: The primary symptom is defecation with bloody stools.
The prognosis varies with the extent of the disease, the location in bowel, the degree of wall infiltration, and whether there is local or distant METASTASIS. In patients with cancer limited to the mucosa, the 5-year survival rate is 70%; with spread to the lymph nodes, it drops to 30%.
From Polyp to Cancer
Polyps are usually benign growths that develop in the colon. They vary in size and appearance; some look like a wart when small, and a cherry on a stem or fig when they grow. They are important because with time they can become cancerous. Polyps of the colon and rectum are almost always benign and usually produce no symptoms. They may, however, cause painless rectal bleeding or bleeding not apparent to the naked eye. The incidence of polyps increases with age. The cumulative risk of cancer developing in an unremoved polyp is 2.5% at 5 years, 8% at 10 years, and 24% at 20 years after the initial discovery and diagnosis. Polyps larger than 1 cm have a greater cancer risk associated with them than smaller polyps. It has been shown that the removal of polyps by colonoscopy reduces the risk of getting colon cancer significantly.
Risk factors for colon cancer include a high meat diet and corresponding low intake of fresh fruits and vegetables.
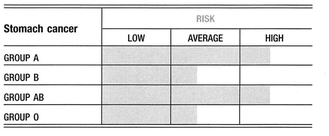
Blood Group Links
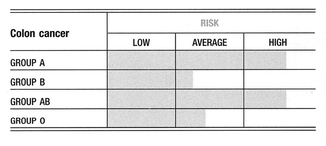
Being either blood group A or blood group AB should be considered an important risk factor for colorectal cancer1.
The amount of blood group antigen made in the colon is highest in the upper colon (the cecum), and diminishes gradually so as to virtually disappear by the very end of the colon (the rectosigmoid). In colon cancer, the exact opposite happens. The cells of the upper colon lose the ability to produce ABO antigens, while the cells of the lower colon start to manufacture them. In immunology the term “horror autotoxicus” is used to indicate that the immune system has an innate disinclination to attack the body; thus, an ability to mimic the markers on cells that are normally used by the immune system to determine whether a cell is healthy or malignant is a potent way to derail the immune response. For example, the expression of blood group antigens was found to correlate with the degree of effectiveness of the immune system’s natural killer (NK) cells. The higher the amount of blood group antigen on the tumor cells, the lower the aggressiveness of the tumor-killing NK cells of the immune system2,3,4.
Cancer cells often manufacture antigens that are typically made only in fetal development and repressed throughout adult life. The opposite of this is also true. That is, when a cell starts to lose genetic control over itself, it can also lose the ability to manufacture the antigens that are made when that same cell is healthy.
Since the production of surface antigens is intimately linked with the process of each cell attaching to its neighbor, it is likely that the addition or deletion of blood group antigens from the surface of colon cancer cells is somehow related to their ability to spread, or metastasize5. It has even been reported that colorectal cancer cells can manufacture blood type antigens of a different blood group than their host. The production of B antigen in normally type A or O individuals with colon cancer has been most often reported. In fact, in one case the detection of an unwarranted B antigen led to the diagnosis of a previously undetected tumor6.
Colon cancer cells of the rectosigmoid, the area that most often develops colon cancer, manufacture large amounts of group A antigen, even in people who are not group A. Also, the amount of group A antigen made seems to correlate with the potential for metastasis, or spread.
Blood Group and Tumor Markers
Physicians often monitor a tumor marker called CEA, or “carcinoembryonic antigen,” when gauging the progression of colon cancer. Like most tumor markers, CEA is a glycoprotein, the same class of molecule as blood group antigens. It appears that expression of blood group A antigen is intimately related to synthesis of CEA.
In 1987 researchers looked at the expression of blood group antigens in relation to the production of CEA in human colon carcinoma cells of different ABO blood groups. All tumor cells made A and B antigens, regardless of the patient’s original blood group. However, tumor cells from group O patients had lower expression of both A and B antigens and high production of CEA. Cells from patients with group A blood had low to undetectable CEA production and high expression of both A and B antigens7.
In 1995 an article published in the Journal of Cell Biochemistry reported that “altered expression of ABO blood group substances is a common feature of human colorectal carcinoma, yet it remains unclear how these structural changes influence the biological properties of tumor cells.”
Certain chemically induced rat colon tumors display many features of human cancer, thereby providing a potentially useful model to study the role of blood group substances in colon cancer progression. Using antibodies developed for chemically induced tumors of the colon, the researchers found that these same antibodies reacted to the epitope (binding site) that is also expressed in blood group A human colon carcinoma cell lines but not in cell lines whose donors were blood group B. The researchers offered that “blood group A-specific lectins may provide a useful tool for early detection of colon cancer”8.
It is interesting that the A antigens made by cancer cells in the lower colon are embryonic variations not seen typically in adults. In one study, four variants of the A antigen were found in human colon cancer cells, but of these only one was found in adults; the other three were variants of blood group A typically only found in fetal tissue9. This is significant, as one of the primary roles blood group antigens play in the developing fetus involves shaping the architecture of developing tissue and organs by serving as cell-to-cell attachment and detachment points. The question is, does the redevelopment of these “fetal” forms of blood group A permit tumor cells to develop the ability to detach from the primary site and spread through the body? Only more research will tell.
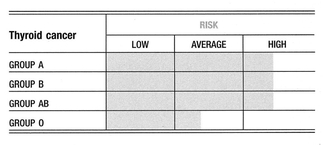
There is a strange but documented link between the expression of blood group antigens in tumors of the thyroid and lower colon10. Blood group A individuals with a history of thyroid tumors, either benign or malignant, may be at especially high risk of developing cancer of the lower colon.
Blood Group and the Tn Antigen
Malignant cells in colon cancer develop a tumor marker called the Thomsen-Friedenreich (T) antigen. This antigen is suppressed in normal healthy cells, and only becomes unsuppressed as a cell moves toward malignancy. It is so rare to find the T antigen in healthy tissue that we actually have antibodies against it. It is even more unusual to find a Tn antigen, the precursor to T or a less well-developed T antigen, on a healthy cell.
As a general rule, an orderly expression of T antigens on a cancer cell usually indicates a cancer with a relatively favorable outlook. However, a prevalence of Tn antigens on a cancer cell usually denotes a highly aggressive, metastatic cancer, irrespective of the organ involved or the form of cancer. In addition to colon cancer, Tn is also expressed in ULCERATIVE COLITIS11, which may help explain why chronic ulcerative colitis is a risk factor for the subsequent development of colon cancer.
The Thomsen-Friedenreich (T) antigen and Tn antigen show some structural similarity to the A antigen (even though it is derived from the M blood type antigen). Not surprisingly, blood group A individuals have the least aggressive antibody immune response against the T and probably Tn antigens. In fact, the T and Tn antigens and the A antigens are actually immunologically considered to be quite similar because of their shared terminal sugar (N-acetyl-galactosamine), and so might be readily confused by the immune system of blood group A individuals. This finding has led researchers to conclude that the Tn antigen is, in a broad sense, an A-like antigen. The hypothesis: because of the lower level of antibody against T and Tn antigens, and because of this tendency for the immune system of group A to be disinclined to attack Tn antigens, blood group A is at an immunological disadvantage in attacking any cell bearing the T and Tn antigenic markers.
Therapies, Colon Cancer
ALL BLOOD GROUPS:
Adhere to the colon cancer screening guidelines as part of your overall physical health screening program. The three types of screening are fecal occult blood testing (FOBT), flexible sigmoidoscopy, or the colonoscopy for detection.
The FOBT looks for blood in the stool that might be associated with cancer. In some instances, the presence of blood is caused by hemorrhoids. In other cases, cancer might not be accompanied by blood in the stool.
The flexible sigmoidoscopy, which examines the bottom third of the colon, could miss a cancer farther up. The New England Journal of Medicine has featured two large studies that reported that the flexible sigmoidoscopy missed too many precancerous growths in a sample of 5,000 patients.
Colonoscopy is the most thorough screening test, so it is also the best test for colon cancer. Colonoscopy is an examination of the entire colon (5 feet in length) with a slender, flexible, lighted tube, looking for abnormal cells that could develop into cancer.
BLOOD GROUP A:
1. Cancer Prevention Protocol
2. Chemotherapy Adjunct Protocol
3. Intestinal Health Protocol
4. Surgery Recovery Protocol
5. Chronic Illness Recovery Protocol
BLOOD GROUP B:
1. Cancer Prevention Protocol
2. Chemotherapy Adjunct Protocol
3. Intestinal Health Protocol
4. Surgery Recovery Protocol
5. Chronic Illness Recovery Protocol
BLOOD GROUP AB:
1. Cancer Prevention Protocol
2. Chemotherapy Adjunct Protocol
3. Intestinal Health Protocol
4. Surgery Recovery Protocol
5. Chronic Illness Recovery Protocol
BLOOD GROUP O:
1. Cancer Prevention Protocol
2. Chemotherapy Adjunct Protocol
3. Intestinal Health Protocol
4. Surgery Recovery Protocol
5. Chronic Illness Recovery Protocol
Related Topics
Cancer (general)
Colitis, ulcerative
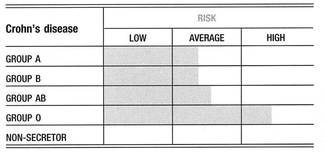
Crohn’s disease
Immunity
Lectins
Polyps, colon
REFERENCES
1. Slater G, Itzkowitz S, Azar S, Aufses AH Jr. Clinicopathologic correlations of ABO and Rhesus blood type in colorectal cancer. Dis Colon Rectum. 1993;36:5—7.
2. Salem RR, Wolf BC, Sears HF, et al. Expression of colorectal carcinoma-associated antigens in colonic polyps. J Surg Res. 1993;55:249—255.
3. Blottiere HM, Burg C, Zennadi R, et al. Involvement of histo-blood-group antigens in the susceptibility of colon carcinoma cells to natural killer-mediated cytotoxicity. Int J Cancer. 1992;52:609—618.
4. Schoentag R, Primus FJ, Kuhns W. ABH and Lewis blood group expression in colorectal carcinoma. Cancer Res. 1987;47:1695—1700.
5. Kawaguchi T. [Adhesion molecules and carbohydrates in cancer metastasis]. Rinsho Byori. 1996;44:1138—1146.
6. Northoff H, Wolpl A, Bewersdorf H, Faulhaber JD. An ABO-blood group abnormality leading to the detection of a colon-carcinoma. Blut. 1983;46:161—164.
7. Cooper HS, Marshall C, Ruggerio F, Steplewski Z. Hyperplastic polyps of the colon and rectum. An immunohistochemical study with monoclonal antibodies against blood groups antigens (sialosyl-Lea, Leb, Lex, Ley, A, B, H). Lab Invest. 1987;57:421—428.
8. Laferte S, Prokopishyn NL, Moyana T, Bird RP. Monoclonal antibody recognizing a determinant on type 2 chain blood group A and B oligosaccharides detects oncodevelopmental changes in azoxymethane-induced rat colon tumors and human colon cancer cell lines. Cancer J Cell Biochem. 1995;57:101—119.
9. Itzkowitz SH. Blood group-related carbohydrate antigen expression in malignant and premalignant colonic neoplasms. J Cell Bioclzem Suppl. 1992;16G:97—101.
10. Vowden P, Lowe AD, Lennox ES, Bleehen NM. Thyroid blood group isoantigen expression: a parallel with ABH isoantigen expression in the distal colon. Br J Cancer. 1986; 53:721—725.
11. Freed DL, Green FH. Do dietary lectins protect against colonic cancer? Lancet. 1975;2:1261—1262.
CANCER, COLORECTAL-See Cancer, colon
CANCER, ENDOMETRIAL-See Caneer, uterine
CANCER, ESOPHAGEAL-Cancer of the esophagus.
Symptoms
• Difficulty swallowing
• Chest pain
• Weight loss
• Vocal cord paralysis and hoarseness
About Esophageal Cancer
About 12,500 people will die of the disease each year. This cancer is about three times more common among men than among women and three times more common among individuals of African ancestry than among Caucasians. There are two main types of esophageal cancer. One type grows in the cells that form the top layer of the lining of the esophagus. These are called squamous cells, and cancer that starts there is known as squamous cell carcinoma. Squamous cell cancer can grow anywhere along the length of the esophagus. It accounts for about half of all esophageal cancer. The other type of cancer of the esophagus starts near the opening to the stomach. It begins in the cells of Barrett’s esophagus and is called adenocarcinoma. Adenocarcinoma cannot start unless squamous cells have been changed by acid reflux.
Esophageal cancer usually presents with progressive dysphagia (difficulty swallowing) over several weeks, as the lumen of the esophagus becomes constricted. The first stage involves difficulty swallowing solid food, and the sensation that food is sticking on the way down the esophagus. The second stage involves difficulty swallowing semisolid food. Finally, there is difficulty swallowing liquid food and saliva; this progression over time suggests the presence of a growing malignant process rather than a spasm, benign ring, or peptic stricture. Chest pain usually radiates to the back.
Weight loss, even when the patient maintains a good appetite, is almost universal. Compression of the recurrent laryngeal nerve may lead to vocal cord paralysis and hoarseness.
Scientists believe that some risk factors such as smoking and drinking cause damage to the cells that line the esophagus. Likewise, long-term irritation of the lining of the esophagus from reflux can promote cancer formation. There is also some evidence that certain viruses can increase the risk of esophageal cancer.
Blood Group Links
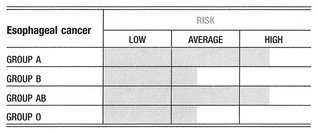
Groups A and AB seem to have a higher incidence of esophageal cancer over the other blood groups.
Up to 20% of those with chronic GERD will go on to develop the precancerous condition called BARRETT’S ESOPHAGUS—and up to 10% of those will have an increased risk of developing esophageal cancer. Furthermore, patients who develop esophageal cancer subsequent to Barrett’s esophagus typically have a poor prognosis because the cancer is often discovered at an advanced stage.
Therapies, Esophageal Cancer
BLOOD GROUP A:
1. Cancer Prevention Protocol
2. Chemotherapy Adjunct Protocol
3. Stomach Health Protocol
4. Surgery Recovery Protocol
5. Chronic Illness Recovery Protocol
BLOOD GROUP B:
1. Cancer Prevention Protocol
2. Chemotherapy Adjunct Protocol
3. Stomach Health Protocol
4. Surgery Recovery Protocol
5. Chronic Illness Recovery Protocol
BLOOD GROUP AB:
1. Cancer Prevention Protocol
2. Chemotherapy Adjunct Protocol
3. Stomach Health Protocol
4. Surgery Recovery Protocol
5. Chronic Illness Recovery Protocol
BLOOD GROUP O:
1. Cancer Prevention Protocol
2. Chemotherapy Adjunct Protocol
3. Stomach Health Protocol
4. Surgery Recovery Protocol
5. Chronic Illness Recovery Protocol
Related Topics
Barrett’s esophagus
Cancer (general)
Gastroesophageal reflux disease (GERD)
Immunity
CANCER, GALLBLADDER-Tumors of the gallbladder.
Symptoms
• Abdominal pain
• Nausea and/or vomiting
• Jaundice
• Gallbladder enlargement
About Gallbladder Cancer
Gallbladder cancer is a disease of the elderly. Between 6,000 and 7,000 new cases of gallbladder cancer are diagnosed each year in the United States, usually among people over 70 years old. They are nearly three times as likely to be women than men. Gallbladder cancer is more common among Caucasian women than among women of African ancestry. It is also more common among individuals of Mexican and Native American ancestry than among the general population as a whole.
Few gallbladder cancers are found early, before they have spread to other tissues and organs. Many early cancers are found incidentally when a person’s gallbladder is removed as treatment for gallstones. At later stages, symptoms may include the following:
Abdominal pain: More than half of all gallbladder cancer patients have abdominal pain at the time of diagnosis. Most often this is in the right upper part of the abdomen.
Nausea and/or vomiting: At the time of their diagnosis, more than half of all people with gallbladder cancer report vomiting as a symptom.
Jaundice: Almost half of all gallbladder cancer patients have jaundice when they are diagnosed.
Gallbladder enlargement: Sometimes bile duct blockage causes bile to accumulate in the gallbladder, causing it to become larger than usual. This enlargement can sometimes be felt by the doctor during a physical exam, and can also be detected by imaging studies such as ultrasound.
Blood Group Links
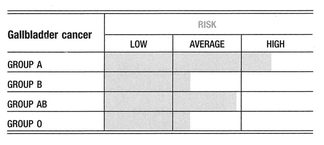
Group B seems to have a higher incidence of gallbladder cancer over the other blood groups. This is probably related to group B’s susceptibility to slow-growing viruses and bacterial diseases, which compromise the immune system.
Therapies, Gallbladder Cancer
BLOOD GROUP A:
1. Cancer Prevention Protocol
2. Chemotherapy Adjunct Protocol
3. Liver Support Protocol
4. Surgery Recovery Protocol
5. Chronic Illness Recovery Protocol
BLOOD GROUP B:
1. Cancer Prevention Protocol
2. Chemotherapy Adjunct Protocol
3. Liver Support Protocol
4. Surgery Recovery Protocol
5. Chronic Illness Recovery Protocol
BLOOD GROUP AB:
1. Cancer Prevention Protocol
2. Chemotherapy Adjunct Protocol
3. Liver Support Protocol
4. Surgery Recovery Protocol
5. Chronic Illness Recovery Protocol
BLOOD GROUP O:
1. Cancer Prevention Protocol
2. Chemotherapy Adjunct Protocol
3. Liver Support Protocol
4. Surgery Recovery Protocol
5. Chronic Illness Recovery Protocol
Related Topics
Cancer (general)
Gallstones
Liver disease (general)
Immunity
CANCER, GLIOMA AND OTHER BRAN TUMORS-Tumors of the brain, spinal cord, and nervous system.
Symptoms
• Numbness and/or weakness of both legs
• Abnormal positioning of the body
• Headache
• Nausea
• Vomiting
• Blurred vision
About Brain Gliomas
Glioma is a term referring to three types of brain tumors:
Astrocytoma: Most tumors that arise within the brain itself start in brain cells called astrocytes. These tumors are called astrocytomas. Most astrocytomas cannot be cured because they spread widely throughout the surrounding normal brain tissue. Sometimes astrocytomas spread along the cerebrospinal fluid pathways. However, with only very rare exceptions, astrocytomas do not spread outside of the brain or spinal cord.
Oligodendroglioma: These tumors start in brain cells called oligodendrocytes. They spread or infiltrate in a manner similar to astrocytomas and, in most cases, cannot be completely removed by surgery. However, a small number of oligodendrogliomas are associated with long-term survivals of 30 or 40 years. Oligodendrogliomas may spread along the cerebrospinal fluid pathways but rarely spread outside the brain or spinal cord.
Ependymoma: These tumors arise from the ependymal cells that line the ventricles. Ependymomas may block the exit of cerebrospinal fluid from the ventricles, causing the ventricle to become very large in a condition called hydrocephalus. Unlike astrocytomas and oligodendrogliomas, ependymomas characteristically do not spread or infiltrate into normal brain tissue. As a result, some but not all ependymomas can be completely removed and cured by surgery. Spinal cord ependymomas have the greatest chance of surgical cure. Ependymomas may spread along the cerebrospinal fluid pathways but do not spread outside the brain or spinal cord.
Whether a brain cancer is detected early usually depends on its location within the brain. Cancers located in more important areas of the brain may cause symptoms earlier than those located in less important areas of the brain. Brain and spinal cord tumors often interfere with the specific functions of the region they develop in. For example, spinal cord tumors often cause numbness and/or weakness of both legs, and tumors of the basal ganglia typically cause abnormal movements and abnormal positioning of the body. Tumors within any part of the brain may cause pressure to rise within the skull. Increased pressure within the skull may cause headache, nausea, vomiting, or blurred vision. Headache is a common symptom of brain tumor, occurring in about 50% of patients.
Blood Group Links
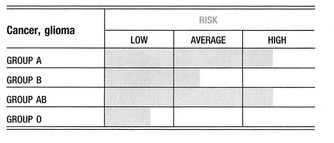
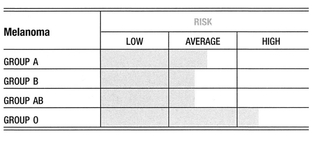
A positive, consistent, and often very strong association has been found between blood group A and brain and nervous system tumors. A weaker association for these forms of cancer exists for group B. Conversely, it has been a consistent finding that being group O is a favorable prognostic factor for brain and nervous system cancers.
Additionally, ABO blood group has found to be of prognostic value in gliomas. In one study of oligodendroglioma patients, those with blood group A had a distinctly poorer prognosis than patients with O or B blood. The survival data from this unselected series indicated that cerebral oligodendrogliomas have a less favorable prognosis than has generally been believed1.
In another study, the distribution of ABO blood groups was analyzed statistically in 271 patients treated for glioblastoma multiforme. The control group included 500 patients treated for craniocerebral trauma. A statistically significant difference was observed in the distribution of ABO blood groups between these patient groups, with higher frequency of group A and lower of group O in the patients with glioblastoma multiforme2.
The efficacy of postoperative chemotherapy and immunochemotherapy was analyzed according to the survival period from study of patients who underwent operation for malignant (III-IV degree malignancy) gliomas. In analyzing the efficacy of various schedules of combined treatment, the investigators took into consideration the patients’ ABO blood group because it is known that many antineoplastic antibiotics contain structures that are marked by cross-reaction with the ABO isoantigens. The results of the study showed that the use of polychemotherapy and levamisole in neuro-oncological patients with A(II) and AB(IV) blood group promising. Levamisole and the antineoplastic antibiotic reumycin proved to be authentically effective in patients with A(II) blood group and ineffective in those with O(I) blood group. The data obtained are recommended for use in individual selection of the schedule of chemotherapy and immunochemotherapy in patients of different ABO blood groups3.
The exception to the overall preference of group A and B is pituitary adenomas. Pituitary adenoma has been shown to be associated with an increased occurrence of group O blood over the other blood groups. In an analysis of 282 brain tumors of all types compared with 55,089 control individuals from the Boston population, the percentage of adenoma of the pituitary was significantly increased in group O (almost 62% of all adenomas) versus all other brain tumors, which showed an overall excess of group A4.
Therapies, Glioma, and Other Brain Tumors
BLOOD GROUP A:
1. Cancer Prevention Protocol
2. Chemotherapy Adjunct Protocol
3. Surgery Recovery Protocol
4. Chronic Illness Recovery Protocol
BLOOD GROUP B:
1. Cancer Prevention Protocol
2. Chemotherapy Adjunct Protocol
3. Surgery Recovery Protocol
4. Chronic Illness Recovery Protocol
BLOOD GROUP AB:
1. Cancer Prevention Protocol
2. Chemotherapy Adjunct Protocol
3. Surgery Recovery Protocol
4. Chronic Illness Recovery Protocol
BLOOD GROUP O:
1. Cancer Prevention Protocol
2. Chemotherapy Adjunct Protocol
3. Surgery Recovery Protocol
4. Chronic Illness Recovery Protocol
Related Topics
Cancer (general)
Immunity
REFERENCES
1. Turowski K, Czochra M. [ABO blood groups in glioblastoma multiforme]. Neurol Neurochir Pol. 1979;13:173-176.
2. Mork SJ, Lindegaard KF, Halvorsen TB, et al. Oligodendroglioma: incidence and biological behavior in a defined population. J Neurosurg. 1985;63:881-889.
3. Romodanov SA, Gnedkova IA, Lisianyi NI, Glavatskii AI. [Efficacy of chemotherapy and immunochemotherapy in neuro-oncologic patients of various blood groups (ABO system)]. Zh Vopr Neirokhir ImNNBurdenko. 1989;(1):17-20.
4. Mayr E, Diamond L, Levine RP, Mayr M. Suspected correlation between blood group frequency and pituitary adenoma. Science. 1956;(9):932-934.
CANCER, GYNECOLOGICAL TUMORS-Neoplasm of the female reproductive tract.
Various cancers can occur in the female reproductive tract: Endometrial cancer, ovarian cancer, cervical cancer, vulvar cancer, vaginal cancer, fallopian tube cancer, and gestational trophoblastic disease.
Blood Group Links
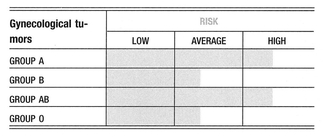
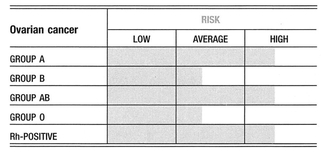
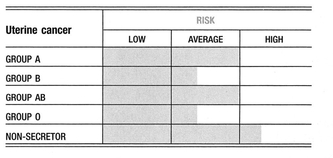
A retrospective analysis of 968 women affected by gynecological tumors was conducted to assess any difference in survival among patients with different blood groups. Data were presented on 237 cases of endometrial cancer, 92 cases of ovarian cancer, and 639 cases of invasive cervix cancer, detailing the women’s ABO blood group antigenic phenotypes, the stage of the cancer, and the treatment they received. With regard to endometrial cancer, a better 5-year and 10-year survival is associated with blood group O when compared with blood group A. This finding is more evident when 5-year survival is considered among patients affected by ovarian cancer. With regard to cervical cancer, analysis showed that a little better than 5-year survival is associated with the O blood phenotype. The study confirmed the evidence of an association between the A blood group and gynecological tumors. Endometrial and ovarian cancer occur more frequently in women with blood group A, than in women with other blood groups. Moreover, for the same tumors, blood group A is associated with a poor prognosis1,2.
Related Topics
Cancer (general)
Immunity
REFERENCES
1. Marinaccio M, Traversa A, Carioggia E, et al. [Blood groups of the ABO system and survival rate in gynecologic tumors]. Minerva Ginecol. 1995;47:69-76.
2. Milunicova A, Jandova A, Skoda V. [The secretion of ABH group substances by women with gynecologic carcinomas]. Z Immunitatsforsch Allerg Klin Immunol. 1969;139:90-93.
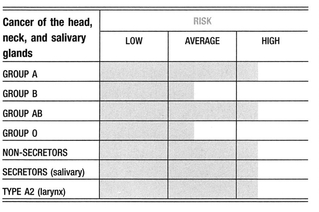
CANCER, HEAD, NECK, AND SALIVARY GLANDS-Primarily oral tumors.
Blood Group Links
NOTE: There have been statistical studies related to these cancers and blood groups, but little is known about their full relationships or the implications for treatment. Many of these studies remain contradictory or are subject to further investigation.
Cancer of the lip is significantly associated with blood group A. Cancers of the tongue, gums, and cheeks also have a blood group A association. Cancers of the salivary glands are strongly associated with blood group A, and weakly with blood group B—although studies are few and have yielded contradictory results‘.
Blood group O seems to have substantial protection against this type of cancer. Cancer of the salivary glands also appear to have an association with being a secretor.
As a general rule, a higher intensity of oral disease is found among ABO non-secretors. So it is not surprising that when it comes to precancerous, or cancerous changes to tissue of the mouth and esophagus, non-secretors seem to fare worse than secretors. This oral disease susceptibility is reflected in the occurrence of epithelial dysplasia, for example, which is found almost exclusively in the non-secretor group2.
Cancers of the larynx and hypopharynx are associated with blood groups A, B, and AB. The A2 blood type (a less common variant of group A) is significantly more frequent in patients with glottis cancer.
Structural changes to squamous cell cancers of the head and neck are quite common. ABO blood groups are expressed in normal tissue of this region. However, once squamous cell cancer develops, the A antigen disappears in about one-third of group A and AB individuals, and the O antigen is expressed in carcinoma cells from all the blood groups. These cancers generally have a poor prognosis. The T and Tn antigens also become commonly expressed in these cancers.
Ductular epithelium and the epithelial components of benign Warthin’s tumors have ABO antigens, whereas the loss of antigen in the epithelial portion of the malignant Warthin’s tumor is characteristic of epithelial cancerous dedifferentiation. Loss of antigen in adenoid cystic and undifferentiated carcinomas of the parotid supports the concept that antigen is absent in epithelially derived malignant tumors3.
Therapies, Cancer of the Head, Neck, and Salivary Glands
BLOOD GROUP A:
1. Cancer Prevention Protocol
2. Chemotherapy Adjunct Protocol
3. Surgical Recovery Protocol
4. Chronic Illness Recovery Protocol
BLOOD GROUP B:
1. Cancer Prevention Protocol
2. Chemotherapy Adjunct Protocol
3. Surgery Recovery Protocol
4. Fatigue-Fighting Protocol
BLOOD GROUP AB:
1. Cancer Prevention Protocol
2. Immune-Enhancing Protocol
3. Surgery Recovery Protocol
4. Fatigue-Fighting Protocol
BLOOD GROUP O:
1. Cancer Prevention Protocol
2. Chemotherapy Adjunct Protocol
3. Surgery Recovery Protocol
4. Chronic Illness Recovery Protocol
Related Topics
REFERENCES
1. Garrett JV, Nicholson A, Whittaker JS, Ridway JC. Bloodgroups and secretor status in patients with salivary-gland tumours. Lancet. 1971;2:1177-1179.
2. Vidas I, Delajlija M, Temmer-Vuksan B, Stipetic-Mravak M, Cindric N, Marieie D. Examining the secretor status in the saliva of patients with oral pre-cancerous lesions. J Oral Rehabil. 1999;26:177-182.
3. Woltering EA, Tuttle SE, James AG, Sharma HM. ABO (H) cell surface antigens in benign and malignant parotid neoplasms. J Surg Oncol. 1983;24:177-179. / /
CANCER, LEUKEMIA-Malignant neoplasms of blood-forming tissues.
Symptoms
• Weight loss
• Fever
• Loss of appetite
• Anemia, a shortage of red blood cells, causes
• Shortness of breath
• Excessive tiredness
• A “pale” color to the skin
About Leukemia
Leukemia is cancer of the white blood cells. This cancer starts in the bone marrow but can then spread to the blood, lymph nodes, the spleen, liver, central nervous system, and other organs. Leukemia is a complex disease with four different types and subtypes. There are four types of leukemia:
Acute means rapidly growing. Although the cells grow rapidly, they are not able to mature properly.
Chronic refers to a condition where the cells look mature but they are not completely normal. The cells live too long and cause a build-up of certain kinds of white blood cells.
Lymphocytic are leukemias that develop from lymphocytes in the bone marrow.
Myelogenous leukemia develops from either of two types of white blood cells: granulocytes or monocytes.
Most signs and symptoms of acute leukemia result from a shortage of normal blood cells due to crowding out of normal blood cell—producing bone marrow by the leukemia cells. As a result, people do not have enough properly functioning red blood cells, white blood cells, and blood platelets. As a result, individuals will experience the following symptoms:
• Anemia, a shortage of red blood cells, causes shortness of breath, excessive tiredness, and a pale color to the skin.
• Leukemia often causes enlargement of the liver and spleen, two organs located on the right and left side respectively, of the abdomen. Enlargement of these organs would be noticed as a fullness, or even swelling, of the belly.
• Leukemia may spread to the lymph nodes, causing swelling; or to the skin, causing rashes; or to the gums, causing swelling, pain, and bleeding.
Blood Group Links
Loss of A, B, and O antigens from the surface of red blood cells has been a recurrent observation in patients with hematologic malignancy (blood cancers), particularly those malignancies in which the myeloid lineage is involved. In fact, a study found that 55% of patients of blood group A, B, or AB had a proportion of red cells with decreased expression of A or B antigens compared with no changes in 127 healthy A, B, and AB individuals. In most cases, the changes were not detected by routine serologic (blood) typing. The loss of A or B antigens was the primary change in 28% of patients. In 17% of patients, loss of A or B antigens was an indirect consequence of loss of the precursor H (O) antigen. Alterations involving both the H (O) and the A or B antigens were seen in 10% of patients. Loss of H was also detected in 21% of group O patients whereas none of 51 healthy O individuals showed changes. Alterations of ABO antigens can now be considered a common event in myeloid malignancy‘.
Acute leukemia is more common in males at almost every age, and this fact remains unexplained. A study was carried out in northeast peninsular Malaysia to examine whether there was a difference in ABO blood group distribution between males and females with acute leukemia. The ABO blood groups of 109 male and 79 female patients with acute leukemia were compared with those of 1,019 normal individuals (controls). In the control population, 39.7% were group O. Among males with acute leukemia, 39.4% were group O, whereas among females with acute leukemia, the proportion was 24.1%. The same trend to a lower proportion of group O among females was seen if the group was divided into adult/ pediatric or lymphoblastic/myeloblastic groups, though these differences were not statistically significant. If these findings can be confirmed by other studies, they suggest the presence of a “sex-responsive” gene near to the ABO gene locus on chromosome 9, which relatively protects group O women against acute leukemia. The existence of such a gene might also partly explain why acute leukemia, and possibly other childhood cancers, are more common in males2.
Among leukemia patients, a significant increase in the frequency of the A2 phenotype was found in chronic lymphocytic leukemia3.
Therapies, Leukemia
BLOOD GROUP A:
1. Cancer Prevention Protocol
2. Chemotherapy Adjunct Protocol
3. Surgery Recovery Protocol
4. Chronic Illness Recovery Protocol
BLOOD GROUP B:
1. Cancer Prevention Protocol
2. Chemotherapy Adjunct Protocol
3. Surgery Recovery Protocol
4. Chronic Illness Recovery Protocol
BLOOD GROUP AB:
1. Cancer Prevention Protocol
2. Chemotherapy Adjunct Protocol
3. Surgery Recovery Protocol
4. Chronic Illness Recovery Protocol
BLOOD GROUP O:
1. Cancer Prevention Protocol
2. Chemotherapy Adjunct Protocol
3. Surgery Recovery Protocol
4. Chronic Illness Recovery Protocol
Related Topics
Cancer (general)
Immunity
REFERENCES
1. Marsden KA, Pearse AM, Collins GG, Ford DS, Heard S, Kimber RI. Acute leukemia with t(1;3)(p36;q21), evolution to t(1;3)(p36;q21), t(14;17)(q32;q21), and loss of red cell A and Le(b) antigens. Cancer Genet Cytogenet. 1992;64:80-85.
2. Jackson N, Menon BS, Zarina W, Zawawi N, Naing NN. Why is acute leukemia more common in males? A possible sex-determined risk linked to the ABO blood group genes. Ann Hematol. 1999;78:233-236.
3. Janardhana V, Propert DN, Green RE. ABO blood groups in hematologic malignancies. Cancer Genet Cytogenet. 1991; 51:113-120.
4. Bianco T, Farmer BJ, Sage RE, Dobrovic A. Loss of red cell A, B, and H antigens is frequent in myeloid malignancies. Blood. 2001;97:3633-3659.
CANCER, LIVER-Tumors of the liver.
Symptoms
Liver cancer is often not found early because there are seldom any signs or symptoms. In later stages, symptoms include:
• Weight loss
• Lack of appetite
• Pain in the stomach area
• Sudden jaundice
About Liver Cancer
Many cases of liver cancer can be prevented. Worldwide, preventing infection with HEPATITIS B AND C can reduce the risk of liver cancer. There is now a vaccine to prevent hepatitis B, and children especially should be vaccinated. Using protection during sex and not sharing needles lowers the chances of getting hepatitis. Not abusing alcohol will help lower the number of cases of CIR RHOSIS of the liver, a risk factor for liver cancer. Changing the way certain grains are stored in other countries could reduce contact with aflatoxins. Also, laws to protect workers from harmful chemicals can lower the risk. These measures have already been taken in many countries.
Also beware of:
• Anabolic steroids: These are male hormones. They are sometimes given for medical reasons, but some athletes also take them to increase their strength.
• Arsenic: In some parts of the world, drinking water contains traces of arsenic. This can increase the risk of liver cancer.
• Tobacco: Some studies suggest a link between tobacco use and liver cancer. Tobacco is carcinogenic, and smoking is clearly linked to many other cancers.
Blood Group Links
Liver cancer may show an increased incidence of group A. Liver hemangiomas are known to be more common in group A over the other blood groups.
Therapies, Liver Cancer
ALL BLOOD GROUPS:
Most liver cancer can be prevented by adhering to the Blood Type Diet, moderating alcohol intake, and reducing your exposure to toxic substances.
BLOOD GROUP A:
1. Cancer Prevention Protocol
2. Chemotherapy Adjunct Protocol
3. Liver Support Protocol
4. Surgery Recovery Protocol
5. Chronic Illness Recovery Protocol
BLOOD GROUP B:
1. Cancer Prevention Protocol
2. Chemotherapy Adjunct Protocol
3. Liver Support Protocol
4. Surgery Recovery Protocol
5. Chronic Illness Recovery Protocol
BLOOD GROUP AB:
1. Cancer Prevention Protocol
2. Chemotherapy Adjunct Protocol
3. Liver Support Protocol
4. Surgery Recovery Protocol
5. Chronic Illness Recovery Protocol
BLOOD GROUP O:
1. Cancer Prevention Protocol
2. Chemotherapy Adjunct Protocol
3. Liver Support Protocol
4. Surgery Recovery Protocol
5. Chronic Illness Recovery Protocol
Related Topics
Cancer (general)
Immunity
Liver disease
CANCER, LUNG-Tumors of the lung.
Symptoms
• A cough that does not go away
• Chest pain, often made worse by deep breathing
• Hoarseness
• Weight loss and loss of appetite
• Bloody or rust-colored sputum (spit or phlegm)
• Shortness of breath
• Fever without a known reason
• Recurring infections such as bronchitis and pneumonia
• New onset of wheezing
About Lung Cancer
Lung cancer is the leading cause of cancer death for both men and women though it is slightly more common in men. More people die of lung cancer than of colon, breast, and prostate cancers combined. Lung cancer is fairly rare in people under the age of 40. The average age of people found to have lung cancer is 60.
The lung is a site for both primary tumors and, very often, METASTASES from other organs (breast, colon, kidney, thyroid, testes, bone, and prostate). Most lung cancers are clearly associated with cigarette smoking, which explains the continual rise in lung cancers in women paralleling their increase in smoking. Primary lung malignancy is the most common cause of death from cancer.
Since most people with early lung cancer do not have any symptoms, only about 15% of lung cancers are found in the early stages. Although most lung cancers do not cause symptoms until they have spread, you should report any of the preceding symptoms to your doctor right away. Often these problems are caused by some other condition, but if lung cancer is found, prompt treatment could extend your life and relieve symptoms.
Blood Group Links
Lung cancer is closely associated with cigarette smoking although additional environmental or individual factors might regulate a person’s susceptibility to that disease. As a third genetic host factor, the ABO blood group frequencies were evaluated in 263 lung cancer patients. The frequency ratio of group A to group O was significantly higher as compared to 41,423 blood donors. The ratio of A to tended to be especially high in young patients not older than 50 years. In bronchial cancer, the ratio of B to O was significantly higher than expected. The conclusion was that ABO blood groups seem to influence lung cancer susceptibility1.
Therapies, Lung Cancer
NOTE: Although there appear to be secondary and tertiary links between lung cancer and blood groups, it would be misleading to suggest that blood group is a significant factor in the development of lung cancer. The primary cause of lung cancer is smoking, followed by the regular inhalation of toxic substances.
BLOOD GROUP A:
1. Cancer Prevention Protocol
2. Chemotherapy Adjunct Protocol
3. Surgery Recovery Protocol
4. Chronic Illness Recovery Protocol
BLOOD GROUP B:
1. Cancer Prevention Protocol
2. Chemotherapy Adjunct Protocol
3. Surgery Recovery Protocol
4. Chronic Illness Recovery Protocol
BLOOD GROUP AB:
1. Cancer Prevention Protocol
2. Chemotherapy Adjunct Protocol
3. Surgery Recovery Protocol
4. Chronic Illness Recovery Protocol
BLOOD GROUP O:
1. Cancer Prevention Protocol
2. Chemotherapy Adjunct Protocol
3. Surgery Recovery Protocol
4. Chronic Illness Recovery Protocol
Related Topics
REFERENCES
1. Roots I, Drakoulis N, Ploch M, et al. Debrisoquine hydroxylation phenotype, acetylation phenotype, and ABO blood groups as genetic host factors of lung cancer risk. Klin Wochenschr. 1988;66(suppl):87-97.
CANCER, LYMPHOMA-Tumors of the lymphoid tissue.
Symptoms
About Lymphomas
There are two main types of lymphomas. Hodgkin’s lymphoma or Hodgkin’s disease is named after Dr. Thomas Hodgkin, who first described it as a new disease in 1832. All other types of lymphoma are called non-Hodgkin’s lymphomas. These two types of lymphoma can usually be distinguished from each other by examining the cancerous tissue under a microscope. In some cases, additional tests to identify specific chemical components of the lymphoma cells or tests of the cells’ DNA may be needed.
Hodgkin’s lymphoma: Hodgkin’s disease is a type of cancer that starts in lymphatic tissue. Lymphatic tissue includes the lymph nodes and other organs that are part of the body’s lymph system, which forms blood and protects against germs. Because lymphatic tissue is found in many parts of the body, Hodgkin’s disease can start almost anywhere. This disease causes the lymphatic tissue to become enlarged and press on nearby structures. The cancer can spread through the lymphatic vessels. If it gets into the blood vessels it can then spread to almost any other place in the body.
There are no screening tests to find Hodgkin’s disease early, and some people with the disease have no symptoms at all. When they do occur, symptoms can include enlarged, painless lymph nodes. But in most people, especially children, enlarged lymph nodes are caused by an infection or other illness, not cancer. If you (or your child) have lymph nodes over an inch in size and no recent infection, it is best to have them checked by the doctor.
Other symptoms of Hodgkin’s disease can be caused by the swelling of lymph nodes inside the chest, creating pressure on the windpipe. These symptoms can include coughing or shortness of breath. Some people have fever, drenching night sweats, or weight loss. The fever can come and go over periods of several days or weeks.
Non-Hodgkin’s lymphoma: Non-Hodgkin’s lymphoma is cancer that starts in lymphoid tissue (also called lymphatic tissue). The lymphatic system is important for filtering germs and cancer cells as well as fluid from the extremities and internal organs. Other types of cancer—lung or colon cancers, for example—can develop in other organs and then spread to lymphoid tissue. But these cancers that can spread to lymph nodes are not lymphomas. Lymphomas start in the lymphoid tissue and can spread to other organs.
Non-Hodgkin’s lymphoma is the fifth most common cancer in this country, excluding non-MELANOMA skin cancers. Since the early 1970s, the incidence rates for non-Hodgkin’s lymphoma have nearly doubled; the increase was a result of both better methods of detection and an actual increase in the number of new cases. During the 1990s, the rate of increase appeared to slow and may be beginning to decline.
Although some types of non-Hodgkin’s lymphoma are among the most common childhood cancers, over 95% of non-Hodgkin’s lymphoma cases occur in adults. The average age at diagnosis is in the early 40s. The risk of developing non-Hodgkin’s lymphoma increases throughout life, and the elderly have the highest risk. The increasing average age of the American population is expected to contribute to the increase in non-Hodgkin’s lymphoma cases during the next few years. Non-Hodgkin’s lymphoma is more common in men than in women. Caucasians are affected more often than people of African or Asian ancestry.
Non-Hodgkin’s lymphoma that involves easily seen or palpated lymph nodes close to the surface of the body (lymph nodes on the sides of the neck, in the groin or underarm areas, above the collar bone, etc.) are usually noticed by the patient, a family member, or a health care professional.
When the lymphoid tissue inside the abdomen is involved, the abdomen can be swollen, sometimes so much it may resemble pregnancy in a woman. This may be due to large collections of fluid or tumor. Sometimes the cancer damages the lining layer of the abdominal cavity and causes large amounts of fluid to build up. When lymphoma causes swelling of lymphoid tissue near the intestines, passage of feces through the compressed area may be blocked. The pressure or blockage can also cause discomfort or abdominal pain.
When lymphoma starts in the thymus, irritation or compression of the nearby trachea can cause coughing, shortness of breath, or even suffocation. The superior vena cava (SVC) is the large vein that carries blood from the head and arms back to the heart. It passes near the thymus and lymph nodes inside the chest. Growth of lymphoma may compress this vein. This causes swelling of the head and arms known as SVC syndrome. This can also affect the brain and can be life threatening. Patients with SVC syndrome need to be treated as soon as possible.
In addition to symptoms and signs resulting from local effects of cancer growth, non-Hodgkin’s lymphoma can produce generalized symptoms such as unexplained weight loss, fever, profuse sweating (enough to soak clothing), particularly at night, or severe itchiness. Oncologists sometimes call these generalized effects “B symptoms.” The presence of B symptoms is associated with a poor prognosis and is related to an increased tumor burden (more cancer cells) in some patients.
Blood Group Links
There are only highly preliminary data linking blood groups to lymphoma. EPSTEIN-BARR VIRUS may play a role in the development of Hodgkin’s disease, placing blood group B at a somewhat elevated risk. Group O is associated with the best clinical outcome. More investigation is needed before conclusions can be reached.
Therapies, Lymphoma
BLOOD GROUP A:
1. Cancer Prevention Protocol
2. Chemotherapy Adjunct Protocol
3. Surgery Recovery Protocol
4. Chronic Illness Recovery Protocol
BLOOD GROUP B:
1. Cancer Prevention Protocol
2. Chemotherapy Adjunct Protocol
3. Antiviral Protocol
4. Surgery Recovery Protocol
5. Chronic Illness Recovery Protocol
BLOOD GROUP AB:
1. Cancer Prevention Protocol
2. Chemotherapy Adjunct Protocol
3. Surgery Recovery Protocol
4. Chronic Illness Recovery Protocol
BLOOD GROUP O:
1. Cancer Prevention Protocol
2. Chemotherapy Adjunct Protocol
3. Surgery Recovery Protocol
4. Chronic Illness Recovery Protocol
Related Topics
Cancer (general)
Epstein-Barr virus
Immunity
CANCER, MELANOMA-Tumors of the skin.
Symptoms
• A change in any pigmented lesion, including hypo or hyperpigmentation, bleeding, scaling, size change, and any texture change.
About Melanoma
The median age for skin cancers is 53 years old, but it has the highest annual incidence rate of any cancer among Caucasians between the ages of 25 to 29. More than 50% of all individuals with melanoma are between the ages of 20 to 40. The only genetic predisposition is in the familial dysplastic nevus syndrome. If an affected person’s family history includes one relative with melanoma, the risk of developing melanoma is 100%. Skin pigmentation is the only other risk factor transmitted genetically up. Also there is twice the risk for people who had adolescent blistering sunburns; or a fair complexion, freckling, blue eyes and blond hair; and those who have had any previous pigmented lesions (especially for dysplastic nevi) and intermittent ultraviolet light exposure.
An important symptom is any change in any pigmented lesion, including hypopigmentation or hyperpigmentation, bleeding, scaling, size, and texture. The overwhelming majority of melanoma arises in the skin, but it may also present as a primary lesion in any tissue pigmentation. Metastatic spread may be to any region in the body. Lentigo maligna is a cutaneous lesion that is the slowest growing malignant melanoma and has the least tendency to metastasize. It occurs most often on the face, beginning as a circumscribed macular patch of model pigmentation showing shades of dark brown, tan, or black. There is also an ocular melanoma that effects the eye; it is a progressive lesion of malignant origin.
Blood Group Links
When the records of 168 patients with malignant melanoma were reviewed with regard to the incidence of the major blood groups, blood group A was encountered in 39.9%, group B in 7.7%, group AB in 3%, and group O in 49.4%. Although blood group O had a higher frequency compared to that of the general Caucasian population of various series, the difference was not significant. Patients with blood group A had a median survival of 67.7 months, whereas patients with blood group O had a median survival of 46.6 months. The improved survival for blood group A remained significant only in female patients, when the sexes were considered separately. However, group A had an incidence of 24% in early Clark’s lesions while that incidence in group O was 9.8%. Female patients had longer median survival (86 months) than male patients (44 months)1.
Fifteen polymorphic systems of the blood (ABO, MNSs, Rhesus, P, Kell, Duffy, Kidd, Hp, Gc, Gm, Inv, aP, PGM1, EsD, and 6-PGD) were examined in 191 unrelated male and female patients suffering from malignant melanoma. These polymorphic systems were compared with the corresponding phenotype and gene frequencies of controls from the same geographical area (Rhineland-Palatinate). The only associations discovered were the ABO and Gm polymorphisms: The incidence of O and Gm(-1) phenotypes in patients was obviously higher than in controls. These observations agree with the findings in other population samples from Germany and Bulgaria 2.
Therapies, Melanoma
ALL BLOOD GROUPS:
PREVENTION
Avoid solar radiation from exposure to the sun. The best protection against the sun is proper clothing. Wear clothes made of tightly woven fabrics. Clothing made of loosely woven fabric or wet clothes can expose your skin to 30% more ultraviolet radiation. Wear long sleeves and pants. Wear a hat with a broad brim to protect your neck and ears (a baseball cap only protects your forehead). Apply sunscreen to sun-exposed skin.
Don’t go out without sunscreen. Sunscreen lotion is generally divided into two categories: physical sunscreens and chemical sunscreens. Physical sunscreens reflect ultraviolet radiation away from the skin while chemical sunscreens absorb ultraviolet radiation. Examples of physical sunscreens include zinc oxide and titanium dioxide. Although physical sunscreens are generally better at blocking the different kinds of ultraviolet radiation, they tend to be more “messy”—some can melt with heat and/or discolor clothing. Chemical sunscreens tend to become invisible when they are rubbed on the skin; because they are invisible it’s easy to miss an area of skin, which limits their effectiveness. Many products also come in a water-resistant formulation. Many chemical sunscreens also can cause eye irritation, and there is a higher chance of allergic reactions to chemical sunscreens, as opposed to physical sunscreens.
Choose a sunscreen with an SPF (sun protection factor) of at least 15. That means it provides 15 times more protection against ultraviolet radiation than no sunscreen at all. For persons with a fair complexion, a sunscreen with an SPF of 30 to 40 may be preferable.
TREATMENT
The standard method of early treatment for melanomas is excisional biopsy—the surgical removal of a mole that is thought to be melanoma. Your doctor will cut out the suspected melanoma, being careful to remove the depth of the mole as well as the portion above the skin. The excised tissue will be sent to a lab where a pathologist will look at it under a microscope to determine the nature of the tissue. If the body tissue removed in the biopsy is determined to be melanoma, your doctor will excise the remaining skin around the original site that was cut out to ensure clear margins. This means that the skin around the melanoma is noncancerous.
BLOOD GROUP A:
1. Cancer Prevention Protocol
2. Chemotherapy Adjunct Protocol
3. Skin Health Protocol
4. Surgery Recovery Protocol
5. Chronic Illness Recovery Protocol
BLOOD GROUP B:
1. Cancer Prevention Protocol
2. Chemotherapy Adjunct Protocol
3. Skin Health Protocol
4. Surgery Recovery Protocol
5. Chronic Illness Recovery Protocol
BLOOD GROUP AB:
1. Cancer Prevention Protocol
2. Chemotherapy Adjunct Protocol
3. Skin Health protocol
4. Surgery Recovery Protocol
5. Chronic Illness Recovery Protocol
BLOOD GROUP O:
1. Cancer Prevention Protocol
2. Chemotherapy Adjunct Protocol
3. Skin Health Protocol
4. Surgery Recovery Protocol
5. Chronic Illness Recovery Protocol
Related Topics
Cancer (general)
Immunity
REFERENCES
1. Karakousis CP, Evlogimenos E, Suh O. Blood groups and malignant melanoma. J Surg Oncol. 1986;33:24-26.
2. Walter H, Brachtel R, Hilling M. On the incidence of blood group O and Gm(-1) phenotypes in patients with malignant melanoma. Hum Genet. 1979;49:71-81.
CANCER, OVARIAN-Tumors of the ovary.
Symptoms
• Swelling of the abdomen
• Unusual vaginal bleeding
• Pelvic pressure
• Back pain
• Leg pain
• Digestive problems such as gas, bloating, indigestion, or long-term stomach pain
About Ovarian Cancer
Ovarian cancer is the sixth most common cancer in women. It ranks fifth as the cause of cancer death in women. Ovarian cancer causes more deaths than any other reproductive organ cancer. Most women who have ovarian cancer are postmenopausal.
The chances of survival from ovarian cancer are better if the cancer is found early. If the cancer is found and treated before it has spread outside the ovary, 95% of women will survive at least 5 years. However, only 25% of ovarian cancers are found at this early stage.
There is a familial risk factor for ovarian cancer. A woman who has a first-degree relative, i.e., a mother or a sister with a history of ovarian cancer, her risk for disease increases from 1.4% to 5.0%. With two or more such relatives, the lifetime risk becomes 7%. In families with hereditary ovarian cancer syndrome, the risk rises to 40% to 50%.
Small ovarian tumors are difficult for even the most skilled examiner to feel. Early cancers of the ovaries tend to cause symptoms that are relatively nonspecific. These symptoms include swelling of the abdomen (due to a mass or accumulation of fluid), unusual vaginal bleeding, pelvic pressure, back pain, leg pain, and digestive problems such as gas, bloating, indigestion, or long-term stomach pain. Most of these symptoms can also be caused by other less serious conditions. By the time ovarian cancer is considered as a possible cause of these symptoms, it may have already spread beyond the ovaries. Also, some types of ovarian cancer can rapidly spread to the surface of nearby organs. Nonetheless, prompt attention to symptoms can improve the odds of early diagnosis and successful treatment. If you have symptoms of ovarian cancer, report them to your health care provider right away.
Blood Group Links
The distribution of ABO blood group and Rh factor was studied in 175 women with ovarian tumors and cysts, compared with healthy controls. An increased probability of the majority of the ovarian tumor types among AB blood group females was noted, compared with other groups (O, A, and B). The probability of ovarian tumors becoming malignant proved to be least in B group females. A considerably increased relative ovarian tumor and cyst morbidity was noted among Rh-positive females compared with Rh-negative ones‘.
In the period from 1955 to 1979, 337 patients with carcinoma of the ovary were reported to the Icelandic Cancer Registry. ABO blood groups for 192 of the patients were available and showed a significant excess of blood group A; significantly fewer patients of blood group B were found than expected2.
Therapies, Ovarian Cancer
ALL BLOOD GROUPS:
Screening: Women with a high risk of developing epithelial ovarian cancer, such as those with a very strong family history of this disease, may be screened with transvaginal sonography (an ultrasound test performed with a small instrument placed in the vagina) and blood tests. Transvaginal sonography is helpful in finding a mass in the ovary, but it does not accurately predict which masses are cancers and which are benign diseases of the ovary. Blood tests for ovarian cancer may include measuring the amount of CA-125 (also known as OC-125). The amount of this protein is increased in the blood of many women with ovarian cancer. However, some noncancerous diseases of the ovaries can also increase the blood levels of CA-125 and some ovarian cancers may not produce enough CA-125 to cause a positive test. When these tests are positive, it may be necessary to do more x-ray studies or to take samples of fluid from the abdomen or tissue from the ovaries to find out whether a cancer is really present.
BLOOD GROUP A:
1. Cancer Prevention Protocol
2. Chemotherapy Adjunct Protocol
3. Surgery Recovery Protocol
4. Chronic Illness Recovery Protocol
BLOOD GROUP B:
1. Cancer Prevention Protocol
2. Chemotherapy Adjunct Protocol
3. Surgery Recovery Protocol
4. Chronic Illness Recovery Protocol
BLOOD GROUP AB:
1. Cancer Prevention Protocol
2. Chemotherapy Adjunct Protocol
3. Surgery Recovery Protocol
4. Chronic Illness Recovery Protocol
BLOOD GROUP O:
1. Cancer Prevention Protocol
2. Chemotherapy Adjunct Protocol
3. Surgery Recovery Protocol
4. Chronic Illness Recovery Protocol
Related Topics
Cancer (general)
Cancer, gynecological tumors
Immunity
REFERENCES
1. Rybalka AN, Andreeva PV, Tikhonenko LF, Koval‘chuk NA. [ABO system blood groups and the rhesus factor in tumors and tumorlike processes of the ovaries]. Vopr Onkol. 1979;25:28-30.
2. Bjarnason O, Tulinius H. Tumours in Iceland. 9. Malignant tumours of the ovary. A histological classification, epidemiological considerations and survival. Acta Pathol Microbiol Immunol Scand [A]. 1987;95:185-192.
CANCER, PANCREATIC-Tumors of the pancreas.
Symptoms
• Jaundice
• Pain in the abdominal (stomach) area or the middle to upper back area
• Weight loss over a number of months, accompanied by loss of appetite and severe tiredness
• Digestive problems, with pale, bulky, and greasy stools
• Swollen gallbladder
• Blood clots
• Diabetes
About Pancreatic Cancer
The incidence of pancreatic cancer has tripled over the last 40 years. There is no known cause, although chronic pancreatitis, smoking, certain chemicals (metal, and gas), coffee, alcohol, and DIABETES MELLITUS (in women) are risk factors. It is a rare cancer, accounting for only 2% to 3% of all cancers; however, it accounts for 10% of all fatal abdominal malignancies, and is now the fourth most common fatal cancer in the United States. It is three to four times more common in men, and is seen between the years of 35 and 70, with the peak at about 60.
Pancreatic cancer is a quickly metastasizing malignancy that is usually surgically untreatable by the time of diagnosis. The prognosis is extremely unfavorable; the 5-year survival rate is only 9%, and it is rare for a patient to live beyond 5 years.
By the time a person has symptoms of pancreatic cancer, the tumor has often reached a large size and the cancer may have spread to other organs.
Blood Group Links
Blood group B and blood group A have the highest incidence of pancreatic cancer. Group O is associated with the lowest risk (29). Since pancreatic juice is liberally laced with blood type antigens, the expression of group A and group B antigens must have some effect in inhibiting the immune response.
Few investigations have discussed an association between ABO blood groups and pancreatic cancer. In one study, the ABO blood groups distribution of 224 patients with histologicallyconfirmed pancreatic cancer was compared with that of two control groups: 7,086 patients with various diseases and 7,320 voluntary blood donors. An increased number of pancreatic cancers were found among the patients with blood group B and a decreased number in patients with blood group O, when compared with the two control groups.
Therapies, Pancreatic Cancer
ALL BLOOD GROUPS:
Especially if there is a family history of pancreatic cancer:
1. Avoid exposure to metals.
2. Limit or avoid consumption of alcohol and coffee.
3. Don’t smoke.
BLOOD GROUP A:
1. Cancer Prevention Protocol
2. Chemotherapy Adjunct Protocol
3. Liver Support Protocol
4. Surgery Recovery Protocol
5. Chronic Illness Recovery Protocol
BLOOD GROUP B:
1. Cancer Prevention Protocol
2. Chemotherapy Adjunct Protocol
3. Liver Support Protocol
4. Surgery Recovery Protocol
5. Chronic Illness Recovery Protocol
BLOOD GROUP AB:
1. Cancer Prevention Protocol
2. Chemotherapy Adjunct Protocol
3. Liver Support Protocol
4. Surgery Recovery Protocol
5. Chronic Illness Recovery Protocol
BLOOD GROUP O:
1. Cancer Prevention Protocol
2. Chemotherapy Adjunct Protocol
3. Liver Support Protocol
4. Surgery Recovery Protocol
5. Chronic Illness Recovery Protocol
Related Topics
Cancer (general)
Immunity
Liver disease
CANCER, PROSTATE-The development of abnormal cells in the prostate that multiply at an uncontrollable rate.
Symptoms
The most common signs of a prostate problem, which may be early signs of cancer, include:
• Painful, frequent, urgent, or hesitant urination
• Trouble fully emptying the bladder
• Frequent need to urinate during the night
• Sluggish urine flow
• Sharp pain in the pelvic region or rectum
• Blood in the urine
About Prostate Cancer
Prostate cancer is the most common cancer, excluding skin cancers, in American men. One man in six will be diagnosed with prostate cancer during his lifetime, but only one man in 30 will die of this disease. Men of African ancestry are more likely to have prostate cancer than are Caucasians or men of Asian ancestry.
Prostate cancer usually takes its time, advancing very slowly through four stages.
Stage A: The cancer is microscopic, there are no symptoms, and it cannot be detected by a digital rectal exam. A prostate-specific antigen (PSA) test might show elevated antigen levels, as will a biopsy.
Stage B: There are probably no notable symptoms, although a doctor can normally detect a lump or an unnaturally hard area on the prostate’s outer lobes. This information is obtained during a rectal examination. At this point, the cancer hasn’t advanced beyond the prostate. Stage B cancers are usually divided into two subgroups. Stage B-1 means that the cancer is confined to one lobe of the prostate. In Stage B-2, the cancer has spread to both lobes.
Stage C: By this stage, there’s a good chance that the cancer has advanced beyond the prostate, and has initially invaded surrounding tissues. There might be some urinary problems. Stage C prostate cancer can be detected by a digital rectal or other standard exam. Stage C-1 is the less severe of the two C stages, because the cancer’s involvement with other tissues is still minimal. In Stage C-2, the cancer has caused some blockage of the urethra, and has made its presence known by impeding the flow of the urine.
Stage D: The cancer is no longer confined to the prostate, and has spread widely throughout the body, potentially involving the lymph nodes, lungs, and bones. This is the most serious stage of all cancers. It has four subgroupings. In Stage D-0, other than elevated prostate enzyme levels, there are no other visible manifestations that the cancer has progressed. In Stage D-1, there is clear evidence that the cancer has spread to the lymph nodes in the immediate area surrounding the prostate. In Stage D-2, the cancer has metastasized to other sites in the body, notably the other lymph nodes, the bones, or other organs.
General Risk Factors and Causes
Prostate disease, especially cancer, is sometimes confusing, since even the known risk factors are so poorly understood. For example, it is known that African-Americans have a higher incidence of prostate cancer than Caucasians, but this may be environmental, not genetic, since African nationals have a low incidence. (Men of Hispanic and Japanese ancestry also have a low incidence of prostate cancer.) Cadmium miners have a higher rate of prostate cancer than the general population, but the reason is not known. It’s also still a mystery why men who have had vasectomies seem to experience more prostate cancer than the general population.
Blood Group Links
Men of blood groups A and AB have a higher risk of developing prostate cancer. Secretors tend to have a greater incidence of prostate cancer than non-secretors.
Therapies, Prostate Cancer
ALL BLOOD GROUPS:
The prostate-specific antigen (PSA) test is a simple and inexpensive blood test. The protein in the seminal fluid is produced in the prostate, but circulated throughout the body. When PSA levels are elevated in the bloodstream, there’s the possibility of a problem. The reading shows how many nanograms (1 nanogram equals 1 billionth of a gram) of PSA are present per milliliter of blood. As a rule, up to four nanograms is considered acceptable, although doctors also watch for rapid changes in the PSA over short periods. For example, if your PSA reading is 2.5 one year, then rises to 3.5 the next, it might raise a red flag even though it is within the normal range.
BLOOD GROUP A:
1. Cancer Prevention Protocol
2. Chemotherapy Adjunct Protocol
3. Surgery Recovery Protocol
4. Chronic Illness Recovery Protocol
BLOOD GROUP B:
1. Cancer Prevention Protocol
2. Chemotherapy Adjunct Protocol
3. Surgery Recovery Protocol
4. Chronic Illness Recovery Protocol
BLOOD GROUP AB:
1. Cancer Prevention Protocol
2. Chemotherapy Adjunct Protocol
3. Surgery Recovery Protocol
4. Chronic Illness Recovery Protocol
BLOOD GROUP O:
1. Cancer Prevention Protocol
2. Chemotherapy Adjunct Protocol
3. Surgery Recovery Protocol
4. Chronic Illness Recovery Protocol
Related Topics
Cancer (general)
Prostatic hyperplasia, benign
CANCER, RECTAL-See Cancer, colon
CANCER, STOMACH-Tumors of the stomach.
Symptoms
• Weight loss
• Iron-deficiency anemia
About Stomach Cancer
Cancer of the stomach is responsible for approximately 13,000 deaths and 24,000 new cases per year in the United States. Worldwide, over half a million deaths result from stomach cancer annually. Although the stomach cancer rate in the United States and the number of deaths from this disease fell dramatically from the 1940s to the 1990s, it is still a potent killer. The disease is found most often in people over age 55. It affects men twice as often as women, and is more common in individuals of African ancestry than in Caucasians. Also, stomach cancer is more common in some parts of the world—such as Japan, Korea, parts of Eastern Europe, and Latin America—than in the United States. The diets in these parts of the world contain many foods that are preserved by drying, smoking, salting, or pickling. Scientists believe that eating foods preserved in these ways may play a role in the development of stomach cancer. Fresh foods, especially fresh fruits and vegetables, may protect against this disease.
Stomach cancer may initially manifest with mild symptoms. All the common signs associated with stomach problems may arise but are usually not as severe as those caused by ulcers. The most common first sign is of a metastasis while the primary malignancy remains silent. Weight loss and iron-deficiency anemia may be present.
Blood Group Links
Stomach cancer was one of the earliest diseases ever shown to be associated with blood groups, an association first discovered in the early 1950s1-3. Originally, it was thought that the tendency for blood group A toward low stomach acid may have been the provocative factor, but other causes may be more important. The genetic ability for cells of the stomach to turn cancerous has been shown to be linked to the ABO gene locus itself, on chromosome 9q34. In this case, the A allele itself is contributing to the genetic mutations in the stomach, another instance of the link between a blood group and disease not being the result of a physical expression of blood type, per se, but rather a genetic linkage.
The Thomsen-Friedenreich Antigen
Many malignant cells, such as those found in stomach cancer, develop a tumor marker called the Thomsen-Friedenreich (T) antigen. This antigen is suppressed in normal healthy cells, but becomes unsuppressed as a cell moves toward malignancy. It is so rare to find the Tn antigen in healthy tissue that most people actually have an antibody to it.
The T antigen is exuberantly expressed in cells of the stomach that turn cancerous. About one-third of all Japanese express some T antigen in apparently normal stomach tissue, and this may also help explain why stomach cancer rates in Japan are among the highest in the world4.
The Thomsen-Friedenreich antigen shows some structural similarity to the A antigen. Perhaps this explains why, of all the blood groups, blood group A stomach cancer patients carry the least amount of the anti-T antibody. Since gastric juice is typically loaded with blood group antigens anyway, it is not unlikely that groups A and AB would be at a disadvantage at recognizing the T antigens as cancer markers, or mounting a defense if they did5,6.
Therapies, Stomach Cancer
BLOOD GROUP A:
1. Cancer Prevention Protocol
2. Chemotherapy Adjunct Protocol
3. Surgery Recovery Protocol
4. Chronic Illness Recovery Protocol
BLOOD GROUP B:
1. Cancer Prevention Protocol
2. Chemotherapy Adjunct Protocol
3. Surgery Recovery Protocol
4. Chronic Illness Recovery Protocol
BLOOD GROUP AB:
1. Cancer Prevention Protocol
2. Chemotherapy Adjunct Protocol
3. Surgery Recovery Protocol
4. Chronic Illness Recovery Protocol
BLOOD GROUP O:
1. Cancer Prevention Protocol
2. Chemotherapy Adjunct Protocol
3. Surgery Recovery Protocol
4. Chronic Illness Recovery Protocol
Related Topics
Barrett’s esophagus
Cancer (general)
Immunity
REFERENCES
1. Aird I, Bental HH, Mehigan JA, Roberts JA Fraser. Brit Med J. 1954;(2):315.
2. Walther WW, Raeburn C, Case J. Lancet. 1956;(2):970.
3. Wallace J. Brit Med J. 1954;(2):254.
4. Hirohashi S. Tumor-associated carbohydrate antigens related to blood group carbohydrates. Gan To Kagaku Ryoho. 1986;13(2):1395-1401.
5. Kurtenkov O, Klaamas K, Miljukhina L. The lower level of natural anti-Thomsen-Friedenreich antigen (TFA) agglutinins in sera of patients with gastric cancer related to ABO(H) blood group phenotype. Int J Cancer. 1995;60:781-785.
6. Clausen H, Stroud M, Parker J, Springer G, Hakomon S. Monoclonal antibodies directed to the blood group A associated structure, galactosyl-A: specificity and relation to the Thomsen-Friedenreich antigen. Mol Imrnunol. 1988;25:199-204.
CANCER, THYROID-Tumors of the thyroid.
Symptoms
• A lump or swelling in the front of the neck or in other parts of the neck.
About Thyroid Cancer
Cancer of the thyroid is a disease in which malignancies are found in the tissues of the thyroid gland. It is more common in women than in men. Most patients are between 25 and 65 years old. People who have been exposed to large amounts of radiation, or who have had radiation treatment for medical problems in the head and neck have a higher chance of getting thyroid cancer, although the cancer may not appear until 20 years or longer after radiation treatment.
There are four main types of cancer of the thyroid: papillary, follicular, medullary, and anaplastic.
• Papillary: The most commonly seen thyroid cancer, it is found two to three times more often in women than in men. Although seen more frequently in the young, it is a more serious malignancy in the elderly. It is often seen in patients who have had radiation exposure or HASHIMOTO’S THYROIDITIS.
•
Follicular: Responsible for approximately 25% of thyroid tumors, it is seen more often in the elderly patient. It also is more common in females and has a history of radiation associated with its onset. As follicular cancer spreads through the blood, it more easily causes distant metastases and is therefore more malignant.
• Medullary: Most likely to have a strong genetic factor, it is related to a gene abnormality.
• Anaplastic: It is faster growing and more inclined to spread rapidly to other organs.
Blood Group Links
Blood group A has a propensity for thyroid cancer, while blood group O appears to be protected. Similar to many other cancers, the fine structure of various antigens is altered between healthy and cancerous cells. As a general rule, loss of A and B antigens, and the appearance of greater numbers of Tn antigens are characteristic of thyroid cancers, and are associated with a tendency for malignancy 1-3.
Therapies, Thyroid Cancer
ALL BLOOD GROUPS:
Four types of conventional treatment are used:
• Surgery (taking out the cancer and/or thyroid gland)
• Radiation therapy (using high-dose x-rays or other high-energy rays to kill cancer cells)
• Hormone therapy (using hormones to stop cancer cells from growing)
• Chemotherapy (using drugs to kill cancer cells)
In addition, the following dietary guidelines are recommended. Consume foods rich in iodine, silicon, and phosphorus: kelp, dulse, Swiss chard, turnip greens, egg yolks, wheat germ, cod roe, lecithin, sesame seed butter, seeds and nuts, and raw goat’s milk.
BLOOD GROUP A:
1. Cancer Prevention Protocol
2. Chemotherapy Adjunct Protocol
3. Surgery Recovery Protocol
4. Chronic Illness Recovery Protocol
BLOOD GROUP B:
1. Cancer Prevention Protocol
2. Chemotherapy Adjunct Protocol
3. Surgery Recovery Protocol
4. Chronic Illness Recovery Protocol
BLOOD GROUP AB:
1. Cancer Prevention Protocol
2. Chemotherapy Adjunct Protocol
3. Surgery Recovery Protocol
4. Chronic Illness Recovery Protocol
BLOOD GROUP O:
1. Cancer Prevention Protocol
2. Chemotherapy Adjunct Protocol
3. Surgery Recovery Protocol
4. Chronic Illness Recovery Protocol
Related Topics
Cancer (general)
Immunity
Thyroid disease, Graves’
Thyroid disease, Hashimoto’s thyroiditis
Thyroid disease, hyperthyroidism
Thyroid disease, hypothyroidism
REFERENCES
1. Vowden P, Lowe AD, Lennox ES, Bleehen NM. Thyroid blood group isoantigen expression: a parallel with ABH isoantigen expression in the distal colon. Br J Cancer. 1986; 53:721-725.
2. Klechova L, Gosheva-Antonova T. [ABO and Rh blood group factors in thyroid gland diseases]. Vutr Boles. 1980; 19:75-79.
3. Toyoda H, Kawaguchi Y, Shirasawa K, Muramatsu A. Familial medullary carcinoma of the thyroid through 3 generations. Acta Pathol Jpn. 1977;27:111-121.
CANCER, UTERINE-Tumors of the uterus.
Symptoms
• Postmenopausal bleeding
About Uterine Cancer
Uterine cancer is the most common gynecological cancer in women and the third most common female cancer overall (after breast and colorectal). It is seen primarily in postmenopausal women between 50 to 60 years old. Predisposing factors include OBESITY, DIABETES, HIGH BLOOD PRESSURE, INFERTILITY, a history of IRREGULAR PERIODS, late onset of MENOPAUSE (over 52 years old), and use of estrogen therapy (unopposed estrogens) in a woman who still has her uterus. Overall, the general 5-year survival rate for endometrial (uterine lining) cancer is somewhat favorable.
Almost 63% of women will be alive after 5 years of treatment with no sign of the cancer; 28% will die within 5 years; and 9% will be alive but have the disease. However, in the United States, the 5-year survival rate for stage I tumors reaches 90%.
Postmenopausal bleeding is the most frequent sign of endometrial cancer. See a doctor if any spotting occurs.
Blood Group Links
Normal endometrial tissue does not contain blood group antigens. However, over one-half of endometrial cancers have detectable blood group antigens. An increased rate of expression of Lewis group antigens, particularly Lewis (b) antigen, is also observed in endometrial cancers compared with its expression in normal endometria.
Therapies, Uterine Cancer
BLOOD GROUP A:
1. Cancer Prevention Protocol
2. Chemotherapy Adjunct Protocol
3. Surgery Recovery Protocol
4. Chronic Illness Recovery Protocol
BLOOD GROUP B:
1. Cancer Prevention Protocol
2. Chemotherapy Adjunct Protocol
3. Surgery Recovery Protocol
4. Chronic Illness Recovery Protocol
BLOOD GROUP AB:
1. Cancer Prevention Protocol
2. Chemotherapy Adjunct Protocol
3. Surgery Recovery Protocol
4. Chronic Illness Recovery Protocol
BLOOD GROUP O:
1. Cancer Prevention Protocol
2. Chemotherapy Adjunct Protocol
3. Surgery Recovery Protocol
4. Chronic Illness Recovery Protocol
Related Topics
Cancer (general)
Cancer, gynecological tumors
CANCER, VAGINAL-See Cancer, gynecological tumors
CANCER, VULVAR-See Cancer, gynecological tumors
CANDIDA ALBICANS-See Fungal disease, candidiasis
CANDIDIASIS-see Fungal disease, candidiasis
CARBOHYDRATE INTOLERANCE-See Insulin resistance (Syndrome X)
CARDIOVASCULAR DISEASE-Disorders of the heart and blood vessel system, including coronary artery disease (CAD), heart attack, hypertension, and stroke.
Symptoms
Characteristically there are few symptoms of cardiovascular disease, and the condition remains silent until there is a cardiovascular event, such as stenosis (narrowing), thrombosis (clotting), or aneurysm (widening) of an artery or chamber. There are many conditions that exist in relationship to CARDIOVASCULAR DISEASE, including essential HYPERTENSION, coronary ARTERIOSCLEROSIS, congestive heart failure, CEREBROVASCULAR ACCIDENT, atrial arrhythmias, ventricular arrhythmias, and ANEURYSMS.
About Cardiovascular Disease
The human heart weighs only 10.5 ounces (about the size of an average fist) and is hollow. This is not really very big considering the job it does. In some animals such as horses the heart size is much greater. The heart is also bigger in champion endurance athletes as a result of genetics and training.
In each 24-hour period, the human heart beats over 100,000 times and pumps about 2,000 gallons of blood—enough to fill an oil tanker. In a 70-year lifetime, an average human heart beats more than 2.5 billion times. It has been speculated that the lifespan of any particular species is relative to the absolute number of heartbeats over the course of its life; smaller sized, short-lived species have very high heart rates, while larger, longer-lived species have much slower heart rates.
In a sense, the heart is really two hearts, the left heart and the right. Both sides pump the same amount of blood, but to different locations of the body, and at different pressures. The right ventricle pumps low-oxygen blood from the body to the lungs where it is oxygenated. This is a short trip and requires little pressure development, so the right ventricle is rather thin walled, like a fireplace bellows.
The left ventricle is the real workhorse, pumping oxygenated blood that has returned from the lungs to the entire body. This job requires being able to move blood through an incredible maze of blood vessels at tremendously greater pressure. The left heart muscle is thicker as a result.
The circulatory system, composed of heart and blood vessels, contains five quarts of blood. The heart pumps these five quarts through 60,000 miles of veins and arteries. The round trip takes about 1 minute, and sometimes the blood reaches a speed of 10 miles per hour.
The heart is an amazingly resilient organ. Alexis Carrel, the famous experimental biologist who received the 1912 Nobel Prize in Physiology for Medicine, concluded that, given an optimum supply of nutrients and oxygen, the human heart is capable of functioning perfectly for over two centuries! The most common forms of heart disease do not result from a wearing-out of the heart, but rather from a breakdown in the supply of nutrients to the heart, usually the result of disease in the arteries on which the heart relies.
General Risk Factors and Causes
As we age, our vessels become less pliant. Therefore, aging itself is a risk factor for cardiovascular disease. Additional risk factors include HYPERTENSION, tobacco use, DIABETES, OBESITY, physical inactivity, a family history of premature arteriosclerosis, and HIGH CHOLESTEROL—especially decreased high-density lipoproteins (HDL) and increased low-density lipoproteins (LDL).
Although the health of the cardiovascular system relies on a complex blend of several important factors, including diet, exercise, stress, and other lifestyle issues, one unrecognized but very important component of the puzzle involves genetics, in particular blood group genetics.
Blood Group Links
Your blood group influences the workings of the cardiovascular system in several ways. It influences the ability to metabolize dietary fats, which has important effects on the heart and circulation.
ABO blood group directly influences the viscosity or thickness of blood, which has powerful implications for circulation. Blood group genetics have a direct effect upon the chemical response to stress—an important risk factor for heart disease.
Although each blood group can develop elements of cardiovascular disease, they appear to do so for different reasons. Some of the causes of heart disease in one blood group may be obvious, such as the link between blood group A and cholesterol. Others, such as the effects of high triglycerides and INSULIN RESISTANCE in blood group O, are less well known. Yet it makes good sense to look for different causes of action in different people, since the conventional wisdom that heart disease is always the result of a high fat, high protein diet can and has been effectively challenged by a number of studies.
The ability to understand your risk factors for cardiovascular disease in light of the specifics of your blood group gives a powerful predictive ability that is absent when you are evaluating the abundant and often contradictory literature on lifestyle and heart disease.
Not every risk factor for heart disease has a blood group component. For example, untreated high blood pressure, diabetes, or smoking will increase your odds of developing cardiovascular disease no matter what your blood type is. Quitting smoking and undergoing frequent blood pressure and diabetes screenings with appropriate treatment, if necessary, will contribute to your success in modifying other, blood group—specific risk factors.
Heart Disease and Blood Group Differences
Blood group O and blood group B are less likely to generate heart disease as a result of high cholesterol. Their pathway is CARBOHYDRATE INTOLERANCE. Blood groups A and AB follow a more conventional path, through high cholesterol. Each of these pathways leads to a very different lifestyle plan and diet to stay heart healthy.
A and AB: The Blood Group-Cholesterol Factor
A number of studies show that blood groups A and AB are more likely to be at risk of heart disease and death by virtue of elevated cholesterol.
The relationship between blood type and total serum cholesterol level was examined in a Japanese population to determine whether elevated cholesterol levels are associated with blood group A, as had been demonstrated in many Western European populations. The results showed that cholesterol levels were very significantly elevated in the group A group compared to the others1.
A study examining a total of 380 marker/risk factor combinations found positive associations between blood group A and both total serum cholesterol and LDL cholesterol, while a negative association was found between blood group B and total serum cholesterol as a risk factor for heart disease2.
A Hungarian study measured the cholesterol of 653 patients who underwent coronary angiography between 1980 and 1985 at the Hungarian Institute of Cardiology. The results showed the numbers of blood group A patients were smaller than would be normally expected from the Hungarian population. The study also found that there were differences between the blood types as to the areas of the vessels where the narrowing of the coronary arteries had occurred3.
Several forms of elevated lipoproteins are inherited. One of the more common forms of hyperlipoproteinemia is called type IIB, and it is characterized by increased LDL and VLDL (very low density lipoproteins). Type IIB hyperlipoproteinemia results in premature hardening of the arteries, obstruction of the carotid artery (the artery that supplies blood to the head and brain), PERIPHERAL ARTERY DISEASE, HEART ATTACK, and STROKE. Since all of these disorders show higher rates of occurrence in blood group A, it is not surprising that studies have found a significant connection between hyperlipoproteinemia IIB and group A in both newborns and in patients who have suffered heart attacks4.
O and B: The Blood Group-Carbohydrate Intolerance Factor
For blood groups O and B, the leading risk factor for heart disease is not so much the fat in the food as the fat on the person. In other words, the elevated risk factor is due to carbohydrate intolerance. When groups O and B adopt low-fat, high carbohydrate, lectin-rich diets, they gain weight. This particular kind of weight gain is a major risk factor for heart disease.
For many years, heart experts have been saying that HIGH TRIGLYCERIDES are not an independent risk for heart disease, but only dangerous in combination with other factors. However, increasing evidence points to elevated triglycerides as a risk factor on their own, and this partially explains the anomaly of the O and B pathway to heart disease 5,6.
Triglycerides are formed by three fatty chains linked to one another. Most fat in food and the human body exist in this form. Diabetics often have high triglyceride levels, and diabetes is believed to be the leading cause of hypertriglyceridemia. In other words, insulin resistance, caused by carbohydrate intolerance, leads to high triglycerides. By current standards, borderline high triglyceride levels are between 200 and 400 mg/dL; high triglyceride levels are between 400 and 1,000 mg/dL; and very high levels are over 1,000 mg/dL.
One study found that men with the highest fasting triglyceride concentrations were more than twice as likely to have heart attacks than men with the lowest levels—even after diabetes, smoking, and sedentary living were factored in. People with triglyceride levels as low as 142 mg/dL were still at risk for heart attacks, which was surprising, because levels below 200 mg/dL were considered normal.
A classic sign of insulin resistance is the “apple-shaped figure,” characterized by a broad girth at the midsection. Fat cells located in the abdomen release fat into the blood more easily than fat cells found elsewhere. For example, “pear-shaped” individuals, with fat located in the hips and thighs, do not have the same health risks. The release of fat from the abdomen begins within 3 to 4 hours after a meal is consumed, compared to many more hours for other fat cells. This easy release shows up as higher triglycerides and free fatty acid levels. Free fatty acids themselves cause insulin resistance, and elevated triglycerides usually coincide with low HDL, or good cholesterol. Overproduction of insulin as a result of insulin resistance syndrome has also been shown to increase the production of very bad cholesterol, VLDL7.
The link between obesity, triglycerides, and bad lipoproteins has been shown for blood group O. In a French study screening blood donors for cardiovascular or cerebrovascular disease, serum triglycerides and lipoproteins were shown to correlate with both obesity and group O. There is also a connection between non-secretors and high triglyceride levels, as well as insulin resistance.
At long last the medical establishment is beginning to recognize the need to step outside of its narrow box and expand its thinking regarding cardiovascular risk factors. Although there is ample evidence that cholesterol plays an important role in ATHEROSCLEROSIS and HEART DISEASE for some people, a large number of people do not fit this limited profile.
O and B: Cholesterol Protection
There is a physiological explanation for the relative protection blood groups O and B enjoy from high-protein diets. Intestinal alkaline phosphatase, an enzyme manufactured in the small intestine, has the primary function of splitting dietary cholesterol and fats, and is naturally high in blood groups O and B—especially among secretors. Conversely, blood groups A and AB have lower levels of this enzyme. Recent studies suggest that it is the inability to break down dietary fat that in part predisposes groups A and AB to higher cholesterol and more heart attacks; the opposite is true for blood groups O and B, who are aided in the breakdown of dietary fat by high amounts of intestinal alkaline phosphatase8,9,10.
Intestinal alkaline phosphatase activity rises following the ingestion of a fat-containing meal, especially if the triglycerides in the meal are long-chain fatty acids. In a study of volunteers given different test meals, the after-meal rise in serum intestinal alkaline phosphatase activity was significantly greater following the long-chain fatty acid meal than following the medium-chain fatty acid meal, and was significantly higher in blood groups O and B over A and AB.
Paradoxically, it appears that intestinal alkaline phosphatase gives groups O and B metabolic advantages when they eat high-protein meals. In fact, consumption of protein further increases the levels of alkaline phosphatase in their intestines. Without protein in their diets, they do not gain the benefits of the specialized fat-busting enzymes in their intestines. This explains why these blood groups can lower their cholesterol by adopting high-protein diets11.
An intriguing study helped to cast some light on why blood groups A and AB have such low levels of alkaline phosphatase activity. Researchers presented evidence that the blood group A antigen may itself inactivate alkaline phosphatase. It may be the case that the lower levels of this enzyme, and the subsequent inability to break down dietary fats, may actually be a physical expression of the A antigen. They found that the red cells of blood groups A and AB bind almost all intestinal alkaline phosphatase, while the red cells of groups O and B do so to a much lesser degree12.
Additional Risk Factors for Cardiovascular Disease
ORAL CONTRACEPTIVES
Birth control pills can worsen cardiovascular risk factors. They raise blood cholesterol levels and increase blood pressure, so women who already have these problems should not take oral contraceptives. Smokers who use oral contraceptives run the risk of developing dangerous BLOOD CLOTS (THROMBOSIS). There is some evidence that group A and AB woman are more at risk for blood clots resulting from oral contraceptive use than groups O or B13.
PHYSICAL INACTIVITY
Researchers have found that people who seldom exercise don’t recover as well from heart attacks as physically active people. Although it is not clear if lack of exercise alone is a risk factor for developing heart disease, in combination with other factors, such as obesity, the risk is higher. Physical activity considerably decreases the bad LDL cholesterol and increases the good HDL cholesterol.
STRESS
Excessive emotional stress over a prolonged period appears to increase the risk of heart disease. STRESS can exacerbate other risk factors, such as overeating, smoking, and high blood pressure. Stress can trigger the release of hormones that promote blood clots. Stress releases fatty acids and glucose into the bloodstream. These can be converted into natural fat and cholesterol and deposited on arterial walls. Such deposits create resistance to the blood flow through the arteries, and contribute to high blood pressure.
Differences between ABO blood groups with regard to stress chemistry may account for some of the observed differences among heart disease victims that appear to be linked to blood group. In one study, blood group O heart attack patients scored significantly higher than blood group A in characteristics associated with “type A” behavior, including behavior scales and anger indexes. Blood group B subjects, as expected, were in the middle14.
Therapies, Cardiovascular Disease
BLOOD GROUP A:
1. Cardiovascular Protocol
2. Metabolic Enhancement Protocol
3. Antistress Protocol
Specifics:
Beware of extra iron: A survey of over 1,900 Finnish men between the ages of 42 and 60 revealed a 4% increase in heart attack risk with each 1% increase in serum ferritin. Ferritin is the protein the body uses to store iron. High iron levels are believed to catalyze the formation of free radicals, which damage the artery wall.
BLOOD GROUP B:
1. Cardiovascular Protocol
2. Metabolic Enhancement Protocol
BLOOD GROUP AB:
1. Cardiovascular Protocol
2. Metabolic Enhancement Protocol
3. Antistress Protocol
BLOOD GROUP O:
1. Cardiovascular Protocol
2. Metabolic Enhancement Protocol
Related Topics
Angina pectoris
Atherosclerosis
Blood clotting disorders
Diabetes mellitus
Heart attack
Hypercholesterolemia
Hypertension
Hypertriglyceridemia
Insulin resistance
Obesity
Stress
Syndrome X
REFERENCES
1. Wong FL, Kodama K, Sasaki H, Yamada M, Hamilton HB. Longitudinal study of the association between ABO phenotype and total serum cholesterol level in a Japanese cohort. Genet Epidemiol. 1992;9:405-418.
2. George VT, Elston RC, Amos CI, Ward LJ, Berenson GS. Association between polymorphic blood markers and risk factors for cardiovascular disease in a large pedigree. Genet Epidemiol. 1987;4:267-275.
3. Tarjan Z, Tonelli M, Duba J, Zorandi A. [Correlation between ABO and Rh blood groups, serum cholesterol and ischemic heart disease in patients undergoing coronarography]. Orv Hetil. 1995;136:767-769.
4. Kipschidse NN, Schawgulidse NA. [Arteriosclerosis and blood lipids]. Z Gesamte Inn Med. 1989;44:175-176.
5. Contiero E, Chinello GE, Folin M. Serum lipids and lipoproteins associations with ABO blood groups. Anthropol Anz. 1994;52:221-230.
6. Borecki IB, Elston RC, Rosenbaum PA, Srinivasan SR, Berenson GS. ABO associations with blood pressure, serum lipids and lipoproteins, and anthropometric measures. Hum Hered. 1985;35:161-170.
7. Lewis GF, Steiner G. Acute effects of insulin in the control of VLDL production in humans. Implications for the insulin-resistant state. Diabetes Care. 1996;19:390-393.
8. Domar U, Hirano K, Stigbrand T. Serum levels of human alkaline phosphatase isozymes in relation to blood groups. Clin Chim Acta. 1991;203:305-313.
9. Nakata N, Tozawa T. [The ABO blood groups-dependent reference intervals for serum alkaline phosphatase isozymes and total activity in individuals 20-39 years of age]. Rinsho Byori. 1995;43:508-512.
10. Day AP, Feher MD, Chopra R, Mayne PD. Triglyceride fatty acid chain length influences the post prandial rise in serum intestinal alkaline phosphatase activity. Ann Clin Biochem. 1992;29:287-291.
11. Stepan J, Graubaum HJ, Meurer W, Wagenknecht C. [Isoenzymes of alkaline phosphatase—reference values in young people and effects of protein diet]. Experientia. 1976;32:832-834.
12. Bayer PM, Hotschek H, Knoth E. Intestinal alkaline phosphatase and the ABO blood group system—a new aspect. Clin Chim Acta. 1980;108:81-87.
13. Jick H, Slone D, Westerholm B. Venous thromboembolic disease and ABO blood type. A cooperative study. Lancet. 1969;1:539-542. No abstract available.
14. Neumann JK, Chi DS, Arbogast BW, Kostrzewa RM, Harvill LM. Relationship between blood groups and behavior patterns in men who have had myocardial infarction. South Med J. 1991;84:214-218.
CAVITIES-See Dental caries
CELIAC DISEASE-Also known as celiac sprue; an immune reaction to gliadin, a protein found in wheat, rye, oats, and barley.
Symptoms
• Chronic diarrhea
• Weight loss
• Iron deficiency
• General nutrient malabsorption
About Celiac Disease
Celiac disease is thought to be an immune reaction to gliadin, a protein found in the four cereal grains: wheat, rye, oats, and barley. About four out of five people with celiac disease have antibodies to gliadin in their blood. It has also been found that, in general, half the patients with gastrointestinal disorders have raised antibodies to gliadin as well. People with celiac disease have a lack of IgA, the protective antibody found in mucus, making their intestinal lining more fragile.
Blood Group Connections
There is a strong association between being a non-secretor and having overt celiac disease. Nonsecretors are about 200% more likely to have celiac disease than secretors1,2. This would make sense, since it has been noted for over 20 years that non-secretors had lower levels of IgA than secretors3,4.
There is some evidence that the improvement in celiac among non-secretors may be linked to the health of their Lewis (a) blood type antigen. The Lewis (a) substance in the gut of nonsecretors is a large molecule. Evidence suggests that this large form of Lewis (a) is converted by the intestinal cells into a small form, which can enter the bloodstream and pass through the kidneys. Non-secretor celiac patients who have not regenerated normal mucosa after treatment have significantly higher levels of small Lewis (a) in their urine when compared with healthy individuals 5. Another study showed that special cells of the intestines called M cells preferentially display the Lewis (a) antigen, and probably provide the material for other cells to metabolize in healthy systems. M cells are important: They are part of the Peyer’s patches of the gut—the place where immune tolerance to foods is built.
Celiac disease predisposes patients to the eventual development of LYMPHOMA, illustrating the intimacy between the immune system and the gut, and how it can be deranged by dietary difficulties.
The Lectin Connection
Lectins have been extensively studied in celiac disease, but the results are inconclusive. Celiac disease seems to effect all blood groups about equally, though perhaps for different reasons. Part of the reason seems to be that gliadin, the perpetrator here, is different from wheat germ lectin, the major everyday problem for blood group O6. For example, studies have reported no ability to bind gliadin or gluten with N-acetyl-glucosamine (NAG), the sugar that so handily binds wheat germ lectin7.
This is not to say that gluten doesn’t appear to be somewhat lectin-like in its own right: Gluten has been shown to bind to carbohydrate-rich tissues much like a lectin. Gluten can even be inhibited by a specific sugar, alpha-D-mannose. Many intestinal influenza viruses bind to alpha-D-mannose as well. This may explain the traditional naturopathic wisdom, noted by lectinologist David Freed, which recommends that a patient fast during gastrointestinal flus8. In addition to bacteria, the lectin from the plant Snowdrop (Galanthus nivalis), which is being used to genetically alter foods, also binds alpha-D-mannose.
Therapies, Celiac Disease
BLOOD GROUP A:
1. Stomach Health Protocol
2. Intestinal Health Protocol
BLOOD GROUP B:
1. Stomach Health Protocol
2. Intestinal Health Protocol
BLOOD GROUP AB:
1. Stomach Health Protocol
2. Intestinal Health Protocol
BLOOD GROUP O:
1. Stomach Health Protocol
2. Intestinal Health Protocol
Related Topics
Cancer, lymphoma
Digestion
Immunity
Lectins
REFERENCES
1. Dickey W, Wylie JD, Collins JS, Porter KG, Watson RG, McLoughlin JC. Lewis phenotype, secretor status, and coeliac disease. Gut. 1994;35:769-770.
2. Heneghan MA, Kearns M, Goulding J, Egan EL, Stevens FM, McCarthy CF. Secretor status and human leucocyte antigens in coeliac disease. Scand J Gastroenterol. 1996;31: 973-976.
3. Shinebaum R. ABO blood group and secretor status in the spondyloarthropathies. PEMS Microbiol Immunol. 1989;1: 589-395.
4. Blackwell CC, May SJ, Brettle RP, MacCallum CJ, Weir DM. Secretor state and immunoglobulin levels among women with recurrent urinary tract infections. J Clin Lab Immunol. 1987;22:133-137.
5. Evans DA, Donohoe WT, Hewitt S, Linaker BD. Lea blood group substance degradation in the human alimentary tract and urinary Lea in coeliac disease. Vox Sang. 1982;43:177-187.
6. Giannasca PJ, Giannasca KT, Leichtner AM, Neutra MR. Human intestinal M cells display the sialyl Lewis A antigen. Infect Immun. 1999;67:946-953.
7. Langman MJ, Banwell JG, Stewart JS, Robson EB. ABO blood groups, secretor status, and intestinal alkaline phosphatase concentrations in patients with celiac disease. Gastroenterology. 1969;57:19-23.
8. Ruhlmann J, Sinha P, Hansen G, Tauber R, Kottgen E. Studies on the aetiology of coeliac disease: no evidence for lectin-like components in wheat gluten. Biochim Biopliys Acta. 1993;1181:249-256.
CEREBRAL ANEURYSM-See Aneurysm, cerebral
CEREBRAL VASCULAR DISEASE-See Aneurysm, cerebral
CEREBROVASCULAR ACCIDENT-See Stroke
CERVICAL CANCER-See Cancer, gynecological tumors
CHEST PAIN-See Angina pectoris; Bronchitis
CHOLERA-See Bacterial disease, cholera
CHOLESTEROL-See Hypercholesterolemia
CHRONIC FATIGUE SYNDROME-A syndrome involving the combination of various symptoms, including headache, muscle ache, fatigue, flu-like symptoms, fever, swelling of the lymph nodes, depression, and digestive ailments.
Symptoms
About Chronic Fatigue Syndrome
What causes chronic fatigue syndrome (CFS)? Some experts think the source is a viral illness that triggers prolonged immune activity; fatigue is a secondary symptom. Others believe that altered neurochemistry associated with depression can cause sleep abnormalities, a lower pain threshold, and depressed immune function. Still other experts are convinced that all fatigue-related symptoms stem from a primary sleep disturbance that causes an alteration of the immune system, irritability, and muscle pain.
Although CFS masquerades as a virus or autoimmune disease, the root cause may be a problem of poor metabolism in the liver. In other words, the liver is unable to detoxify chemicals, and the result is immunologic problems.
Magnesium deficiency and oxidative stress have both been identified in 54% of people with CFS. A detailed review of the literature suggests a number of marginal nutritional deficiencies may have some relevance to CFS. These include deficiencies of various B vitamins, vitamin C, magnesium, sodium, zinc, L-tryptophan, L-carnitine, coenzyme Q10, and essential fatty acids. Any of these nutrients could be marginally deficient in CFS patients, a finding that appears to be primarily due to the illness process rather than to inadequate diets.
The Centers for Disease Control defines CFS by the following criteria:
MAJOR CRITERIA
• New onset of fatigue causing 50% reduction in activity for at least 6 months
• Exclusion of other illnesses that can cause fatigue
MINOR CRITERIA
(presence of 8 of 11 of the following symptoms.):
Symptoms • Mild fever
• Recurrent sore throat
• Painful lymph nodes
• Muscle weakness
• Muscle pain
• Prolonged fatigue after exercise
• Recurrent headache
• Migratory joint pain
• Neurological or psychological complaints
• Sleep disturbances
• Sudden onset of symptom complex
The Immune Connection
Natural killer (NK) cells are a critical component of the immune system when it comes to handling viruses effectively. In a simplified sense, NK cells act like tiny armor-piercing pieces of Velcro, cruising through the immune system. When they encounter a cell infected by a virus, they stick to the cell, and destroy it by a process called lysis. Because of the importance of NK cells in combating a virus, it comes as no surprise that studies in humans consistently point toward the vital role of NK cells in the defense against CFS.
Low levels of NK cell activity are also associated with the severity of CFS. The lower the NK cell activity, the more severe are the symptoms. There may also be a familial association between NK cell activity and CFS. Researchers have discovered that NK cell activity was much lower in individuals who had several extended family members suffering from CFS—even if they themselves did not have the disease1—6.
Blood Group Links
Overall, blood groups O and B have the greatest susceptibility to CFS. For blood group O, CFS may be linked to nutritional deficiencies and liver toxicity.
For blood group B, CFS is likely linked with viral infections and nutritional deficiencies.
The risk for blood groups A and AB heightens during conditions of weakened immunity, especially low levels of NK cells.
Therapies, Chronic Fatigue Syndrome
BLOOD GROUP A:
1. Fatigue-Fighting Protocol
2. Immune-Enhancing Protocol
3. Antistress Protocol
BLOOD GROUP B:
1. Fatigue-Fighting Protocol
2. Antiviral Protocol
3. Liver Support Protocol
BLOOD GROUP AB:
1. Fatigue-Fighting Protocol
2. Immune-Enhancing Protocol
3. Antiviral Protocol
BLOOD GROUP O:
1. Fatigue-Fighting Protocol
2. Immune-Enhancing Protocol
3. Liver Support Protocol
Related Topics
Autoimmune diseases (general)
Cancer (general)
Immunity
Viral diseases (general)
REFERENCES
1. Fulcher KY, White PD. Randomised controlled trial of graded exercise in patients with the chronic fatigue syndrome. BMJ. 1997;314:1647-1652.
2. McCully KK, Sisto SA, Natelson BH. Use of exercise for treatment of chronic fatigue syndrome [review]. Sports Med. 1996;21:35—48.
3. Sharpe M, Hawton K, Simkin S, et al. Cognitive behaviour therapy for the chronic fatigue syndrome: a randomized controlled trial. BMJ. 1996;312:22—26.
4. Shaw DL et al. Management of fatigue: A physiologic approach. Am J Med Sci. 1962;243:758.
5. Crescente FJ. Treatment of fatigue in a surgical practice. J Abdom Surg. 1962;4:73.
6. Cox IM, Campbell MJ, Dowson D. Red blood cell magnesium and chronic fatigue syndrome. Lancet. 1991;337:757—760.
CIRRHOSIS—See Liver disease
CLUSTER HEADACHES—See Headache
COLDS—See Common cold
COLITIS, ULCERATIVE—Inflammation of the innermost lining of the colon wall.
Symptoms
• Bloody diarrhea
• Abdominal pain
• Fever
• Weight loss
About Ulcerative Colitis
As the name suggests, ulcerative colitis involves only the colon and rectum, although some “backwash” may occur in the last part of the small intestine. In contrast to CROHN’S DISEASE, ulcerative colitis involves the mucosa, or the innermost lining of the colon wall. Crohn’s disease involves all layers of bowel, and may involve any part of the gut, from mouth to anus. Like Crohn’s, ulcerative colitis increases the risk for colon cancer.
Blood Group Links
Evidence suggests that patients with ulcerative colitis have elevated antibodies to opposing blood types1. This probably represents the production of blood type—like substances from the mucins of the intestinal lining by bacteria in the gut. If so, it may help explain why sulfa drugs, which work by suppressing the levels of gut bacteria, are generally effective in controlling inflammation. The intestinal microflora are known to be disturbed by inflammatory bowel disease, and it has been thought that ulcerative colitis patients were mounting some sort of allergic hypersensitivity to gut bacteria2.
Depressing the levels of bacteria reduces the immune provocation. However, specific strains of bacteria may be capable of degrading the mucin of colitis patients into antigens that mimic an opposing blood type, which are the true cause of the immune reaction in inflammatory bowel disease. For example, bacteria producing the B antigen in the mucus of a colitis patient who is blood group A will cause the immune system of the patient to mistake the body’s colon lining for a bad blood transfusion, and attack it as foreign. This has been reported in the literature, and several strains of bacteria, including species of Proteus vulgaris, a common gut bacteria, are capable of synthesizing a blood group B antigen (pseudo-B) out of colon mucus.
Gut bacteria are capable of manufacturing large amounts of both A and B blood group antigens by degrading colon mucin. Because of this, it might be hypothesized that blood group O, the only blood group to manufacture both anti-A and anti-B, would have a greater incidence of ulcerative colitis. The literature is mildly supportive of this, although larger and more inclusive studies need to be done3. However, it is true that blood group O, especially non-secretors, does mount a more intensive immune reaction than the other blood groups4. In my own practice, I have found that individuals with blood group O who have inflammatory bowel disease tend to develop a more aggressive form than the other blood groups, but do well when they follow the appropriate diet for their type.
Therapies, Ulcerative Colitis
ALL BLOOD GROUPS:
Conventional treatment involves the use of aminosalicylates (ASA) and corticosteroids. The old sulfa antibiotics and newer 5-ASA preparations such as Asacol (mesalamine), Pentasa (mesalamine), and Dipentum (olsalazine sodium) are still the mainstay of treatment. Acute relapses are often treated with corticosteroids, though their long-term use has substantial toxicity. In patients requiring long-term steroids, immunosuppressive agents such as Imuran (azathioprine) and 6-MP (6-mercaptopurine) are helpful in reducing the required dose of steroids. However, several months may pass before any positive reaction manifests to these drugs.
Although many gastroenterologists continue to ignore food as a provocative factor, it is doubtful that drug therapy alone can successfully cure inflammatory bowel disease.
Many gums found in commercially processed foods can intensify the effects of dietary lectins. One gum in particular, carrageenan, is used to induce ulcerative colitis—like symptoms in test animals. Because carrageenan is effective at stabilizing milk proteins, it is often used in milk and diary products. However, the studies on humans are conflicting: Given large doses, no inflammatory lesions were noticed in healthy human volunteers 5. However, it should be noted that milk proteins as well as gums intensify the effects of dietary lectins. So, giving large doses of carrageenan, independent of milk products and lectin-containing foods, may not be sufficient to induce problems in healthy individuals.
Blood group O tends to develop the more ulcerative form of colitis that causes bleeding with elimination. This is probably due to the lack of adequate clotting factors in group O blood. Blood groups A, B, and AB tend to develop more of a mucous colitis, which is not as bloody.
In either case, follow the diet for your blood type. You will be able to avoid many of the food lectins that can aggravate the condition, and you may find your symptoms easing.
BLOOD GROUP A:
1. Intestinal Health Protocol
2. Stomach Health Protocol
3. Antistress Protocol
BLOOD GROUP B:
1. Intestinal Health Protocol
2. Anti-Inflammation Protocol
3. Antistress Protocol
BLOOD GROUP AB:
1. Intestinal Health Protocol
2. Stomach Health Protocol
3. Antistress Protocol
BLOOD GROUP O:
1. Intestinal Health Protocol
2. Anti-Inflammation Protocol
3. Yeast/Fungus Resistance Protocol
Specifics: Avoid gums, such as carrageenan, ghatti, and acacia, which are often used as food stabilizers. Look for foods that are stabilized by other methods.
Related Topics
Crohn’s disease
Digestion
Immunity
REFERENCES
1. Chiba M, Nakajima K, Arakawa H, Masamune O, Narisawa T. Anti-erythrocyte antibodies in ulcerative colitis: case report and discussion on the pathophysiology of anti-erythrocyte antibody. Gastroenterol Jpn. 1988;23:564—569.
2. Hentges DJ ed. Human Intestinal Microflora in Health and Disease. New York: Academic Press, 1983.
3. Vesely KT. Frequency of blood groups of the ABC and Rho(D) system in patients with viral hepatitis, cirrhosis of the liver, bile stones, pancreatitis, urolithiasis, and ulcerative colitis. Rev Czech Med. 1970;16:60-71.
4. Tandon OP, Bhatia S, Tripathi RL, Sharma KN. Phagocytic response of leucocytes in secretors and non-secretors of ABH (O) blood group substances. Indian J Physiol Pharmacol. 1979;23:321-324.
5. Bonfils S. Carrageenan in the human gut. Lancet. 1970;ii: 414.
COLON CANCER—See Cancer, colon
COLON POLYPS—See Polyps, colon
COMMON COLD—A general term deseribing a number of common viruses that infect the upper respiratory tract.
Symptoms
• General malaise
• Fever
• Congestion
• Sneezing
• Sore throat
Blood Group Links
There are hundreds of different strains of cold virus, and it would be impossible to see a blood type specificity in all of them. However, studies of British military recruits showed a slightly lower overall incidence of cold viruses in recruits who were blood group A, which is consistent with our findings that blood group A was developed to be resistant to these common viruses. Viruses also have less impact on blood group AB. The A antigen, carried by both blood group A and AB, blocks the attachment of various strains of flu to the membranes of the throat and respiratory passages.
INFLUENZA, a more serious virus, also strikes blood groups O and B in preference to groups A and AB. In its early stages, influenza may have many of the symptoms of a common cold. However, the flu causes dehydration, muscle pains, and serious weakness.
The symptoms of a common cold or flu are miserable, but they are actually a sign that your immune system is trying hard to fight off the offending virus.
Therapies, Common Cold
ALL BLOOD GROUPS:
1. Maintain general good health with adequate rest and exercise, along with learning to cope with the stresses of life. Stress is a major factor in the depletion of immune system resources. This may protect you from frequent infections and may even shorten the duration of the colds and flu you do get.
2. Follow the basic dietary guidelines for your blood type. This will optimize your immune response and help shorten the course of your cold or flu.
3. Take a bit of extra vitamin C, or increase the sources of vitamin C in your diet. Small doses of the herb echinacea (Echinacea purpurea) helps prevent colds, or at least helps to shorten their duration.
4. Increase the humidity in your room with a vaporizer or humidifier to prevent a dry throat and nasal tissue.
5. If your throat is sore, gargle with salt water. One-half teaspoon of ordinary table salt and a tall glass of comfortably warm water provides a soothing and cleansing rinse. Another good gargle, especially if you are prone to tonsillitis, is a tea of equal parts goldenseal root (Hydrastis canadensis) and sage. Gargle with this mixture every few hours.
6. If your nose is runny or stuffy, use an antihistamine to reduce the reaction of tissues to the infecting virus and relieve nasal congestion. Be especially careful with ephedra-type antihistamines, such as those found in health food stores and in some over-the-counter decongestants. These can raise the blood pressure, keep you awake at night, and complicate prostate problems in men.
7. Antibiotics are not effective against viruses.
BLOOD GROUP A:
1. Immune-Enhancing Protocol
2. Antiviral Protocol
BLOOD GROUP B:
1. Immune-Enhancing Protocol
2. Antiviral Protocol
BLOOD GROUP AB:
1. Immune-Enhancing Protocol
2. Antiviral Protocol
BLOOD GROUP O:
1. Immune-Enhancing Protocol
2. Antiviral Protocol
Related Topics
Immunity
Infectious disease
Influenza
Viral disease
Constipation
Constipation occurs when the stools are unusually hard or a person’s bowel patterns have changed and become less frequent. Most chronic constipation is caused by poor bowel habits and irregular meals, with a diet low in bulk and water content. Some other causes are a habitual use of laxatives, a rushed and stressful daily schedule, and travel that requires abrupt adjustments of eating and sleeping patterns. Lack of physical exercise, acute illness, painful rectal conditions, and some medications may also cause constipation.
Every blood group is susceptible to constipation under the proper circumstances. Constipation is not so much a disease as a warning that something is not right with your digestive system. You’ll find most of the clues in your diet:
• Are you eating enough foods in your diet that are high in fiber content?
• Are you drinking enough fluids—in particular, water and juices?
• Are you exercising regularly?
Many people simply take a laxative when they’re constipated. But that doesn’t solve the natural systemic causes of constipation. The long-term solution is in the diet. Blood groups A, B, and AB can supplement their diets with fibrous, unprocessed bran. Group O, in addition to eating plenty of the fibrous fruits and vegetables on their diets, can take a supplement of butyrate, a natural bulk-forming agent, as a substitute for bran (because bran is not advised for them).
Related Protocols
BLOOD GROUP A:
1. Intestinal Health Protocol
2. Liver Support Protocol
3. Detoxification Protocol
BLOOD GROUP B:
1. Intestinal Health Protocol
2. Liver Support Protocol
3. Detoxification Protocol
BLOOD GROUP AB:
1. Intestinal Health Protocol
2. Liver Support Protocol
3. Detoxification Protocol
BLOOD GROUP O:
1. Intestinal Health Protocol
2. Liver Support Protocol
3. Detoxification Protocol
Related Topics
Cancer, colon
Colitis, ulcerative
Crohn’s disease
Digestion
Hemorrhoids
Lectins
Polyps, colon
Stress
CORONARY ARTERY DISEASE (CAD)— Arterial plaque build-up producing blockages in the arteries serving the heart.
Symptoms
Coronary artery disease (CAD) results from arterial plaque build-up, which produces blockages in the arteries serving the heart. It is characteristically insidious in onset, is often irregularly distributed in different vessels, and can abruptly interfere with blood flow to segments of the heart, most often when the arterial blockages rupture and spread. The major complications of CAD are angina pectoris, unstable angina, heart attack, and sudden cardiac death due to arrhythmias.
About Coronary Artery Disease
Heart disease is the leading cause of death for people over the age of 45 living in the United States. Each day, 4,100 Americans suffer a heart attack; while hundreds of thousands survive, they are left with a damaged heart. Some 7 million Americans suffer from CAD, the most common form of heart disease. This particular disease is caused by a narrowing of the coronary arteries that feed the heart. CAD is the number one killer of both men and women in the United States. Each year, more than 500,000 Americans die of heart attacks caused by CAD.
Like any other tissue in the body, the heart needs its own supply of oxygen and nutrients, which are supplied by coronary arteries. The word coronary comes from the Greek word meaning “wreath.” And like a wreath, the three coronary arteries radiate from the heart’s main artery and cover the heart. These arteries provide the constant supply of fresh, oxygenated blood to the heart muscle. If this supply is restricted or cut off for any reason, the heart muscle literally begins to starve, and can die. When heart muscle begins to die, it is called a heart attack, or MYOCARDIAL INFARCTION. If enough tissue dies, the heart no longer has the strength to pump, and it will falter or stop.
General Risk Factors and Causes
Risk factors are conditions that increase your chances of developing heart disease. Some can be changed, and some cannot. Although each of these factors raise the risk of CAD, they do not clearly define all the causes of the disease. Even with an absence of these risk factors present, some people still develop CAD.
Most risk factors for CAD are preventable with lifestyle changes. They include HIGH BLOOD PRES-SURE, HIGH CHOLESTEROL, smoking, OBESITY, and physical inactivity—all of which can be controlled. Problems arise when we try to portray risk factors as being identical for entirely different groups of people. It is here that many of the recent assertions about the connections between lifestyle and cardiovascular disease begin to break down; while large numbers of people do fit the risk profile for heart disease, equally large numbers do not. It is the extra level of interpretation that genetic information such as blood type provides that adds the needed “biomarkers” to accurately pinpoint who will suffer from cardiovascular disease from one risk factor, and who may develop it for a completely different reason.
Homocysteine has recently been identified as a risk factor for coronary, peripheral, and cerebral vascular disease. Patients with homocystinuria, a rare recessive disease, have plasma homocysteine levels 10 to 20 times above normal (hyperhomocystinemia) and accelerated, premature vascular disease. Homocysteine has a direct toxic effect on endothelium and promotes THROMBOSIS (clotting) and oxidation of LDL. Modest elevations of total plasma homocysteine have multiple causes, including low levels of folic acid and vitamins B6 and B12, renal insufficiency, use of certain drugs, and genetically determined variations in homocysteine metabolic enzymes. Patients with homocysteine values in the top 5% have a 3.4 greater risk of myocardial infarctions or cardiac death than those in the lower 90% after adjustment for other risk factors. Increased homocysteine levels are associated with increased risk of a heart condition, regardless of its cause. Recent studies suggest a graded risk even for those who have the normal of range homocysteine; thus, a reduction in the normal plasma levels may be advantageous as well.
Blood Group Links
In a study of 191 coronary artery bypass candidates, investigators paradoxically found an excess of blood group O over blood group A. When they examined the data more closely, they concluded that that the tendency of blood group A individuals to develop blood clots more readily (“thrombotic proneness”) caused a poorer prognosis. In essence, the individuals from blood group A were missing from the study because they had already died in greater numbers, leaving a disproportionate number of blood group O patiens among the long-term survivors‘.
ABO non-secretors have a higher incidence of heart disease, but paradoxically with the subtypes’ higher incidence of alcoholism, the effects of alcohol are most protective in this group as well.
Therapies, Coronary Artery Disease
ALL BLOOD GROUPS:
The most simple and effective way to reduce plasma homocysteine is administration of folic acid 1 to 2 mg/day, which has essentially no side effects except in untreated vitamin B12 deficiency.
BLOOD GROUP A:
1. Cardiovascular Protocol
2. Metabolic Enhancement Protocol
3. Antistress Protocol
BLOOD GROUP B:
1. Cardiovascular Protocol
2. Metabolic Enhancement Protocol
3. Antistress Protocol
BLOOD GROUP AB:
1. Cardiovascular Protocol
2. Metabolic Enhancement Protocol
3. Antistress Protocol
BLOOD GROUP O:
1. Cardiovascular Protocol
2. Metabolic Enhancement Protocol
3. Antistress Protocol
Related Topics
Atherosclerosis
Cardiovascular disease
Diabetes mellitus, type II
Heart attack
Hypercholesterolemia
Hypotension
Insulin resistance
Stress
REFERENCES
1. Erikssen J, Thaulow E, Stormorken H, Brendemoen O, Hellem A. ABO blood groups and coronary heart disease (CHD). A study in subjects with severe and latent CHD. Thromb Haemost. 1980;43:137-140.
Cough
Cough is a symptom of many diseases. Most coughs come from simple viral infections, such as the common cold or bronchitis. Sometimes mucus is produced with the cough and sometimes not. If the color is green or yellow, it may be a hint of a more serious bacterial infection, although this is not a reliable indicator. If the color is red, there may be bleeding in the lungs.
Cough can also occur in some autoimmune diseases, such as rheumatoid arthritis.
Any cough that produces blood or blood-stained mucus, as well as any cough that lasts more that 2 weeks, requires a visit to a medical professional for an examination.
Related Protocols
BLOOD GROUP A:
1. Pulmonary Support Protocol
2. Antibacterial Protocol
3. Allergy Control Protocol
BLOOD GROUP B:
1. Pulmonary Support Protocol
2. Antibacterial Protocol
3. Allergy Control Protocol
BLOOD GROUP AB:
1. Pulmonary Support Protocol
2. Antibacterial Protocol
3. Allergy Control Protocol
BLOOD GROUP O:
1. Pulmonary Support Protocol
2. Antibacterial Protocol
3. Allergy Control Protocol
Related Topics
Allergies, environmental/hay fever
Arthritis, rheumatoid
Bacterial disease (general)
Bronchitis
Cancer, lung
Common cold
Influenza
CROHN’S DISEASE—Inflammation and ulceration in deep layers of the intestinal wall.
Symptoms
• Cramps and discomfort upon eating
• Watery to loose stools with rectal bleeding
• Weight loss
• Anorexia
• Recurrent fevers
• Growth failure in younger patients
• Urinary tract infections
• Aphthous ulcers
About Crohn’s Disease
Crohn’s disease is a chronic inflammatory and ulceration process that occurs in the deep layers of the intestinal wall. It has periods of remission and relapse. The most common area affected is the lower part of the small intestine, called the ileum, and the first part of the colon. This type of Crohn’s disease is called ileocolitis1.
The cause of Crohn’s disease is unknown, although alterations in intestinal permeability may play a primary role. Increased intestinal permeability in Crohn’s disease may promote the local disease, and also provide the route for food antigen entry2.
The Lectin Connection
There is ample evidence that dietary LECTINS can induce increased permeability of the gut. Intestinal permeability has been theorized to account for much of the FOOD INTOLERANCE found in people today. The intestines are very selective as to the size and quality of what is normally absorbed through their lining. In one study, rats fed on diets containing kidney beans showed increased intestinal permeability to serum proteins that had been injected into their bloodstream. Afterward, kidney bean proteins injected into their bloodstream were detected in both the lumen (open space) and the walls of the small intestine. It was suggested that dietary lectins may, at least in part, be responsible for loss of serum proteins, and may contribute to other food intolerance secondary to the loss of gut integrity.
Blood Group Links
Crohn’s disease, like ulcerative colitis, shows a preference to blood group O. Groups A, B, and AB often develop inflammatory bowel conditions when there is a stress component.
Therapies, Crohn’s Disease
BLOOD GROUP A
1. Intestinal Health Protocol
2. Anti-Inflammation Protocol
BLOOD GROUP B:
1. Intestinal Health Protocol
2. Anti-Inflammation Protocol
BLOOD GROUP AB:
1. Intestinal Health Protocol
2. Anti-Inflammation Protocol
BLOOD GROUP O:
1. Intestinal Health Protocol
2. Anti-Inflammation Protocol
Related Topics
Colitis, ulcerative
Digestion
Immunity
REFERENCES
1. Mayberry JF, Rhodes J. Epidemiological aspects of Crohn’s disease: a review of the literature. Gut. 1984;25:886-899.
2. Hollander D, Vadheim CM, Brettholz E, et al. Increased intestinal permeability in patients with Crohn’s disease and their relatives. A possible etiologic factor. Ann Intern Med. 1986;105:883-885.
CYSTIC FIBROSIS—See Birth defects
CYSTITIS—Chronic bladder infections.
Symptoms
• Painful, frequent urination of small amounts
• Pelvic pull or pressure
• Lower back pain (accompanied by other symptoms)
• Low-grade fever (accompanied by other symptoms)
About Cystitis
Cystitis is the result of bacteria in the bladder. The primary causes seem to be sexual activity and poor hygiene. Cystitis often follows the beginning of sexual activity, a change in partners, or unusually frequent intercourse. In fact, one study showed that cystitis was 12.8 times more common in the general population than it was among nuns (who were assumed to be celibate).
Cystitis can also occur when the normal bacteria that live in the bowel and the vagina invade the bladder. This happens if fecal matter gets into the vagina. The issue here is hygiene, although it can happen to even the most scrupulous person. In fact, Escherichia coli bacteria, the most common in the bowel, are said to be the cause of most bladder infections.
Cystitis can be recurrent—the same bacteria from a previous episode, incompletely treated. Or it can be a reinfection—a new episode with a new bacteria. Risk factors include previous urinary tract infections; DIABETES; pregnancy; more frequent or vigorous sexual activity than usual; use of a spermicide or diaphragm; and underlying abnormalities of the urinary tract such as tumors, strictures, and incomplete bladder emptying.
A more severe form of cystitis, called interstitial cystitis, is poorly understood. Causes are unknown but some possible causes are subclinical urinary infection, and increased bladder wall permeability to irritants such as urea.
Blood Group Links
There is good evidence that blood groups B and AB are more susceptible to cystitis. That’s because the most common bacteria that produce infections, such as E. coli, Pseudomonas, and Klebsiella, possess a B-like appearance, and groups B and AB produce no anti-B antibodies. A form of synergy also appears to exist between urinary tract infection risk, secretor status, and the lack of ability to create anti-B isohemagglutinin. Essentially, blood groups B and AB and the non-secretor phenotype seem to work together to increase the relative risk of recurrent urinary tract infections among these women1.
Evidence also indicates that women and children with renal scarring subsequent to recurrent urinary tract infection and pyelonephritis are more likely to be ABO non-secretors2—4. As many as 55% to 60% of all non-secretors have been found to develop renal scars, even with the regular use of an antibiotic treatment for urinary tract infections whereas as few as 16% of ABH secretors will develop similar renal scarring5.
This tendency to scarring does not seem to be dictated as much by the aggressiveness of the bacterial infection, but by the more aggressive inflammatory response created by ABH nonsecretors against the bacterial infection. The levels of C-reactive protein, erythrocyte sedimentation rate, and body temperature are significantly higher in non-secretors than in secretors with recurrent urinary tract infection. As a consequence, non-secretors seem to self-inflict to a degree the renal scarring occurs secondary to their acute phase inflammatory response6.
ABO non-secretors appear to be at extra risk for recurrent urinary tract infections. In one study of women with recurrent urinary tract infections, 29% were the Lewis (a+b-) non-secretor phenotype, while another 26% were Lewis (a-b-) recessive phenotype. When the women with nonsecretor and recessive phenotypes were combined and considered collectively, the odds ratio (an estimate of relative risk of recurrent urinary tract infection) for those without the secretor phenotype (Lewis (a-b+)) was 37—11.
When women with urinary tract infections were evaluated, the profile of ABO antigen expression was generally consistent with the ABO phenotype of the individual and appeared to be influenced by the secretor status. Secretors expressed higher levels of A, B, and O determinants than non-secretors. In addition, Lewis antigens were detected on vaginal cells and in mucus. Samples from non-secretors expressed higher levels of Le(a) and Le(x) antigens, whereas secretors expressed higher levels of Le(b) and Le(y) antigens. The levels of antigen expression varied widely among individuals with the same blood type and secretor status. Comparisons between patient and control groups showed no significant differences in ABO or Lewis antigen expression overall, or when controlling for ABO or secretor phenotypes, respectively. These findings confirm our previous observations on healthy women, and document the heterogeneity of blood group antigen expression on vaginal wall cells and mucus from women with a history of urinary tract infections12.
Blood groups of 137 patients with acute pyelonephritis and chronic upper tract infection, cystitis, and asymptomatic bacteriuria were compared with those of a normal uninfected control population. The identified uropathogens were categorized according to the patient’s blood group. There was a significant association between the diagnosis of chronic upper tract infection and blood group B as compared with controls. Analysis of the bacterial isolates showed that more patients with blood group B had infections with Pseudomonas sp organisms, Klebsiella pneumoniae, and Proteus sp bacteria than was expected; and fewer patients with blood group A had infections with Pseudomoreas than predicted13.
There was an increased number of patients in blood group AB with infections caused by Escherichia coli and Klebsiella pneumoniae. These results suggest that an individual’s blood group may be a significant factor in the host-response to bacterial invasion and may influence the development of infection with certain gram-negative bacilli.
The ABO and rhesus blood groups of 280 patients of a center of nephrology were compared with a control group consisting of 4,089 blood donors. Female patients with pyelonephritis showed with a probability of error of less than 5% more frequently the blood group A (56.7 vs 41.8%) and more infrequently the blood group O (20.0% versus 39.9%). The increase of frequency of female patients of the blood group B with cystic kidneys (30.0% versus 12.6%) reached the significance level.
Early studies confirmed these findings. Robinson and co-workers15 reported that individuals of blood groups B and AB had a significantly greater susceptibility to gram-negative enteric pathogens (other than Shigella). This was especially true for Escherichia coli and Salmonella bacteria in populations of African and Puerto Rican ancestry. Individuals in blood groups B and AB, regardless of ethnic background, had a 55% greater chance of developing E. coli infections than did individuals of blood groups A and O. They found increased risk (not statistically significant) of E. coli pyelonephritis in patients in groups B and AB, compared with patients in groups A and O. Cruz-Cooke and co-workers16 found that patients in blood group B had a 50% greater probability of having urinary tract infections caused by E. coli compared with non-B subjects. Kinane and coworkers’ reported that women who lacked anti-B (blood group B and AB) and were non-secretors were at higher risk of recurrent urinary tract infections than women who lacked either anti-B or secretor substance.
Therapies, Cystitis
BLOOD GROUP A:
1. Antibacterial Protocol
2. Urinary Tract Health Protocol
BLOOD GROUP B:
1. Antibacterial Protocol
2. Urinary Tract Health Protocol
BLOOD GROUP AB:
1. Antibacterial Protocol
2. Urinary Tract Health Protocol
BLOOD GROUP O:
1. Antibacterial Protocol
2. Urinary Tract Health Protocol
Related Topics
Bacterial disease (general)
Cancer, bladder
Immunity
Lectins
REFERENCES
1. Kinane DF, Blackwell CC, Brettle RP, Weir DM, Winstanley FP, Elton RA. ABO blood group, secretor state, and susceptibility to recurrent urinary tract infection in women. Br Med J (Clin Res Ed). 1982;285(6334):7—9.
2. May SJ, Blackwell CC, Brettle RP, MacCallum CJ, Weir DM. Non-secretion of ABO blood group antigens: a host factor predisposing to recurrent urinary tract infections and renal scarring. FEMS Microbiol Immunol. 1989;1:383—387.
3. Lomberg H, Hellstrom M, Jodal U, Svanborg Eden C. Secretor state and renal scarring in girls with recurrent pyelonephritis. FEMS Microbiol Immunol. 1989;1:371—375.
4. Lomberg H, de Man P, Svanborg Eden C. Bacterial and host determinants of renal scarring. APMIS. 1989;97:193—199.
5. Jacobson SH, Lomberg H. Overrepresentation of blood group non-secretors in adults with renal scarring. Scand J Urol Nephrol. 1990;24:145—150.
6. Lomberg H, Jodal U, Leffler H, De Man P, Svanborg C. Blood group non-secretors have an increased inflammatory response to urinary tract infection. Scand J Infect Dis. 1992; 24:77—83.
7. Sheinfeld J, Schaeffer AJ, Cordon-Cardo C, Rogatko A, Fair WR. Association of the Lewis blood-group phenotype with recurrent urinary tract infections in women. N Engl J Med. 1989;320:773—777.
8. Similar findings by other researchers support this over representation of recurrent UTI among non-secretors both in women and children.
9. May SJ, Blackwell CC, Brettle RP, MacCallum CJ, Weir DM. Non-secretion of ABO blood group antigens: a host factor predisposing to recurrent urinary tract infections and renal scarring. FEMS Microbiol Immunol. 1989;1:383—387.
10. Stapleton A, Nudelman E, Clausen H, Hakomon S, Stamm WE. Binding of uropathogenic Escherichia coli R45 to glycolipids extracted from vaginal epithelial cells is dependent on histo-blood group secretor status. J Clin Invest. 1992;90:965—972.
11. Jantausch BA, Criss VR, O‘Donnell R, et al. Association of Lewis blood group phenotypes with urinary tract infection in children. J Pediatr. 1994;124:863—868.
12. Lavas EL, Venegas MF, Duncan JL, et al. Blood group antigen expression on vaginal cells and mucus in women with and without a history of urinary tract infections. J Urol. 1994;152(pt1):345—349.
13. Ratner JJ, Thomas VL, Forland M. Relationships between human blood groups, bacterial pathogens, and urinary tract infections. Am J Med Sci. 1986;292:87—91.
14. Voigtmann B, Burchardt U. ABO blood groups in patients with nephropathies [in German]. Z Gesamte Inn Med. 1991;46:156—159.
15. Robinson MG, Tolchin D, Halpern C. Enteric bacterial agents and the ABO blood groups. Am J Hum Genet. 1971; 23:135-145.
16. Cruz-Cooke R. Blood groups and urinary microorganisms. J Med Genet. 1965;(2):185—188.