

Air pollution goes hand-in-hand with industrialization as can be seen in this satellite image taken over Beijing, China. The air is a uniform brownish gray, showing that the region is thick with smog. Although legislation protects the people who live in developed nations from at least some of the problems caused by air pollution, people in many developing nations have no such protection. In China, an ongoing battle to improve the standard of living of its people has resulted in unrestrained growth and little protection from environmental damage. At least 75% of the people who live in China’s cities live with air quality that is below the national standard. Beijing is an enormous city of more than 10 million people, where many of the bicycles that have transported people for decades are being traded in for cars. Along with the increase in electricity generation from coal, and many other factors, the result is unhealthy living conditions.
There is, unfortunately, still plenty of smog in developed nations as can be seen in this chapter.
Earth’s atmosphere provides living creatures on the planet with the gases they need for photosynthesis and respiration. In addition, the ozone layer protects organisms from the Sun’s ultraviolet radiation. The importance of the atmosphere for Earth’s life cannot be overestimated yet people also use the atmosphere as a dump for waste gases and particles.
Pollutants include materials that are naturally occurring but are added to the atmosphere so that they are there in larger quantities than normal. Pollutants may also be human-made compounds that have never before been found in the atmosphere. Pollutants dirty the air, change natural processes in the atmosphere, and harm living things.
Air pollution started to be a problem when early people burned wood for heat and cooking fires in enclosed spaces such as caves and small tents or houses. But the problems became more widespread as fossil fuels such as coal began to be burned during the Industrial Revolution ( Figure below ).

Figure 22.1
A film crew recreates London smog in the Victorian Era.
Air pollution became a crisis in the developed nations in the mid-20th century. Coal smoke and auto exhaust combined to create toxic smog that in some places caused lung damage and sometimes death ( Figure below ). In Donora, Pennsylvania, in October 1948, 20 people died and 4,000 became ill when coal smoke was trapped by an inversion.

Figure 22.2
In London in December 1952, the
Photochemical smog , a different type of air pollution, first became a problem in Southern California after World War II. The abundance of cars and sunshine provided the perfect setting for a chemical reaction between some of the molecules in auto exhaust or oil refinery emissions, and sunshine ( Figure below ). Photochemical smog consists of more than 100 compounds, most importantly ozone.

Figure 22.3
Smog over Los Angeles as viewed from the Hollywood Hills.
The terrible events in Pennsylvania and London, plus the recognition of the hazards of photochemical smog, led to the passage of the Clean Air Act in 1970 in the United States. The act now regulates 189 pollutants. The six most important pollutants regulated by the Act are ozone, particulate matter, sulfur dioxide, nitrogen dioxide, carbon monoxide, and the heavy metal lead. Other important regulated pollutants include benzene, perchloroethylene, methylene chloride, dioxin, asbestos, toluene, and metals such as cadmium, mercury, chromium, and lead compounds.
What is the result of the Clean Air Act? In short, the air in the United States is much cleaner. Visibility is better and people are no longer incapacitated by industrial smog. However, despite the Act, industry, power plants, and vehicles put 160 million tons of pollutants into the air each year. Some of this smog is invisible and some contributes to the orange or blue haze that affects many cities.
Air quality in a region is not just affected by the amount of pollutants released into the atmosphere in that location but by other geographical and atmospheric factors. Winds can move pollutants into or out of a region and a mountain range can trap pollutants on its leeward side. Inversions commonly trap pollutants within a cool air mass. If the inversion lasts long enough, pollution can reach dangerous levels.
Pollutants remain over a region until they are transported out of the area by wind, diluted by air blown in from another region, transformed into other compounds, or carried to the ground when mixed with rain or snow.
Table below lists the smoggiest cities in 2011: eight of the 10 are in California. Why do you think California cities are among those with the worst air pollution?
The state has the right conditions for collecting pollutants including mountain ranges that trap smoggy air, arid and sometimes windless conditions, agriculture, industry, and lots and lots of cars.
Table 22.1
| Rank | City, State |
| 1 | Los Angeles, California |
| 2 | Bakersfield, California |
| 3 | Visalia-Porterville, California |
| 4 | Fresno, California |
| 5 | Sacramento, California |
| 6 | Hanford, California |
| 7 | San Diego, California |
| 8 | Houston, Texas |
| 9 | Merced, California |
| 10 | Charlotte, North Carolina |
The two types of air pollutants are primary pollutants, which enter the atmosphere directly, and secondary pollutants, which form from a chemical reaction.
Some primary pollutants are natural, such as volcanic ash. Dust is natural but exacerbated by human activities; for example, when the ground is torn up for agriculture or development. Most primary pollutants are the result of human activities, the direct emissions from vehicles and smokestacks. Primary pollutants include:

Figure 22.4
High CO

Figure 22.5
Particulates from a brush fire give the sky a strange glow in Arizona.
Any city can have photochemical smog, but it is most common in sunny, dry locations. A rise in the number of vehicles in cities worldwide has increased photochemical smog. Nitrogen oxides, ozone, and several other compounds are some of the components of this type of air pollution.
Photochemical smog forms when car exhaust is exposed to sunlight. Nitrogen oxides is created by gas combustion in cars and then into the air ( Figure below ). In the presence of sunshine, the NO 2 splits and releases an oxygen ion (O). The O then combines with an oxygen molecule (O 2 ) to form ozone (O 3 ). This reaction can also go in reverse: Nitric oxide (NO) removes an oxygen atom from ozone to make it O 2 . The direction the reaction goes depends on how much NO 2 and NO there is. If NO 2 is three times more abundant than NO, ozone will be produced. If nitrous oxide levels are high, ozone will not be created.

Figure 22.6
The brown color of the air behind the Golden Gate Bridge is typical of California cities, because of nitrogen oxides.
Ozone is one of the major secondary pollutants. It is created by a chemical reaction that takes place in exhaust and in the presence of sunlight. The gas is acrid-smelling and whitish. Warm, dry cities surrounded by mountains, such as Los Angeles, Phoenix, and Denver, are especially prone to photochemical smog ( Figure below ). Photochemical smog peaks at midday on the hottest days of summer. Ozone is also a greenhouse gas.

Figure 22.7
Counties with such high ozone levels that they do not attain federal air quality standards.
Most air pollutants come from burning fossil fuels or plant material. Some are the result of evaporation from human-made materials. Nearly half (49%) of air pollution comes from transportation, 28% from factories and power plants, and the remaining pollution from a variety of other sources.
Fossil fuels are burned in most motor vehicles and power plants. These nonrenewable resources are the power for nearly all manufacturing and other industries. Pure coal and petroleum can burn cleanly and emit only carbon dioxide and water, but most of the time, these fossil fuels do not burn completely and the incomplete chemical reactions produce pollutants. Few sources of these fossil fuels are pure, so other pollutants are usually released. These pollutants include carbon monoxide, nitrogen dioxide, sulfur dioxide, and hydrocarbons.
In large car-dependent cities such as Los Angeles and Mexico City, 80% to 85% of air pollution is from motor vehicles ( Figure below ). Ozone, carbon monoxide, and nitrous oxides come from vehicle exhaust.
See the relative amounts of CO 2 released by different fossil fuels in this animation http://www.nature.nps.gov/GEOLOGY/usgsnps/oilgas/CO2BTU_3.MPG

Figure 22.8
Auto exhaust like this means that the fuels is not burning efficiently.
A few pollutants come primarily from power plants or industrial plants that burn coal or oil ( Figure below ). Sulfur dioxide (SO 2 ) is a major component of industrial air pollution that is released whenever coal and petroleum are burned. SO 2 mixes with H 2 O in the air to produce sulfuric acid (H 2 SO 4 ).
Mercury is released when coal and some types of wastes are burned. Mercury is emitted as a gas, but as it cools, it becomes a droplet. Mercury droplets eventually fall to the ground. If they fall into sediments, bacteria convert them to the most dangerous form of mercury: methyl mercury. Highly toxic, methyl mercury is one of the metal’s organic forms.

Figure 22.9
A power plant and its emissions before emission control equipment was added.
Fossil fuels are ancient plants and animals that have been converted into usable hydrocarbons. Burning plant and animal material directly also produces pollutants. Biomass is the total amount of living material found in an environment. The biomass of a rainforest is the amount of living material found in that rainforest.
The primary way biomass is burned is for slash-and-burn agriculture ( Figure below ). The rainforest is slashed down and then the waste is burned to clear the land for farming. Biomass from other biomes, such as the savannah, is also burned to clear farmland. The pollutants are much the same as from burning fossil fuels: CO 2 , carbon monoxide, methane, particulates, nitrous oxide, hydrocarbons, and organic and elemental carbon. Burning forests increases greenhouse gases in the atmosphere by releasing the CO 2 stored in the biomass and also by removing the forest so that it cannot store CO 2 in the future. As with all forms of air pollution, the smoke from biomass burning often spreads far and pollutants can plague neighboring states or countries.

Figure 22.10
A forest that has been slash-and-burned to make new farmland.
Particulates result when anything is burned. About 40% of the particulates that enter the atmosphere above the United States are from industry and about 17% are from vehicles. Particulates also occur naturally from volcanic eruptions or windblown dust. Like other pollutants, they travel all around the world on atmospheric currents.
Volatile organic compounds (VOCs) enter the atmosphere by evaporation. VOCs evaporate from human-made substances, such as paint thinners, dry cleaning solvents, petroleum, wood preservatives, and other liquids. Naturally occurring VOCs evaporate off of pine and citrus trees. The atmosphere contains tens of thousands of different VOCs, nearly 100 of which are monitored. The most common is methane, a greenhouse gas ( Figure below ). Methane occurs naturally, but human agriculture is increasing the amount of methane in the atmosphere.

Figure 22.11
Methane forms when organic material decomposes in an oxygen-poor environment. In the top image, surface methane production is shown. Stratospheric methane concentrations in the bottom image show that methane is carried up into the stratosphere by the upward flow of air in the tropics.
1. What is the difference between the type of smog experienced by cities in the eastern United States and that found in Southern California?
2. London has suffered from terrible air pollution for at least seven centuries. Why is the city so prone to its famous “London fog?” What did London do to get rid of its air pollution?
3. Imagine two cities of the same size with the same amount of industrialization and the same number of motor vehicles. City A is incredibly smoggy most of the time and City B usually has very little air pollution. What factors might go into creating these two different situations?
4. What might be a reason why the city of San Francisco and its metropolitan area is not on the list of smoggiest cities for 2009?
5. Why are naturally occurring substances, such as particulates or carbon dioxide, sometimes considered pollutants?
6. How does ozone form from vehicle exhaust?
7. What are the necessary ingredients for ozone creation, excluding those that are readily available in the atmosphere? Why could there be a city with a lot of cars but relatively little ozone pollution?
8. Some people say that we need to phase out fossil fuel use and replace it with clean energy. Why is fossil fuel use becoming undesirable?
9. Mercury is not particularly toxic as a metal but it is very dangerous in its organic form. How does mercury convert from the metal to the organic form?
10. In what two ways does deforestation contribute to air pollution?
People in developing countries often do not have laws to protect the air that they breathe. The World Health Organization estimates that 22 million people die each year from complications caused by air pollution. Even in the United States, more than 120 million Americans live in areas where the air is considered unhealthy. This lesson looks at the human health and environmental problems caused by different types of air pollution.
All air pollutants cause some damage to living creatures and the environment. Different types of pollutants cause different types of harm.
Particulates reduce visibility. In the western United States, people can now ordinarily see only about 100 to 150 kilometers (60 to 90 miles), which is one-half to two-thirds the natural (pre-pollution) range on a clear day. In the East, people can only see about 40 to 60 kilometers (25-35 miles), about one-fifth the distance they could see without any air pollution ( Figure below ).

Figure 22.12
Smog in New York City.
Particulates reduce the amount of sunshine that reaches the ground, which may reduce photosynthesis. Since particulates form the nucleus for raindrops, snowflakes, or other forms of precipitation, precipitation may increase when particulates are high. An increase in particles in the air seems to increase the number of raindrops, but often decreases their size.
By reducing sunshine, particulates can also alter air temperature. In the three days after the terrorists attacks on September 11, 2001, jet airplanes did not fly over the United States. Without the gases from jet contrails blocking sunlight, air temperature increased 1°C (1.8°F) across the United States ( Figure below ) . Imagine how much all of the sources of particulates combine to reduce temperatures. What might this effect be on global warming?

Figure 22.13
Jet contrails block sunlight.
Ozone damages some plants. Since ozone effects accumulate, plants that live a long time show the most damage. Some species of trees appear to be the most susceptible. If a forest contains ozone-sensitive trees, they may die out and be replaced by species that are not as easily harmed. This can change an entire ecosystem, because animals and plants may not be able to survive without the habitats created by the native trees.
Some crop plants show ozone damage ( Figure below ). When exposed to ozone, spinach leaves become spotted. Soybeans and other crops have reduced productivity. In developing nations, where getting every last bit of food energy out of the agricultural system is critical, any loss is keenly felt.

Figure 22.14
The spots on this leaf are caused by ozone damage.
Oxide air pollutants also damage the environment. NO 2 is a toxic, orange-brown colored gas that gives air a distinctive orange color and an unpleasant odor. Nitrogen and sulfur-oxides in the atmosphere create acids that fall as acid rain.
Human health suffers in locations with high levels of air pollution.
Different pollutants have different health effects:
Many but not all cases of asthma can be linked to air pollution. During the 1996 Olympic Games, Atlanta, Georgia, closed off their downtown to private vehicles. This action decreased ozone levels by 28%. At the same time, there were 40% fewer hospital visits for asthma. Can scientists conclude without a shadow of a doubt that the reduction in ozone caused the reduction in hospital visits? What could they do to make that determination?
Lung cancer among people who have never smoked is around 15% and is increasing. One study showed that the risk of being afflicted with lung cancer increases directly with a person’s exposure to air pollution ( Figure below ). The study concluded that no level of air pollution should be considered safe. Exposure to smog also increased the risk of dying from any cause, including heart disease.

Figure 22.15
A lung tumor is highlighted in this chest x-ray.
One study found that in the United States, children develop asthma at more than twice the rate of two decades ago and at four times the rate in Canada. Adults also suffer from air pollution-related illnesses that include lung disease, heart disease, lung cancer, and weakened immune systems. The asthma rate worldwide is rising 20% to 50% every decade.
Do you know why you are only supposed to eat large predatory fish like tuna infrequently? It is because of the bioaccumulation of mercury in those species.
Some pollutants remain in an organism throughout its life, a phenomenon called bioaccumulation. In this process, an organism accumulates the entire amount of a toxic compound that it consumes over its lifetime. Not all substances bioaccumulate. Can you name one that does not? Aspirin does not bioaccumulate; if it did, a person would quickly accumulate a toxic amount in her body. Compounds that bioaccumulate are usually stored in the organism’s fat.
Mercury is released into the atmosphere when coal is burned ( Figure below ). But breathing the mercury is not harmful. In the atmosphere, the mercury forms small droplets that are deposited in water or sediments.
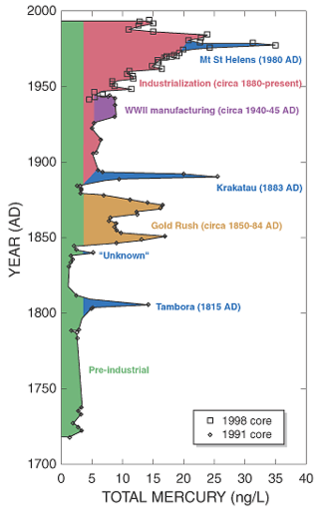
Figure 22.16
Historic increases of mercury in the atmosphere: blue is volcanic eruptions; brown, purple, and pink are human-caused. The red region shows the effect of industrialization on atmospheric mercury.
In the sediments, bacteria convert the droplets to the hazardous compound methyl mercury. Bacteria and plankton store all of the mercury from all of the seawater they ingest ( Figure below ). A small fish that eats bacteria and plankton accumulates all of the mercury from all of the tiny creatures it eats over its lifetime. A big fish accumulates all of the mercury from all of the small fish it eats over its lifetime. For a tuna at the top of the food chain, that’s a lot of mercury.

Figure 22.17
Methyl mercury bioaccumulates up the food chain.
So tuna pose a health hazard to anything that eats them because their bodies are so high in mercury. This is why the government recommends limits on the amount of tuna that people eat. Limiting intake of large predatory fish is especially important for children and pregnant women. If the mercury just stayed in a person’s fat, it would not be harmful, but that fat is used when a woman is pregnant or nursing a baby. A person will also get the mercury into her system when she (or he) burns the fat while losing weight.
Methyl mercury poisoning can cause nervous system or brain damage, especially in infants and children. Children may experience brain damage or developmental delays ( Figure below ). Like mercury, other metals and VOCS can bioaccumulate, causing harm to animals and people high on the food chain.

Figure 22.18
The phrase
Acid rain is caused by sulfur and nitrogen oxides emanating from power plants or metal refineries. The smokestacks have been built tall so that pollutants don’t sit over cities ( Figure below ).

Figure 22.19
Tall smokestacks allow the emissions to rise high into the atmosphere and travel up to 1,000 km (600 miles) downwind.
As they move, these pollutants combine with water vapor to form sulfuric and nitric acids. The acid droplets form acid fog, rain, snow, or they may be deposited dry. Most typical is acid rain ( Figure below ).

Figure 22.20
Pollutants are deposited dry or in precipitation.
Acid rain water is more acidic than normal rain water. Acidity is measured on the pH scale . Lower numbers are more acidic and higher numbers are less acidic (also called more alkaline ) ( Figure below ). Natural rain is somewhat acidic with a pH of 5.6; acid rain must have a pH of less than 5.0. A small change in pH represents a large change in acidity: rain with a pH of 4.6 is 10 times more acidic than normal rain (with a pH of 5.6). Rain with a pH of 3.6 is 100 times more acidic.

Figure 22.21
A pH scale goes from 1 to 14; numbers are shown with the pH of some common substances. A value of 7 is neutral. The strongest acids are at the low end of the scale and the strongest bases are at the high end.
Regions with a lot of coal-burning power plants have the most acidic rain. The acidity of average rainwater in the northeastern United States has fallen to between 4.0 and 4.6. Acid fog has even lower pH with an average of around 3.4. One fog in Southern California in 1986 had a pH of 1.7, equal to toilet-bowl cleaner.
In arid climates, such as in Southern California, acids deposit on the ground dry. Acid precipitation ends up on the land surface and in water bodies. Some forest soils in the northeast are five to ten times more acidic than they were two or three decades ago. Acid droplets move down through acidic soils to lower the pH of streams and lakes even more. Acids strip soil of metals and nutrients, which collect in streams and lakes. As a result, stripped soils may no longer provide the nutrients that native plants need.
Acid rain takes a toll on ecosystems ( Figure below ). Plants that are exposed to acids become weak and are more likely to be damaged by bad weather, insect pests, or disease. Snails die in acid soils, so songbirds do not have as much food to eat. Young birds and mammals do not build bones as well and may not be as strong. Eggshells may also be weak and break more easily.

Figure 22.22
Acid rain has killed trees in this forest in the Czech Republic.
As lakes become acidic, organisms die off. No fish can live if the pH drops below 4.5. Organic material cannot decay, and mosses take over the lake. Wildlife that depend on the lake for drinking water suffer population declines.
Crops are damaged by acid rain. This is most noticeable in poor nations where people can’t afford to fix the problems with fertilizers or other technology.
Acid rain damages cultural monuments like buildings and statues. These include the U.S. Capital and many buildings in Europe, such as Westminster Abbey.( Figure below ).

Figure 22.23
A statue damaged by acid rain
Carbonate rocks neutralize acids and so some regions do not suffer the effects of acid rain nearly as much. Limestone in the midwestern United States protects the area. One reason that the northeastern United States is so vulnerable to acid rain damage is that the rocks are not carbonates.
Because pollutants can travel so far, much of the acid rain that falls hurts states or nations other than ones where the pollutants were released. All the rain that falls in Sweden is acidic and fish in lakes all over the country are dying. The pollutants come from the United Kingdom and Western Europe, which are now working to decrease their emissions. Canada also suffers from acid rain that originates in the United States, a problem that is also improving. Southeast Asia is experiencing more acid rain between nations as the region industrializes.
At this point you might be asking yourself, “Is ozone bad or is ozone good?” There is no simple answer to that question: It depends on where the ozone is located ( Figure below ).

Figure 22.24
(1) Solar energy breaks apart oxygen molecules into two oxygen atoms. (2) Ozone forms when oxygen atoms bond together as O
Human-made chemicals are breaking ozone molecules in the ozone layer. Chlorofluorocarbons (CFCs) are the most common but there are others including halons, methyl bromide, carbon tetrachloride, and methyl chloroform. CFCs were once widely used because they are cheap, nontoxic, nonflammable, and non-reactive. They were used as spray-can propellants, refrigerants, and in many other products.
Once they are released into the air, CFCs float up to the stratosphere. Air currents move them toward the poles. In the winter, they freeze onto nitric acid molecules in polar stratospheric clouds (PSC) ( Figure below ). In the spring, the sun’s warmth starts the air moving, and ultraviolet light breaks the CFCs apart. The chlorine atom floats away and attaches to one of the oxygen atoms on an ozone molecule. The chlorine pulls the oxygen atom away, leaving behind an O 2 molecule, which provides no UV protection. The chlorine then releases the oxygen atom and moves on to destroy another ozone molecule. One CFC molecule can destroy as many as 100,000 ozone molecules.

Figure 22.25
PSCs form only where the stratosphere is coldest, and are most common above Antarctica in the wintertime. PSCs are needed for stratospheric ozone to be destroyed.
Ozone destruction creates the ozone hole where the layer is dangerously thin ( Figure below ). As air circulates over Antarctica in the spring, the ozone hole expands northward over the southern continents, including Australia, New Zealand, southern South America, and southern Africa. UV levels may rise as much as 20% beneath the ozone hole. The hole was first measured in 1981 when it was 2 million square km (900,000 square miles). The 2006 hole was the largest ever observed at 28 million square km (11.4 million square miles). The size of the ozone hole each year depends on many factors, including whether conditions are right for the formation of PSCs.
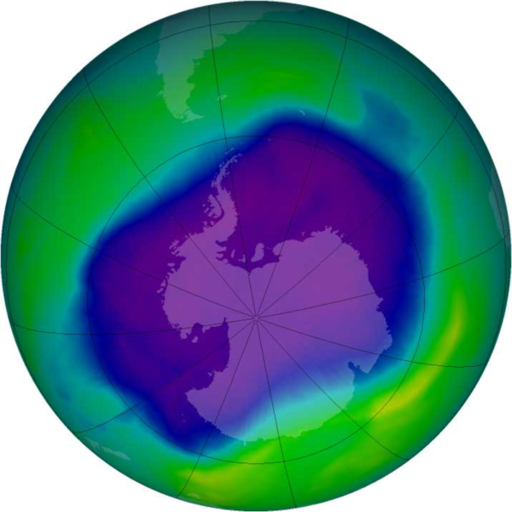
Figure 22.26
The September 2006 ozone hole, the largest observed (through 2009). Blue and purple colors show particularly low levels of ozone.
Find out how the ozone hole forms and view the hole over time on this National Geographic video: http://news.nationalgeographic.com/news/2008/11/081103-ozone-video-vin.html
Ozone loss also occurs over the North Polar Region, but it is not enough for scientists to call it a hole. Why do you think there is less ozone loss over the North Pole area? The region of low ozone levels is small because the atmosphere is not as cold and PSCs do not form as readily. Still, springtime ozone levels are relatively low. This low moves south over some of the world’s most populated areas in Europe, North America, and Asia. At 40 o N, the latitude of New York City, UV-B has increased about 4% per decade since 1978. At 55 o N, the approximate latitude of Moscow and Copenhagen, the increase has been 6.8% per decade since 1978.
This video explains an importance of the stratospheric ozone layer to life on Earth
(8c)
:
http://www.youtube.com/watch?v=I1wrEvc2URE&feature=related
(1:52).
This NASA video discusses the ingredients of ozone depletion of Antarctica and the future of the ozone hole, including the effect of climate change
(8c)
:
http://www.youtube.com/watch?v=qUfVMogIdr8&feature=related
(2:20).
Ozone losses on human health and environment include:
When the problem with ozone depletion was recognized, world leaders took action. CFCs were banned in spray cans in some nations in 1978. The greatest production of CFCs was in 1986, but it has declined since then. This will be discussed more in the next lesson.
1. Why is visibility so reduced in the United States?
2. Why do health recommendations suggest that people limit the amount of tuna they eat?
3. Why might ozone pollution or acid rain change an entire ecosystem?
4. Why does air pollution cause problems in developing nations more than in developed ones?
5. Why are children more vulnerable to the effects of air pollutants than adults?
6. Describe bioaccumulation.
7. How does pollution indirectly kill or harm plants?
8. What do you think the effect is of jet airplanes on global warming?
9. Why is air pollution a local, regional, and global problem?
10. How do CFCs deplete the ozone layer?
The Clean Air Act of 1970 and the amendments since then have done a great job in requiring people to clean up the air over the United States. Emissions of the six major pollutants regulated by the Clean Air Act, carbon monoxide, lead, nitrous oxides, ozone, sulfur dioxide, and particulates, have decreased by more than 50%. Cars, power plants, and factories individually release less pollution than they did in the mid-20th century. But there are many more cars, power plants, and factories. Many pollutants are still being released and some substances have been found to be pollutants that were not known to be pollutants in the past. There is still much work to be done to continue to clean up the air.
How can air pollution be reduced? Using less fossil fuel is one way to lessen pollution. Some examples of ways to conserve fossil fuels are:
All these actions reduce the amount of energy that power plants need to produce.
Developing alternative energy sources is important. Think back to the chapter, Earth's Energy. What are some of the problems facing wider adoption of alternative energy sources?
Sometimes technological approaches are what is needed.
National Geographic videos exploring energy conservation are found in Environment Videos, Energy: http://video.nationalgeographic.com/video/player/environment/.
What you can do to your home to help reduce energy use: http://www.youtube.com/watch?v=6h8QjZvcp0I&feature=related A very simple thing you can do to conserve energy is discussed in “This Bulb,” http://www.youtube.com/watch?v=FvOBHMb6Cqc
Reducing air pollution from vehicles can be done in a number of ways.

Figure 22.27
Catalytic converters are placed on modern cars in the United States.
A plug-in hybrid is plugged into an electricity source when it is not in use, perhaps in a garage, to make sure that the battery is charged. Plug-in hybrids run for a longer time on electricity and so are less polluting than regular hybrids. Plug-in hybrids are beginning to become available in 2010.

Figure 22.28
A hydrogen fuel-cell car looks like a gasoline-powered car.
Pollutants are removed from the exhaust streams of power plants and industrial plants before they enter the atmosphere. Particulates can be filtered out, and sulfur and nitric oxides can be broken down by catalysts. Removing these oxides reduces the pollutants that cause acid rain.
Particles are relatively easy to remove from emissions by using motion or electricity to separate particles from the gases. Scrubbers remove particles and waste gases from exhaust using liquids or neutralizing materials ( Figure below ). Gases, such as nitrogen oxides, can be broken down at very high temperatures.

Figure 22.29
Scrubbers remove particles and waste gases from exhaust.
Gasification is a developing technology. In gasification, coal (rarely is another organic material used) is heated to extremely high temperatures to create syngas, which is then filtered and the energy goes on to drive a generator. Syngas releases about 80% less pollution than regular coal plants, and greenhouse gases are also lower. Clean coal plants do not need scrubbers or other pollution control devices. Although the technology is ready, clean coal plants are more expensive to construct and operate. Also, heating the coal to high enough temperatures uses a great deal of energy, so the technology is not energy efficient. In addition, large amounts of the greenhouse gas CO 2 are still released with clean coal technology. Nonetheless, a few of these plants are operating in the United States and around the world.
One success story in reducing pollutants that harm the atmosphere concerns ozone-destroying chemicals. In 1973, scientists calculated that CFCs could reach the stratosphere and break apart. This would release chlorine atoms, which would then destroy ozone. Based only on their calculations, the United States and most Scandinavian countries banned CFCs in spray cans in 1978.
More confirmation that CFCs break down ozone was needed before more was done to reduce production of ozone-destroying chemicals. In 1985, members of the British Antarctic Survey reported that a 50% reduction in the ozone layer had been found over Antarctica in the previous three springs. Two years later, the "Montreal Protocol on Substances that Deplete the Ozone Layer" was ratified by nations all over the world.
The Montreal Protocol controls the production and consumption of 96 chemicals that damage the ozone layer ( Figure below ). Hazardous substances are phased out first by developed nations and one decade later by developing nations. More hazardous substances are phased out more quickly. CFCs have been mostly phased out since 1995, although were used in developing nations until 2010. Some of the less hazardous substances will not be phased out until 2030. The Protocol also requires that wealthier nations donate money to develop technologies that will replace these chemicals.

Figure 22.30
Ozone levels over North America decreased between 1974 and 2009. Models of the future predict what ozone levels would have been if CFCs were not being phased out. Warmer colors indicate more ozone.
Had CFCs not been phased out, by 2050 there would have been 10 times more skin cancer cases than in 1980. The result would have been about 20 million more cases of skin cancer in the United States and 130 million cases globally.
Since CFCs take many years to reach the stratosphere and they can survive there a long time before they break down, the ozone hole will probably continue to grow for some time before it begins to shrink. The ozone layer will reach the same levels it had before 1980 around 2068 and 1950 levels in one or two centuries.
Climate scientists agree that climate change is a global problem that must be attacked by a unified world with a single goal. All nations must come together to reduce greenhouse gas emissions. However, getting nations to agree on anything has proven to be difficult. A few ideas have been proposed and in some nations are being enacted.
The first attempt to cap greenhouse gas emissions was the Kyoto Protocol, which climate scientists agree did not do enough in terms of cutting emissions or in getting nations to participate. The Kyoto Protocol set up a cap-and-trade system . Cap-and-trade provides a monetary incentive for nations to develop technologies that will reduce emissions and to conserve energy. Some states and cities within the United States have begun their own cap-and-trade systems.
The United Nations Climate Change Conference meets in a different location annually. Although recommendations are made each year, the group has not gotten the nations to sign on to a binding agreement. By doing nothing we are doing something - continuing to raise greenhouse gas levels and failing to prepare for the coming environmental changes.
How bad could a few degrees be? National Geographic has a set of videos about what to expect if temperature rises by each of these amounts by degree Celsius.
The easiest and quickest way is to reduce greenhouse gas emissions is to increase energy efficiency. One effective way to encourage efficiency is financial. A carbon tax can be placed on CO 2 emissions to encourage conservation. The tax would be placed on gasoline, carbon dioxide emitted by factories, and home energy bills so people or businesses that emit more carbon would pay more money. This would encourage conservation since when people purchase a new car, for example, they would be more likely to purchase an energy efficient model. The money from the carbon tax would be used for research into alternative energy sources. All plans for a carbon tax allow a tax credit for people who cannot afford to pay more for energy so that they do not suffer unfairly.
New technologies can be developed, such as renewable sources that were discussed in the chapter,Earth's Energy. Biofuels can replace gasoline in vehicles, but they must be developed sensibly ( Figure below ). So far much of the biofuel is produced from crops such as corn. But when food crops are used for fuel, the price of food goes up. Modern agriculture is also extremely reliant on fossil fuels for pesticides, fertilizers, and the work of farming. This means that not much energy is gained from using a biofuel over using the fossil fuels directly. More promising crops for biofuels are now being researched. Surprisingly, algae is being investigated as a source of fuel! The algae can be grown in areas that are not useful for agriculture, and it also contains much more usable oil than crops such as corn.

Figure 22.31
A bus that runs on soybean oil shows the potential of biofuels.
If climate change becomes bad enough, people can attempt to remove greenhouse gases from the atmosphere after they are emitted. Carbon sequestration occurs naturally when carbon dioxide is removed from the atmosphere by trees in a forest. One way to remove carbon would be to plant more trees, but unfortunately, more forest land is currently being lost than gained.
Carbon can also be artificially sequestered. For example, carbon can be captured from the emissions from gasification plants and then stored underground in salt layers or coal seams. While some small sequestration projects are in development, large-scale sequestration has not yet been attempted.
This type of carbon capture and sequestration comes under the heading of geoengineering. There are many other fascinating ideas in geoengineering that people have proposed that are worth looking at. One wild example is to shadow the planet with large orbiting objects. A large mirror in orbit could reflect about 2% of incoming solar radiation back into space. These sorts of solutions would be expensive in cost and energy.
Just as individuals can diminish other types of air pollution, people can fight global warming by conserving energy. Also, people can become involved in local, regional, and national efforts to make sound choices on energy policy.
1. Since the Clean Air Act was passed in 1970, why is the air still not clean?
2. What are some ways that you can conserve energy?
3. How does reducing air pollutants, as described in the Clean Air Act of 1970, affect greenhouse gas emissions?
4. What has to be done before alternative energy sources can replace fossil fuels?
5. What are catalytic converters?
6. Why are hybrid vehicles more energy efficient than regular vehicles powered by internal combustion engines?
7. Why aren’t fuel-cell vehicles widely available yet?
8. How does a cyclone reduce particulate pollution?
9. How can coal power be made so that it has nearly zero carbon contribution to the atmosphere?
10. Why is it that the ozone hole will not be healed for several decades?
11. Many people think that biofuels are the solution to a lot of the problems of climate change, but others disagree. What requirements would biofuels have to meet if they were to be really effective at replacing gasoline in motor vehicles?
Opening image courtesy of MODIS Land Rapid Response Team and NASA, http://earthobservatory.nasa.gov/IOTD/view.php?id=6344 , and is in the public domain.