Brains (or Not) in Animals from Sponges to Us
“A sponge sees everything? A sponge sees nothing.”
—Lawrence Tierney, actor
Specific sensory milestones arose as the tree of life’s branches and twigs spread out. The very earliest milestones include the evolution of cell-to-cell communication through molecular processes such as quorum sensing. These milestones also included the evolution of sets of genes that perform specific functions in cells called molecular toolboxes for the transfer of information through single cells. Multicellular organisms that evolved later communicated between cells using processes like signal transduction. Once multicellularity arose, more options for how cells communicate opened up, and this development heavily influenced the biology of our direct eukaryotic ancestors. These milestones include action potential, synapses, differentiated nerve cells, neural webs, neural nuclei, and specialized nervous systems. And although these milestones, all reached in the evolution of our lineage, might seem like a streak toward perfection, they are mere stopping points. Other lineages were evolving, too, and our neural milestones more than likely mean nothing to these other lineages.
Charles Darwin likened organismal relations to what he called “the great tree of life” to represent the interconnectedness of all organisms on the planet through common ancestry and divergence. The great tree of life has become a common tool for biologists to trace the evolution of traits. By following the divergence events of organisms and of the traits that might be considered neural, we can get a pretty precise view of how brains in general, and our brain specifically, evolved.
The very early branches in the animal part of the tree of life include sponges and a small, pancake-shaped animal called placozoa. Some researchers think that all sponges are related to one another through a common ancestor to the exclusion of all other animals. Others suggest that there are two great lineages of sponges. What is certain is that sponges have only eight or so cell types, and none of them are neural, so they do not have brains. Placozoa, for their part, have four cell types, and none of them are neural, so they likewise cannot have brains. But what is interesting about these two early branching animals is that they have many of the genes necessary for making a neural cell but simply don’t use them for neural cells. Apparently, they have found other uses for the genes that the rest of animals use to make nerve cells. Both sponges and placozoa can sense environmental changes and respond to them, making these organisms sensing animals without brains or a nervous system. Sponges can “sneeze” by sensing particles that they come into contact with. Their sneezes are spectacular events, in which the sneeze builds up over a period of an hour or so and needs to be viewed in time lapse. Placozoa can forage for nourishment and are very efficient at detecting food, and this without a brain.
Comb jellies, or ctenophores, are incredibly interesting animals. They look darn cool, and they have been proposed as the first animal group to diverge from the single-celled eukaryotic ancestor of all animals. They have several cell types, one of them neural, as well as a nervous system, but no centralized set of cells that forms a brain. If ctenophores are the first animal group to branch off the animal tree of life, then this opens some very interesting possibilities. One is that nervous systems are convergent in comb jellies and other animals (except for sponges and placozoa). The alternative is that nervous systems can be lost relatively easily, such as in the lineage that gave rise to sponges and placozoa. This example shows how using the concept of a common ancestor and a tree of life allows us to dissect the potential major changes of the nervous system that occurred in the divergence of animal nervous systems.
The physiological response of cells where the electrical charge rises and falls in a cell is called an action potential. More complex animals will maintain an electrical difference between the environments that are external and internal to their cells. A cell membrane regulates the difference. The membrane keeps the voltage inside the cell at minus 70 millivolts relative to the outside, which is called the resting state. When the cell is stimulated in a specific way (see text), the voltage difference outside and inside the cell rises by about 110 millivolts, so there is a new difference of plus 40 millivolts. The charge inside the cell rises as a result. The voltage then falls rapidly to well below its resting state before settling back to its resting state of 70 millivolts. The rise and fall of an action potential occurs in a very consistent manner and is how nerve impulses or electrical signals get transmitted from cell to cell throughout the nervous system. Action potentials are also important in communication of the nervous system with the sensory and motor systems.
But the way neural cell communication works is common to these lower “squishy” animals and our lineage. The transfer of an electrical charge through action potential seems to be an ancient animal invention.
After ctenophores, sponges, and placozoa branched off, the common ancestor of cnidarians and the group we belong to, called bilateria, diverged. Any organism that has symmetry across a midline drawn down the center of the head to the end of the body is called a bilaterian. The group most closely related to bilateria are the cnidarians, which includes jellyfish, hydroids, corals, and an odd group of organisms called cubozoans or box-jellies because of their boxlike shape. Cnidarians have cells that can be called neural cells and a nervous system best described as a net, but no centralized set of nerve cells that could be called a brain.
Bilateria are divided into two big groups: the protostomes (for example, insects and mollusks) and the deuterostomes (which includes humans). Brains are found in organisms in both major groups of bilateria, so scientists generally consider the brain a bilaterian invention. Most biologists would describe the protostome and deuterostome brains as the same by using the term “homologous.” Darwin first made this meaningful by pointing out that homologous traits are those that occur as a result of direct common ancestry. So bird wings and bat wings are not homologous, because there are many mammals without wings that disrupt the common ancestry of the two kinds of vertebrates. Any traits present in organisms that are not connected by direct common ancestry are considered “analogous” and have arisen as a result of convergence.
But is the brain really a bilaterian invention? The overall structure of protostome brains can be argued to be structurally different from advanced deuterostome brains. The common ancestor of bilateria had a neural network that reached most parts of its body, and in the anterior region, a patch of neural cells that neurobiologists would call a brain existed. Some neuroanatomists suggest that the common ancestor of bilateria even had a complex structure with three parts to it.
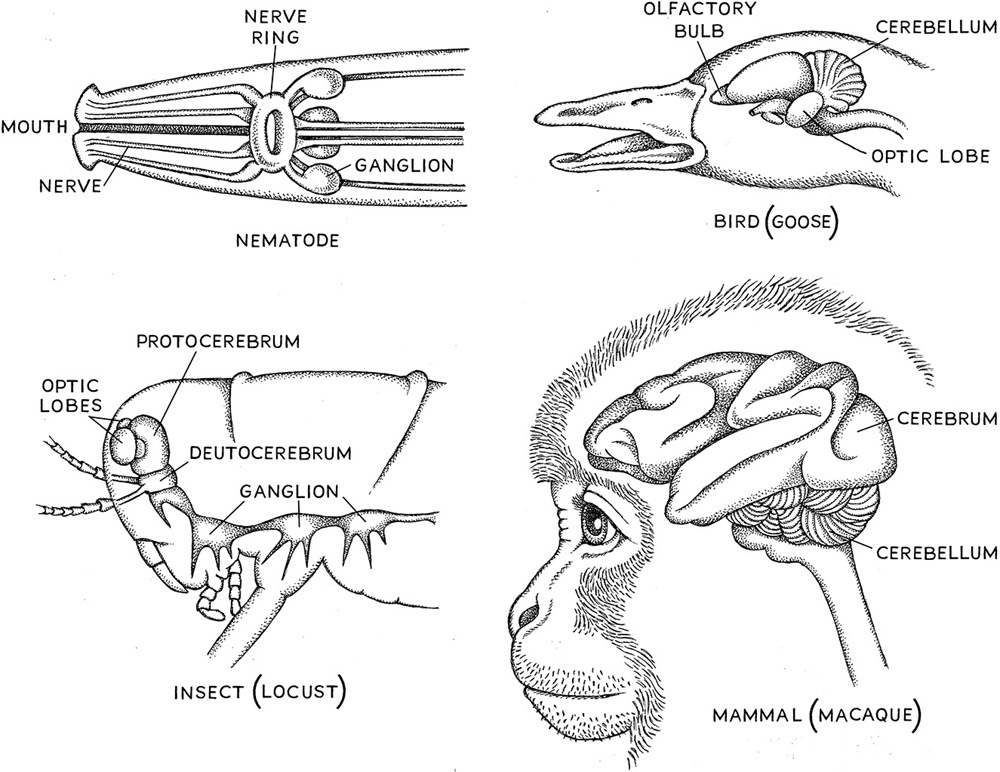
It is possible, though, that the patch of neural cells at the anterior end of the neural tract that traversed the body in the ancestral bilaterian took two different pathways when deuterostomes and protostomes diverged. I like to call the protostome brain a “prain” to differentiate it from what looks to me like a differently evolved and structured deuterostome brain. Although we can use insects as model systems to decipher how sensory information from the outer world is collected and turned into nerve impulses, once the information hits the brain in the case of deuterostomes and the prain in the case of protostomes, the similarities between these two types of neural centers cease. The only satisfying comparisons more than likely stop with the collection of the information by the sense organs and its transfer to the brain or prain, where the information is processed. More than likely, many of the tools in the sensory toolbox existed in the bilaterian ancestor. Since the bilateria diverged from each other these tools were being used diversely as speciation event after speciation event occurred. To be fair, some researchers argue for homology on the basis of another important piece of information. Protostomes like the fruit fly (Drosophila melanogaster) use many of the same genes for neural processing that deuterostomes do, and they also have very similar (but not identical) structures where nerve cells come into contact with each other (called synapses—see box 2.2). But it is an interesting idea to consider that complex brains have evolved at least twice on this planet—once in the protostome lineage and once in the deuterostome lineage (fig. 2.1).
Figure 2.1. Comparison of vertebrate brains (bird and primate) with nematode and insect brains.
If we avoid calling the protostome brain the same kind of brain as ours, or at least not the same triune brain, then we can use the tripartite brain as a heuristic device to make some sense of how the vertebrate brain evolved. The early branching deuterostomes such as the starfish and sea urchins have neural cells, but they are distributed throughout the body with no central cluster of cells to call a brain. Some scientists split hairs and suggest that these organisms, also known as echinoderms, have what is called a distributed brain.
The next group of animals to consider as part of the context for hanging the human brain on the tree of life is a sister group to the echinoderms, the chordates, or phylum Chordata. All organisms in this group have nerve chords. The very earliest branching members are the urochordates and cephalochordates. Urochordates are strange animals that are best represented by the sea squirt, which is placed in a group called tunicates (because they form a tunic or coat of material similar to cellulose to protect themselves as adults). The sea squirt has a larval stage just like some amphibians. In its larval stage, it has what is called a cerebral vesicle, which is a technical way of saying that it has a place for a brain but doesn’t fill it with a brain. Oddly enough, as it develops into an adult, the sea squirt larva feasts on its brain. As a larva it has no external mouth to push food through, and it has to get its nutrition from somewhere. Because as an adult it doesn’t use the tiny clump of nerve cells in the cerebral vesicle, it reabsorbs the tissue so that when it transforms into an adult, it has no brain. It does have what is called a notochord (but no vertebral column—that comes in later in the tree of life). Cephalochordates also have a nerve chord, but again no backbone at all. One of the best representatives of cephalochordates is an organism called the lancelet. It is difficult to make a definitive statement about the existence of a brain in this organism, and in fact anatomists call what could be considered its brain a blister. The blister is literally that, a section of the notochord in the head region that has puffed out a little.
Synapses are contact points that permit one neuron to communicate with another by passing along an electrical signal. The mechanism by which the electric signal occurs is complex (see box 2.1). The cell delivering the electrical charge is called the presynaptic cell, and the one receiving is the postsynaptic cell. Researchers also include the communication from other cell types (such as muscle) to a neuron as being accomplished through synapses.
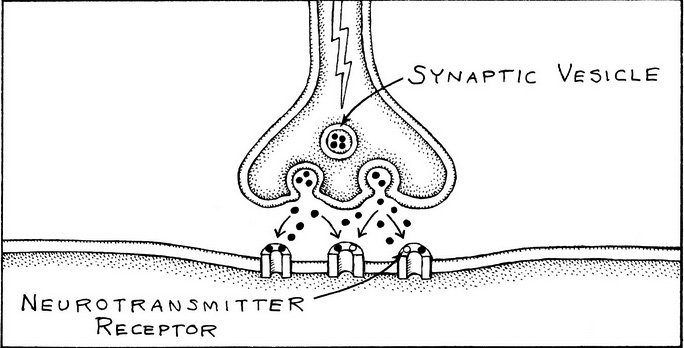
Figure 2.2. The intracellular region of the synapse. The presynaptic cell contains the synaptic vesicle that releases the neurotransmitters, and the postsynaptic cell has the neuroreceptor proteins embedded in the membrane.
Vertebrates have a backbone (or spine) and fully developed nerve chord. Neurobiologists have had a hard time accepting that the vertebrate brain evolved linearly with simple additions of more complex parts. The limbic system and the cortex are two examples of more complex neural real estate that gradually occurred in common ancestors as animals diverged. One would think that more complex structures or processes should evolve from less complex ones. But it is not necessarily true that descendants are always more complex than their ancestors, and it is not true at all that the result of evolution is more complexity. Yet when the starting point is a primitive structure, the only way for it to evolve is to add on. And it seems that the vertebrate brain has evolved by adding layers. Uncovering how these layers were added and when is the problem. Schemes of brain evolution almost always involve accommodating three major brain parts, leading to the image, or heuristic, of a tripartite brain. There are, under some criteria, three major parts of the brain: the stem and cerebellum (also known as the R-complex—“R” for “reptile”), the limbic system, and the cortex. But these divisions are somewhat arbitrary, and this is more than likely why some neuroscientists reject the tripartite sectioning of the brain. However, as a heuristic, using a tripartite model of the brain for vertebrates works fairly well. Even with this disclaimer, there is still controversy as to how the tripartite brain evolved or even whether it is tripartite.
The ancestor of all vertebrates had a very primitive-looking brain with no or very little cortex and a very rudimentary cerebellum. Most of this ancestor’s control of its behavior resided in the stem of the brain and the cerebellum, where very basic bodily functions are controlled, such as heartbeat and breathing. Some basic capacity to respond to the environment also existed in this ancestor’s brain, such as sensing smells, sights, and sounds. This ancestor had what is referred to in the classical tripartite scheme as a reptilian brain. In the classical tripartite brain scheme, the final two layers added are the paleomammalian brain and the neomammalian brain. Most of us who work in evolutionary biology would infer from these names that the next two most important common ancestors, then, are long-extinct mammals (the paleomammal and the neomammal). The problem with this scheme is that it omits birds, which have pretty sophisticated neural ways of dealing with the world and hence brains that are hard to place in the continuum. There is therefore a need to either revise the names of these ancestral brains or assume as we have before that the bird brain that lies beyond the lizard part of the brain is not the same as the mammalian brain. We could rename the reptile brain our inner fish brain and try to move outward on the tree of life. We could also suggest that something like a reptile/bird brain came next, and finally the proto-neo-mammalian brain. The problem with this way of thinking is that fish are not a real group of organisms to some evolutionary biologists. Why? Although the name “fish” is descriptive, it does not define a group of organisms to the exclusion of all others, which is the way taxonomists usually name. The solution to this problem is either to name the major lineages of fish something else or to name all descendants of the common ancestor of what we call fish, fish. And this would include humans as fish. At some point, we need to know when to cut bait and when to fish. So let’s cut bait on this scheme and look at the triune brain from a different perspective.
If one were to review all the major names that neurobiologists have given these three parts of the brain, one would encounter both a certain arbitrariness and a high degree of subjectivity. All are dependent on which species have which structures. What is clear from this exercise is that many scientists really like the idea of there being three major parts of the brain (fig. 2.3).
Consider the divergence of vertebrates and how this might add some objectivity to the discussion. After the major anatomical structures at the base of the brain evolved in the common ancestor of fish, amphibians, reptiles, birds, and mammals (the inner fish brain), the next major common ancestor that evolved was one giving rise to all higher vertebrates (reptiles, birds, and mammals), and this ancestor added a layer of the brain to accommodate more complex ways of processing the signals from the senses gleaned from the environment (our reptile brain). This led to the development of an inner region of the brain that includes what is commonly called the limbic system. This way of thinking is a reasonable way to keep a triune brain scheme alive, since all of the abovementioned vertebrates have a limbic system. The limbic system then started to undertake some very interesting tasks with respect how this ancestor reacted to the environment. The final addition was the cortex, which expanded in various ways in the descendants of this common ancestor. It is significant that the neocortex changes in specific ways in the descendants of this common ancestor, as evidenced by the expansion of the neocortex in birds and mammals and the lack of expansion in reptiles. In short, the neocortex of birds is a different kind of cortex expansion than the neocortex expansion in mammals.
Figure 2.3. The triune brain.
What this all means is that a complex limbic system probably arose in the common ancestor of birds, reptiles, and mammals. But so did a tiny primordial cortex. It is difficult to tease apart the possibility of two ancestors (an ancestor with a tiny primordial cortex and no limbic system or an ancestor without a tiny cortex and a limbic system), and so even though the function and anatomy of the brain appear tripartite, the additions cannot be interpreted as sequential. Part of the problem resides in how birds are related to other higher vertebrates. And again, birds are probably why neurobiologists who disdain the layering tripartite brain scheme do so. But two other kinds of animals also disturb this scheme: turtles and amphibians. Turtles have a thalamic reticular nucleus, a clump of nerve cells around a part of the limbic system called the thalamus, as do other organisms with a limbic system, but it is not as well defined, nor does it do the same things as the bird or mammalian thalamic reticular nuclei. The other critical class, amphibians, has some structures reminiscent of a limbic system, but they are not as well partitioned as even the most primitive reptile limbic systems. To summarize, the heuristic value of a triune brain is important. So, do we stop thinking in threes, or do we use the tripartite brain as a nice device to make sense of a very complex process? I think as long as we realize the limitations of the tripartite brain and don’t try to force analogous structures as being homologous, then the pathway of using the tripartite brain as a heuristic is a good path to go down.
How did a complex brain that integrates sensory information from the outer world evolve? Modern biologists are careful not to attribute too much of what we see in nature as having evolved through natural selection. Richard Lewontin and Stephen Jay Gould in the late 1970s pointed out that much of evolutionary biology, notably human evolutionary biology, consisted of “just-so stories.” They named this the Panglossian Paradigm, after Dr. Pangloss in Voltaire’s novel Candide. Dr. Pangloss saw purpose in everything (“All is for the best”), and so the adaptationist program that Lewontin and Gould saw was so prevalent in biology at the time was aptly named.
I want to simplify the potential of natural selection in the evolution of the integration of senses with perception. But keep in mind that not all traits we see in nature are the result of natural selection. In addition, evolution does not strive toward perfection, as the ranges of our senses suggest. Quite to the contrary, the evolutionary process simply finds the best solution it can to an environmental challenge, and hence many of the characteristics we see in nature are not perfect solutions but rather stopgaps that rapidly and efficiently solve a challenge from the environment.
Let’s think in threes one more time and oversimplify the environmental encounters organisms have into three basic categories that all organisms deal with. The idea comes from the fertile mind of a paleontologist friend of mine. He was a young graduate student taking a course from me while I was at Yale University as an assistant professor. In his simplistic way of thinking he came up with a probably more than oversimplistic scheme for how animals survive the challenges of the natural world. His scheme started with the animal coming into contact with another organism. This prompts the observer animal to place the intruder organism into one of three bins that will enhance or increase the fitness of the species. The first is easy. If the intruder is a fellow species member of the opposite sex, then it is placed into the “mate bin” (as in, “I mate with that”). If it is something that the observer judges as dangerous, then the intruder usually goes into the “run-away bin” (as in, “I run away from that”). The last bin is the “eat bin” (as in “I eat that”).
Now, of course, an organism will refine its interpretation of what goes into these bins as it gains experience. For instance, our Homo sapiens ancestors altered their criteria for the mate bin so that it didn’t matter if the intruder was the same species. If the intruder had two legs and stood upright, then it went into the mate bin, as indicated by genome sequencing evidence indicating that our species possibly interbred with archaic humans like Neanderthals. The run-away bin is probably the trickiest, because intruders larger than the observer often aren’t dangerous and may even be beneficial, and those smaller than the observer often can be as dangerous, or more so, than large, vicious intruders. In addition, even if the interloper is of the same species, the observer might still want to run away from it. The last bin, the eat bin, is also a difficult one, because actually smelling or tasting the interloper, or at the very least getting a good look at it, is often required to make a decision. The faster and more efficiently organisms place other living creatures in their immediate environment into these three bins, the more likely they are to survive and to pass their genes to the next generation. Populations evolve, but individuals do not (individuals live and die, but they do not evolve). The fact that populations evolve means that for the three categories of binning experiences, there is variation in a population in how the members of the population categorize. Variation is the stuff that natural selection works on. The variation simply comes from the genetic makeup of the population where mutations can arise every so often that produce the variation. The variation would appear in the precision with which organisms encountered are binned and the amount of time it takes to bin and react to a challenge. If an individual in a population is imprecise or slow in binning something, it loses! It is gone, and so are the genes it could have passed to the next generation’s population.
Although the preceding is overly simplistic, the three bins are probably real in some basic way. Of course, there are other bins, and there is leakage from one bin into another. But the point is to make clear that our brains are continually making interpretations such as the purported binning of other organisms I describe (including encounters with plants and microbes), and they have evolved to do it very rapidly. In fact, slight changes in the reaction time to some of the binning decisions organisms make can be the difference between surviving or not. This overly simple example also might make it look like natural selection is the only thing at work in how nature works. As discussed earlier, it is important to avoid the Panglossian view of nature; chance plays a huge role in how evolution works and indeed how these three bins might evolve in different species.
Charles Darwin focused most of his intellectual energy on natural selection, and thankfully he did, because he was the first (along with Alfred Russel Wallace) to come up with a mechanism by which evolution could work. He even called his 1859 book On the Origin of Species, one long argument for the existence of evolution by natural selection. This view of how life on the planet evolved was a pervasive part of the evolutionary paradigm until the 1960s and 1970s, when Motoo Kimura and others began to infuse into evolutionary biology that random factors are also involved in driving organismal change on the planet. This force of nature is called genetic drift.
The idea behind genetic drift comes from probability theory and suggests that evolutionary processes are like sampling problems. In any sampling process, there is a finite probability that a biased sample will result. In most cases, the finite probability is tiny compared to the force of natural selection, but in some cases sampling drift, as it is called, can occur with high probability. These cases occur when populations are very small. Think of it this way. If I were to bet you that I could flip one hundred heads in row with a fair coin, it would be a terrible bet for me almost every time I would make it. The probability of my flipping one hundred heads in row, while finite, is very small. But if I bet you I could flip a coin and get two heads in a row, that is a much, much better bet for me. Researchers easily started to see genetic or sampling drift in numerous natural cases, and its impact on how organisms evolve became integrated with ease. It is now thought that sampling drift and natural selection work in concert to mold genetic and phenotypic variation in nature.
The senses and brains of organisms have not escaped the forces of selection and drift. Moreover, they have been molded by these forces—so much so, in fact, that to leave both drivers of evolution out of the picture would be to miss most of the story.