The Limits of Smell and Taste in Humans
“I find people who devote their whole lives to taste a little strange.”
—Jonathan Safran Foer, author
The variation in our senses is substantive and a good way to investigate how they work in humans. Some of the examples you’re about to read could be straight out of The Guinness Book of World Records and indeed are more than likely listed in that entertaining record of human limits.
Joy Milne can smell Parkinson’s disorder. When randomly handed six T-shirts worn by people with Parkinson’s and six worn by unaffected individuals, she correctly identified who had Parkinson’s for eleven of the twelve shirts. Not bad, but even better, because she actually achieved twelve out of twelve. The one she missed was a false positive: she identified one of the shirts from the control group as belonging to someone with Parkinson’s, and this person was later diagnosed with the disease. The smell of Parkinson’s was heavy on Milne’s mind because her husband was showing more and more extreme symptoms of this devastating neurological disease. She developed her unique ability when her husband’s condition worsened and she noticed that he exuded a musky odor. Sadly, her husband has since died from the symptoms of Parkinson’s disorder. Although one might expect the odor to emanate from the armpits or some other sweaty region of the body, it actually comes from the sebaceous glands of the back, chin, forehead, and neck. These glands secrete a product called sebum that leaves a shiny veneer to the skin in the areas where it is secreted. Apparently, something in the sebum rubs off onto the shirts of the people that Milne smelled.
In another smell-related neurological disorder, evidence presented in 2015 suggests that diagnosing Alzheimer’s disease might benefit from assessing the sense of smell. Alzheimer’s is a brain disorder that usually has a late onset in humans. It is characterized clinically by loss of memory and confusion in early stages and by extreme neurological degeneration in later stages. People with the disorder develop large plaquelike structures in their brains; the plaques are thought to be one of the causes of the neurological problems associated with Alzheimer’s. The disease is the only top-ten deadly illness that has no cure or efficient way to slow it down. It afflicts mostly people of western European ancestry. Forty-four million people worldwide have the disease, including five million Americans. In addition, seven hundred thousand people are estimated to die of the disease each year. Scientists have known since the 1980s that some, though not all, Alzheimer’s patients develop a very poor sense of smell. In addition, mice fed tiny amounts of beta-amyloid, a protein found in the brains of people suffering from Alzheimer’s, showed the formation of plaques in their brains, linking plaque formation to the beta-amyloid intake. When these mice were studied for their olfactory acumen, it was found that they spent more time sniffing around objects than normal mice and also could not remember odors. Given that mice have evolved to use smell to interpret their outer world, the loss of this sense correlated with the beta-amyloid intake is significant, and researchers started to look for similar phenomena in humans. A fascinating outcome of this study in mice is that the researchers next removed the beta-amyloid from the mice and the sense of smell returned.
Correlating the loss or alteration of the ability to smell with Alzheimer’s is tricky. If one tests people with the disease for reduced capacity to smell and sees a correlation, then it wouldn’t mean much for early diagnosis. It is crucial to catch the diminished capacity to smell before the onset of the disorder for loss of smell to be a good diagnostic tool. Researchers at several institutions in New York City developed a survey to do just what is needed to make the loss of smell a diagnostic before the onset of the symptoms. Nearly four hundred older people (averaging eighty years) without Alzheimer’s symptoms were enrolled into a study and given the University of Pennsylvania Smell Identification Test (UPSIT). The test is basically a scratch-and-sniff affair with questions about the odors emanating from the scratch areas of a forty-page test. The answers were tabulated and compared with a panel of answers from four thousand control individuals with normal olfactory capacity. In addition, the 387 participants were examined with magnetic resonance imaging (MRI) to assess the thickness of the entorhinal cortex of the brain. This is the first part of the brain to be affected by the conversion to Alzheimer’s, and so it is a logical place to examine for anatomical changes. The study participants were contacted again in a follow-up four years later; 20 percent showed signs of diminished mental capacity, and nearly 13 percent had developed Alzheimer’s. The trick, then, is to go back to the UPSIT data and the MRI data to see if either correlate with the development of Alzheimer’s disease.
Surprisingly, low smell-test scores, indicating diminished olfactory capacity, were strongly correlated with the development of Alzheimer’s, whereas the thicker entorhinal cortex in MRIs was not. In another study of eighty-four elderly individuals, the UPSIT test was administered in an attempt to understand the loss of olfaction and its co-occurrence with Alzheimer’s. This time, the research team, instead of taking MRIs of the brain, made positron emission tomography (PET) scans and analyzed the cerebrospinal fluid of these older adults (average age was seventy-one). The PET scan can detect plaques in the brain, and the cerebrospinal fluid can be analyzed biochemically to detect amyloid. Both are diagnostic of Alzheimer’s. In a follow-up six months later, 67 percent of the participants showed signs of cognitive decline. But testing positive for amyloid (using either PET or cerebrospinal fluid) was the better diagnostic. On the other hand, participants who scored low on the UPSIT scale at a particular threshold were three times more prone to develop cognitive decline as those with scores above the threshold. Both studies support the idea that the UPSIT approach could be a good early indicator of the onset of this terrible disease. Low UPSIT scores as a diagnostic tool could lead to much earlier intervention to curb the progression of this debilitating disorder.
Smelling Parkinson’s and not detecting smells well because of Alzheimer’s are excellent examples of the range of human olfaction. In one case (detecting Parkinson’s disease by smell), the individual increased her acumen for olfaction; she became a “supersmeller.” In the other instance, individuals lose the ability to smell. All of this goes on in the nose, which then communicates with the brain. In fact, the information from the nose goes to and is processed in a small region of the brain called the olfactory lobe.
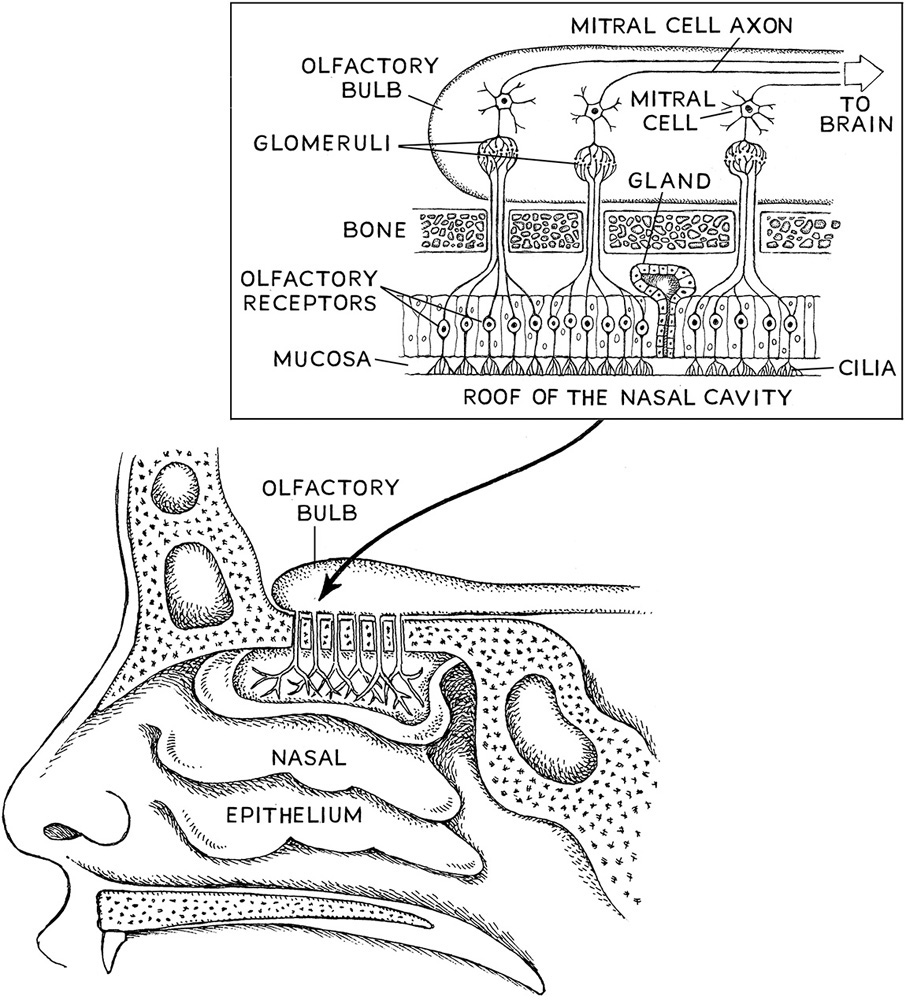
Consider Joy Milne. From photographs, she clearly has an ordinary-looking nose on the outside and more than likely has the same ordinariness on the inside of her nose (fig. 6.1). Externally, she has two nostrils through which the small molecules that constitute odors are inhaled. Internally, she probably has a perfectly normal nasal passage lined by fine hairs to keep out large particles of dust and other junk in the air. Her nasal epithelium, where the initial olfactory action occurs, would also look very normal. Even viewing the nasal epithelium with an electron microscope would show two normal-looking kinds of cell, the first being stem cells that generate new nasal nerve cells throughout her lifetime. The other kind of cell is more complex, but these cells in Milne’s nasal epithelium would look much like the cells in anyone’s nasal epithelium.

Figure 6.1. Structure of the nasal passage and nasal epithelium. Inset: the glomeruli and their connections to the olfactory bulb.
There are millions of nerve cells in Joy Milne’s nasal epithelium. On the end of each of these millions of nerve cells facing outward into the air that is passing through the nasal cavity are small, hairlike structures called cilia. The cilia have a lot of mucus around them and are kind of swimming in it. On the end of the cell pointing inward are projections called axons that run directly to the specific part of the brain called the olfactory bulb. In this bulb, which extends off the brain underneath the frontal cortex and pretty close to the nose, there are twenty-five or so cells that bundle the axons. Each one of these cells, called mitral cells, can have up to twenty-five thousand axons running through them. Inside the mitral cells there are small microscopic bodies called glomeruli, where the nerve axons congregate to form hubs and ultimately connect the cell to the brain. The glomeruli can bundle multiple axons, and Milne’s glomeruli and olfactory would look pretty normal, too.
But the olfactory bulb isn’t the end of the line for the information bundled into the glomeruli. Neurons travel out of the glomeruli and connect the bulb to the olfactory cortex, which is located in the cerebral cortex at the boundary of this part of the brain with the temporal lobe. It is this pathway from the bulb to the cortex that researchers think is responsible for storing memories about odors. In essence, it is probably where Marcel Proust’s famous madeleine cake memory (box 6.1) resided in his brain. But the information from the cilia travel a little farther into the brain to the orbitofrontal cortex, where the information from olfaction is integrated with other higher brain functions. Although what Joy Milne’s brain looks like is not public information, I would bet that she more than likely has a pretty normal olfactory bulb, olfactory cortex, and orbitofrontal cortex.
So what is different about Joy Milne’s olfactory apparatus that gives her the supersense? Perhaps by looking at the odor itself, that musky smell she detected, we can shed light on her supersense and, in the process, on how the sense of smell works. What causes the Parkinson’s odor is a mystery. The culprit could be a small protein called alpha-synuclein that is important in the expression of Parkinson’s disease. This protein is a whopping 140 amino acids long (which is a couple orders of magnitude larger than most molecules responsible for odors), and its three-dimensional structure resembles an unbent paper clip. Alpha-synuclein is also responsible for loss of smell in people with Parkinson’s because it forms clumps in the olfactory bulb. Because of this clumping in the olfactory bulb and other regions of the brain, it was immediately suspected of being involved in the musky odor that Milne detected. But the odor itself emanates from the sebum and could be some other smaller molecule.
The following quotation from Marcel Proust’s Remembrance of Things Past is the origin of the oft-quoted and referenced madeleine, a small cake baked in the shape of a scallop shell. The madeleine has become a sort of metaphor for stored memories, how vivid and wonderful they can be, and how they can be triggered by simple sensory stimulation such as smell: “She sent out for one of those short, plump little cakes called petites madeleines, which look as though they had been moulded in the fluted scallop of a pilgrim’s shell. And soon, mechanically, weary after a dull day with the prospect of a depressing morrow, I raised to my lips a spoonful of the tea in which I had soaked a morsel of the cake. No sooner had the warm liquid, and the crumbs with it, touched my palate than a shudder ran through my whole body, and I stopped, intent upon the extraordinary changes that were taking place. . . . At once the vicissitudes of life had become indifferent to me, its disasters innocuous, its brevity illusory.”
In fact, musky smells have been used by humans for a long time and are so well understood that most musky-smelling solids or liquids are now made synthetically. More than likely, the first musk was obtained from musk deer. It comes from a sac that looks much like a scrotum on the underside of male deer of this species. (The word “musk” is actually derived from the Sanskrit word for testicle.) Anyone who has been around musk deer or beavers can attest that natural musk is not a pleasant-smelling compound. It needs to be diluted and treated with alcohol for it to attain its pleasant odor—otherwise it is pretty foul-smelling. Many molecules are responsible for musk odor. All are part of the liquid that the musk deer stores and eventually sprays from the musk sac. Most of these molecules contain seventeen or eighteen carbon atoms (as opposed to the huge alpha-synuclein’s more than twelve hundred carbon atoms), sometimes arranged in an irregular ring. They have much smaller and very different shapes than alpha-synuclein.
And the shape of the musk molecule is everything when it comes to how the nerve cells in the nasal epithelium work. Embedded in the membranous part of the cilia in the nasal epithelial nerve cells are relatively large molecules appropriately called odorant receptor proteins. These proteins are securely embedded in the membrane because the protein loops in and out of the membrane seven times. It starts on the outside of the cell membrane and goes in, out, in, out, in, out, and finally back into the inside of the cell. The protein itself has two “business ends”: one on the inside of the cell and one on the outside. The part of the protein that sticks out of the membrane is shaped by the amino acids that are at that end of the protein. And the shapes are unique from one odorant receptor to the next. Remember that mammals have varying numbers of these odorant receptor genes in their genomes. Our species, for instance, has about a thousand total genes, but only about four hundred of them actually work, putting the other six hundred genes in the pseudogene category. So, we have four hundred differently shaped odorant receptor proteins that weave in and out of the membranes of the many cilia in our nasal epithelium, all of them having different shapes sticking out of the nerve cell. Each odorant receptor cell has a unique complement of proteins and hence a unique complement of the different shapes sticking out of the cell. The business end of the protein on the outside of the cell is what recognizes the odorant molecule. But the big question is how.
Most biologists would immediately point out that the musk odorant simply collides with a receptor into which it can fit and with which it can interact with a type of lock and key or, as some call it, a hand-in-glove mechanism. Once the odorant molecule fits in with the odorant receptor protein, this changes the three-dimensional structure of the receptor on the end of the protein inside the cell, which in turn triggers reactions in the interior of the cell.
The biophysicist Luca Turin, for his part, made the interesting and unorthodox suggestion that the odorant molecule (in our case, the musk odorant) does indeed fit into the receptor protein, but instead of changing the conformation of the protein on the interior of the cell, it does something very different. Turin’s idea is based on the vibration of molecules, because the electrons in the atoms of the protein are moving around as part of chemical bonds. Imagine a chemical bond where two atoms share an electron. As the electron moves from one atom to the other as a result of the bond, it will twitch or vibrate. The vibrational theory suggests that when the odorant molecule fits into the receptor, it changes the vibrational property of the protein. This vibrational change allows electron transfer to the receptor. The electron then travels from one end of the receptor to the other, which is the cause in the change of the vibration of the receptor. The electron transfer or change in vibrational frequency eventually triggers a cascade of events in the interior of the nerve cell.
Some researchers and journalists have called this the “swipe card theory,” as opposed to the more orthodox lock-and-key mechanism favored by many olfactory researchers. It is an idea that is actually nearly 150 years old, because an unnamed scientist proposed this hypothesis in 1869 in Scientific American, the premier American science journal at the time. Turin’s role in the resurrection and development of the theory is substantial, however, and has led to the development of some clever experimental approaches (box 6.2). When these tests are done, some of the predictions of the vibrational hypothesis bear out, but some don’t. Although the results of the tests are suggestive, they are very difficult to interpret, as Lesley Voshall and Andreas Keller have pointed out, because the experiments that lent credence to the vibrational theory have been challenged. In 2015, Eric Block and colleagues performed experiments with specific receptors, one from a human and one from a mouse, using the deuterium approach (box 6.2). They took isotopomers of the musk odorants and tested them for olfactory differences. None were detected, and so this would reject the vibrational hypothesis.
So, the vibrational theory remains an interesting idea, but the hand-in-glove theory based on shape of the odorant and its receptor pocket seems to be more substantiated. Whether the vibrational theory or the shape theory or perhaps a mixture of both wins out, the same process has to happen once the odorant interacts with the receptor: a cascade of protein interactions inside the cell occur that create an action potential, the currency of the nervous system. This impulse in turn transfers the initial information about the odorant to the brain. For several of the senses this mechanism is the same. The process itself is called signal transduction, and it involves a protein complex that I discuss in the context of the other chemosensory sense—taste.
BOX 6.2 | TESTING THE VIBRATIONAL HYPOTHESIS
Several very clever experiments have been devised to test the validity of the vibrational hypothesis. These approaches involve using deuterium, an isotope of hydrogen. Deuterium has a neutron in its nucleus compared with hydrogen, which has none. A compound made using deuterium will have different properties than the same compound made with hydrogen. One of the different properties of a compound incurred by using deuterium is the vibration of the molecule. By substituting deuterium for hydrogen in an odorant molecule, one can significantly alter the vibrational property of the odorant, making the molecule with deuterium what is called an isotopomer of the one with hydrogen. The size and shape of the odorant are hardly altered by the insertion of deuterium for hydrogen, but the vibrational properties are. If the vibrational theory is correct, then the odorant with the deuterium should smell different from the odorant with hydrogen. Validity of the shape hypothesis would lead to both smelling the same because their shapes are the same. Another test would be to find two odorants with the same vibrational properties but different shapes. In this case, if the vibrational theory is correct, then the two odorants should smell the same, as opposed to the shape theory, where the two odorants should smell different.
Taste has many fewer genes that code for receptors in our genomes than for olfaction. Although there are about four hundred functional olfactory receptor genes in humans, an order of magnitude fewer are taste receptors. This small number of taste receptors doesn’t mean that the range of variation in what different humans can taste is narrow, however. It is important to consider how the small molecules that produce taste interact with taste receptors before seeing how broad the taste receptors are. Some of these interactions are quite different from how odorants interact with their receptors.
Cells that detect taste (for that matter, most cells) are fairly complex entities. Their membranes are littered with small molecules, including the receptor proteins that have already been described. Many of these proteins are securely anchored in the membrane by means of varying numbers of loops of the protein in and out of the cell. Another kind of protein embedded in the membrane is called an ion channel. This protein does what its name suggests, by transporting ions (atoms with electrical charges) from the outside of a cell to the inside and vice versa. Other proteins are embedded in the membrane but are of less importance in the recognition of taste or smell. Inside the cell, there is the obligatory nucleus and other organelles that keep the cell working, such as the mitochondria, where energy for the cell is processed, and the endoplasmic reticulum, where proteins are synthesized. But these cells also include small bodies or sacs called vesicles that congregate at the part of the cell where the nerve impulse will be transferred to a neuron for eventual connection to the brain. The vesicles are chock-full of small molecules called neurotransmitters that are integral parts of transmitting electrical messages from one cell to the next. The electrical messages are called action potentials.
As discussed in Chapter 4, taste receptors recognize five major categories of “tastants”—salty, sweet, bitter, sour, and umami. Recognition of each of these five taste categories is implemented by a different kind of small molecule or even part of a molecule called an ion. For instance, table salt (sodium chloride, or NaCl) has two components: a sodium atom that is missing an electron and a chloride atom that is missing a positron, making the sodium atom positive (Na+) and chloride atom negative (Cl−). The two ions are loosely connected by sharing what is called an ionic bond that is pretty weak as chemical bonds go. Saltiness is recognized when the positive ion of a salt (Na+ in the case of table salt) is transported across the taste cell’s membrane via the ion channels discussed above. When a salt like sodium chloride congregates around taste buds, the outside of the taste bud cells is inundated with Na+ ions. These Na+ ions are rapidly transported across the cell membrane in the ion channel, that small, porelike machine that pumps the ion across the membrane. When a sufficient amount of Na+ ions collect in the nerve cell, it does a trick called depolarization, in which it sucks calcium into the inside of the cell via ion channels. The calcium atoms are doubly positively charged (Ca++), and they induce the vesicles to release their contents. Loads of small molecules called neurotransmitters are released into the area between the taste cell and the adjoining neural cell, called a synapse. The cell needs to reset itself, or void itself of all of the positive ions inside, so once the vesicles have done their job, the ion channels back-transport all of the potassium on the inside of the cell. The potassium ions (K+) are positive, and this resets the charge on the inside of the taste cell, creating an electrical charge or action potential that again is the currency of the nervous system. Acid recognition is accomplished in a similar manner as salt detection.
Because all acids have one thing in common—a weak bond involving hydrogen that produces hydrogen ions (H+)—it is a hydrogen ion that triggers the ionic changes on the inside of the taste cell. You might be asking, if it’s the same mechanism, then why isn’t acid just a salty taste? It turns out that the H+ ions also block the movement of K+ ions across ion channels and also enhance the entrance of other positive ions into the cell. Hence, the salty and acidic tastes are created by different kinds of ionic changes that differentiate the two tastes. In addition, with acidic compounds, the cell vesicles recognize this different kind of accumulation of positive ions, and only those vesicles that should respond to H+ are released. As with salts, the cell needs to reset itself, so after vesicle release, the potassium channels are cleared and the K+ ions on the inside of the cell are transported out for the reset.
Unlike salty and acid, the tastants for sweet, bitter, and umami do not enter the cell. Instead, they interact with receptor proteins embedded in the cell membrane, much like the odorant receptors described previously. These receptor proteins interact with what are called G protein complexes (fig. 6.2) that are positioned on the inside of the cell (box 6.3). The G protein–coupled cascade will also activate the vesicle release.
The vibrational theory could also work for taste. As with smell, this general vibrational idea is not new, because that same 1869 Scientific American article mentioned previously proposed the same hypothesis for taste. The recent advances in the potential of the vibrational theory are based on the knowledge of molecular biology that simply didn’t exist in the nineteenth century. Nonetheless, the way that G protein complex receptors work leaves open the possibility that some receptors work better than others, and there is enough variation in human populations for these receptors that there is quite a range of tasting for humans. In addition, the numbers of receptors that exist on the tongue can have a huge impact on human variation for taste.
The G protein complexes have three subcomponents that are bound to one another: alpha, beta, and gamma. This three-protein complex is connected to the actual receptor molecule described as having two business ends. One end is on the outside of the cell and can bind to the odorant molecule or, in the case of taste, the tastant molecule, in a hand-in-glove manner. The other end is on the inside of the cell and interacts with the G protein complex. Once the odorant or tastant molecule binds to the outer business end of the receptor protein of the smell or taste cell, the internal end of the receptor induces a reaction in which the G protein subunits are cleaved into an alpha-only protein and a beta + gamma–complexed protein. These two proteins activate other proteins inside the cell to induce vesicle release into the synapse. Each of three tastes—sweet, bitter, and umami—induce the vesicle opening into the synapse in different ways, resulting in the differentiation of the three tastes by the brain.
In general, most humans can be placed into three major categories of tasters—nontasters, tasters, and supertasters, roughly in the ratio of 25 percent:50 percent:25 percent. There is also a small percentage (less than 1 percent) of humanity categorized in a super-supertaster category. Supertasters are mostly women, and people of European ancestry are usually not supertasters. So what exactly is a supertaster? You might think that a supertaster would have a lot of fun eating and drinking, but it’s more like the opposite. Because supertasters experience tastes more intensely than nontasters and tasters, the effects of different tastes detected by tongues of supertasters are amplified relative to the nontasters and tasters. Super-supertasters have it even worse than supertasters. Taste is a good case of “more is not better.”
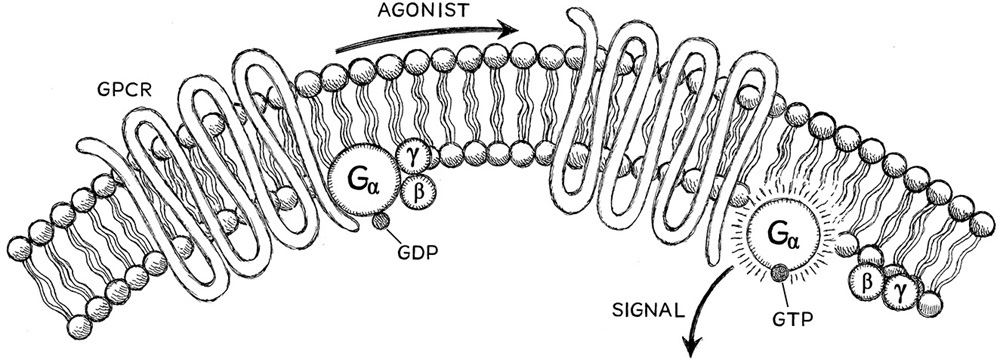
Figure 6.2. G protein–coupled receptors (GPCRs). The GPCR spans the cell membrane seven times and complexes with alpha, beta, and gamma proteins on the inside of the cell. When an agonist (a neurotransmitter) interacts with the protein on the outside of the cell, this cleaves the gamma and beta proteins from the alpha protein and converts GDP (guanosine diphosphate) to GTP (guanosine triphosphate), which then triggers a signal in the cell.
The best way to describe the differences between the categories of tasting is to take one of my favorite beverages to taste—beer—and explain how each of the categories of tasting will respond to this beverage. The Master Brewers Association of the Americas recommend what is called the American Society of Brewing Chemists flavor wheel to help its members assess the taste of their brews. The flavor wheel was created by a coauthor of Sensory Evaluation Techniques, first published in the 1970s and now in its fifth edition. Morten Mielgaard, a professor of the senses and how to measure them, created the taste wheel to lend a more quantitative aspect to beer tasting.
The taste wheel is quite complex and has gone through many iterations since Mielgaard created it, but it does focus on the complexities of the perception of beer. Examples of the more than one hundred possible categories of taste include grapefruit, caramel, farmyard, funky, burnt tire, and baby sick/diapers (which I hope never to taste). It is safe to say that these tastes are the result of many factors, but they all emanate from the very simple contents of beer. In fact, to protect the simple contents of beer, in 1516 Germans created the Bavarian Beer Purity Law, or Reinheitsgebot. The purity law forbids any beverage labeled “beer” to be made with anything but hops, water, and barley. Although yeast is needed in brewing, it is a microbe, and was obviously not recognized as an ingredient five hundred years ago. So, the modern concept of taste in most classical beers comes from only four ingredients. The most interesting aspect of the taste of beer, at least to me, comes from the hops and the sugars in the brew, and of course the alcohol that is the product of fermentation implemented by yeast on the sugars from grain.
Although beer is probably several millennia old, hops have been a part of brewing beer for a little more than a millennium. Its widespread use began in the last eight hundred years in Germany and was cemented in brewing technology with the invention of India pale ale (IPA) in the early to mid-nineteenth century. With the modern advent of microbreweries and the development of custom-made hoppy beers such as the many IPAs that are on the market, this beverage becomes one that has a wide range of bitterness. It might be surprising to note that hops were first used as a preservative in beers. The bitter taste from hops is an afterthought. The manipulation of hops today as an integral ingredient in producing craft beers makes for some pretty wildly hoppy beers. (All of which I enjoy immensely, making me more than likely a normal taster.) Supertasters find beer incredibly bitter, so much so that they will avoid drinking hoppy beers like IPAs and will not be too terribly enamored of even mildly hopped beers, like most lagers. I also am immune to the burn of alcohol in the beer, something that a supertaster will report when his or her lips touch a high-alcohol beer. Suffice it to say, hard liquor is a no-no for supertasters. Nontasters will pretty much drink and eat anything, so their tolerance for hoppiness is extreme. But they more than likely will not be able to tell the difference between a Columbia hopped beer and a Cascade hopped beer. Supertasters more than likely should be able to discern quite well between these two hops by taste, but unless they have been conditioned to drink beer, they more than likely will first and foremost consider both as just really bitter. So it is normal tasters who have all the fun with tasting hoppy beer. All of this does not mean that supertasters and nontasters won’t enjoy alcoholic beverages. A nontaster will have no trouble gulping down a jalapeño-infused tequila, and a supertaster can be conditioned to drink beer or wine and enjoy those beverages. It has been suggested that upscale chefs are supertasters who have conditioned themselves to overcome the overwhelming effects on their taste buds and to use their supertasting as a tool to create novel dishes. Recently, sour beers or farmhouse ales have become very popular. In this case, brewers of these interesting beers take advantage of the sour taste receptors and combine that with a little hoppiness. Anyone who has tasted a really sour farmhouse ale, though, will recognize that their sour taste receptors are going crazy relative to their bitter receptors.
Tasting beer is really a simple reaction of the small chemicals in the brew with receptor molecules on the tongue. But unlike smell, and although taste is combinatorial, the one thing that dictates whether someone can taste, supertaste, or not taste is ultimately caused by the number of taste cells on the tongue. The taste receptor cells are found in bundles of anywhere from thirty to one hundred cells within which the taste receptor proteins reside. The bundles of cells are called taste buds, and most of these reside on physical structures of the tongue called papillae.
There are three forms of papillae on the tongue related to where they reside. The fungiform papillae reside on the anterior region of the tongue, look like little mushrooms budding up from the surface, and can have up to two taste buds per papilla. The circumvillae are located in the posterior region of the tongue, and the foliate papillae are located on the sides of the tongue. Taste papillae are also found on the upper side of the mouth (the palate) and in the throat. Taste cells have also been found in the lungs, but their function in this tissue is unknown.
The density of papillae on the tongue is directly correlated to being a supertaster (more than thirty per 100 mm2), a taster (fifteen to thirty per 100 mm2), and a nontaster (less than fifteen per 100 mm2). In this case, instead of the genes for the taste receptors being the ultimate cause of supertasting, an underlying developmental process is involved. How the tongue develops its papillae has recently been deciphered, and interesting hypotheses about the evolution of the arrangement and number of papillae formed during development are being tested. One immediate result of this work is the discovery of the strange phenomenon that teeth and papillae are patterned with similar genes.
What is an effective technique for examining how many papillae someone has in a given area of the tongue? All of them involve darkening it, and the most enjoyable is to swirl red wine in the mouth and over the tongue. If done correctly, you will be able to see little lumps of tissue on the tongue that are the papillae. Next, take a piece of three-hole notebook paper. The punched holes are about 6 or so millimeters in diameter, and a piece of paper torn off with one of these holes can be placed over the darkened tongue. Now simply count the number of papillae you see in the punched hole. If you have fewer than fifteen papillae, you are more than likely a nontaster, whereas from fifteen to thirty papillae would suggest that you are a taster. Anything over thirty would indicate that you are a supertaster or a super-supertaster (fig. 6.3).
Believe it or not, the reception of tastes is somewhat similar to how pain is perceived. In fact, one of the best ways to describe how pain reception works is to look at foods that have tastes that are painful, such as spicy, hot foods.
Figure 6.3. Tongue papillae and the types of tasters in human populations.
He has bathed in beer, chocolate (apparently milk chocolate), and fifteen hundred pureed Oreos, but his latest bath was a doozy. Cemre Candar, an Internet sensation (whatever that is, and whatever it gets you) has more than fifteen million hits for some of his stunts, and his feat in 2016 drew more than two million viewers over the first week or so it was posted. You see, he completely immersed himself in hot sauce. If that weren’t enough, he topped the bath off with a bucket full of red-hot chili peppers. I don’t recommend doing this or even watching the video of this stunt. It looks excruciatingly painful.
Cemre Candar is indeed a strange human, and his bath in hot sauce was, as he can tell you, very painful. But why? It’s just a liquid, isn’t it? But the hot sauce and hot peppers, while made up of a lot of water and salts, also contain a small molecule called capsaicin. This small chemical found in many peppers reacts with and transduces specific cells in our bodies. The amount of capsaicin in the pepper or hot sauce dictates how hot the sauce actually is. There is a measure for the heat that a pepper can emit, and it is called a Scoville heat unit (SHU). It’s determined by undertaking a little organic chemistry on the pepper, and then feeding decreasing concentrations of the organic extract to a panel of experts until a majority of the panel can no longer detect the heat. The concentration at which the heat can no longer be detected is called the heat level. To demonstrate the scale, consider a jalapeño pepper. If you think these peppers are hot, watch out for the Carolina Reaper (or, rather, stay away from it). A typical jalapeño has a Scoville rating of more than 2,500 SHUs. The hottest Carolina Reaper on record had a rating of 2.5 million SHUs, or more than ten thousand times hotter than the jalapeño. It’s hard to tell from the video, but more than likely, Cemre Candar used a Tabasco-style sauce to fill his bathtub (he used 1,250 bottles of it), topped off by whole red habanero chilis. Tabasco (depending on what variant you use) has a rating of about 5,000 SHUs, and red chilis also rate about 5,000 SHUs. If he had used anything like Blair’s 16 Million Reserve hot sauce, the bath would have been incredibly expensive (this is a top-of-the-line product), and way hotter, as this sauce comes out of the bottle at 16 million SHUs. Even with the relatively mild Tabasco and red chilis, his bath would have been quite concentrated with capsaicin. So, what happened to Cemre Candar’s body as he slowly lowered himself into the red concoction in his bathtub?
As he lowered his body into the tub, the small capsaicin molecule bathed his skin. The skin has all kinds of cells but some also have a transient receptor protein (TRP) embedded in the membrane (the one greatly affected by the hot sauce bath is called TRPV1). This protein is a little like the chemoreceptor proteins discussed previously, except instead of weaving in and out of the membrane seven times, like the other chemoreceptors, the TRP makes only six turns. The big difference, though, is that instead of having ends flapping on the outside and inside of the cell like an olfactory receptor, the TRP makes a channel in the membrane with its six transmembrane domains that is critical for its functioning.
High-temperature, low pH (acid), and small molecules like capsaicin will activate the channel. A compound in hot mustard and wasabi called allyl isothiocyanate will also activate the channel. Each of these insults the skin and will open the channel so that the opening can then regulate the external Ca++ and Na+ (and their intercellular counterparts) concentrations. Without going too deeply into the neurochemistry, we can say that this chemical regulation affects the voltage regulation and is at the heart of the action potential that then sends the information to the brain via the nervous system. In Cemre Candar’s case, the capsaicin hit the TRPV1 channels and opened them up wide, forcing the cell to pump Ca++ back and forth to regulate the concentration of this molecule. This triggered a regulation of voltage in the cell that then ran through Cemre Candar’s nervous system as action potential to his brain, where the response in his brain was one of pain. The further he immersed himself in the red goo, the more of his cells were opening their TRPV1 channels and the more action potential went rushing to his brain to express pain. Oh, and he also realized that it was hot, because the TRPV1 channels would also relay that message to the brain. Although we can’t see him sweat in the red goo, it is well known that a physiological response to overexcitement of TRPV1 channels by capsaicin is sweat, and he was probably doing this profusely while in the tub. The sensing of pain in this case was caused by a silly penchant for the extreme, but there are unfortunate cases where the loss of sensing pain can be injurious.
In 2006, six children were brought to the attention of medical researchers in Pakistan with strange healed and unhealed injuries. Not all of the children were related, but three were from one family and two were from another single family. Their injuries were bizarre, because the children had never complained of being in pain when the injuries occurred. Several of the children were missing the tips of their tongues, having bitten them off in early childhood without even a whimper. The older children of the six did learn to feign being in pain when injuries to them looked particularly bad. Almost all had at least one limb that had been broken and healed without the child mentioning the break to a parent. These remarkable children simply could not feel pain. On the other hand, they could perceive touch and temperature and were ticklish. Because there was a familial pattern to their lack of pain, researchers hypothesized that the problem in the six children was caused by the same phenomenon. In addition, because it was only pain that they could not perceive, scientists hypothesized that a specific kind of receptor in the children was not functioning. James Cox and his colleagues were able to map the loss of pain receptor to a specific location on chromosome 2 because they had family histories and samples of DNA from the families. The researchers then cloned a large chunk of the region where the lesion was localized and examined the chunk for genes that might be related to neurological function in general and pain reception specifically. They focused in on a gene called SCN9A, which codes for a sodium channel in the nervous system. Indeed, when Cox and colleagues examined the SCN9A gene of the six children, instead of finding a single mutation responsible for the lack of pain, they found three different genetic changes in the three different families that produced truncated genes. These truncated genes were effectively nonfunctional and resulted in the lack of functional pain receptor sodium channels in these six children.
In the decade since this study, major work on the genetics of pain receptors in humans has been accomplished. The disruption of normal pain reception and its transmission to the brain has been found to be incredibly complex. Many cell functions are disrupted in anomalous pain reception, including pathways involved in regulation of serotonin, estrogen, GABA, glutamine, and catecholamine. In addition, growth factors and other important proteins in development are involved. It appears that there are many flavors of pain that are mediated by various chemoreceptive and ion channel mechanisms.