Pediatric maxillary and zygomatic fractures are rare due to anatomic protection and increased bone stability.
A thorough history of the inciting trauma, careful examination of the midface, and three-dimensional analysis of computed tomographic imaging are essential to proper diagnosis and treatment planning.
Non-displaced or minimally displaced pediatric maxillary and zygomatic fractures can be treated nonsurgically with activity and diet restrictions.
A maxillary fracture resulting in a subtle malocclusion may be treated with closed reduction via maxillomandibular fixation.
Displaced fractures of the maxilla and zygoma are ideally treated with open reduction with or without internal fixation.
Biodegradable fixation systems alleviate the need for a second surgery to remove hardware.
The benefit of restoring form and function must always be weighed against the consequences of operating on the growing patient.
8.1 Introduction
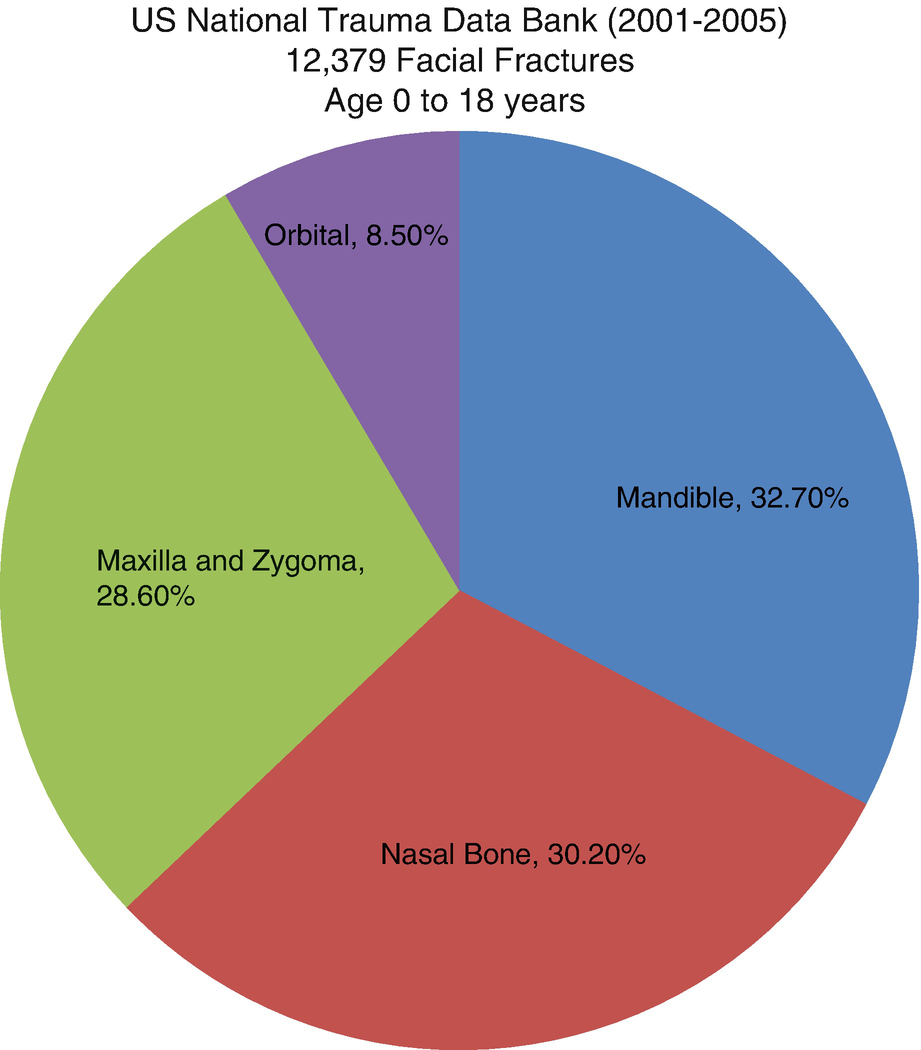
Percentage of pediatric facial fractures by site—National Trauma Data Bank (2001–2005)
High-energy trauma is typically required to fracture the maxilla or zygoma in younger populations [4]. The most common mechanisms of injury for pediatric facial trauma are motor vehicle collisions (55.1%), violence (11.8%), and falls (8.6%) [2]. Isolated fractures are uncommon. The significant forces required to cause midface fractures result in concomitant injuries in greater than 46% of all patients [9–11]. While domestic violence should not be ruled out as a cause of unwitnessed fractures, most authors feel it is an unlikely mechanism of injury [12].
Protective mechanisms decrease the incidence of pediatric facial trauma [2, 13, 14]. Younger children have a low center of gravity and typically fall from low heights. This results in lower forces generated on impact and a reduction in the risk of fracture [13]. Young children are also under close supervision by parents or adults in controlled environments, further reducing their risk [4, 13]. The promotion of safety recommendations and passing of local, state, and national regulations have reduced the risk of facial fractures. In automobiles, these include the rise in use of seat belts and age-appropriate safety seats in cars, as well as vehicle safety modifications [4]. Government regulation in the reduction of speed limits, enforcement of alcohol limits, and use of helmets have also improved outcomes [2, 13, 14]. Additionally, the use of personal protective equipment for children in activities such as biking and contact sports has also decreased the risk of facial fractures in the pediatric population [13].
8.2 Midface Growth
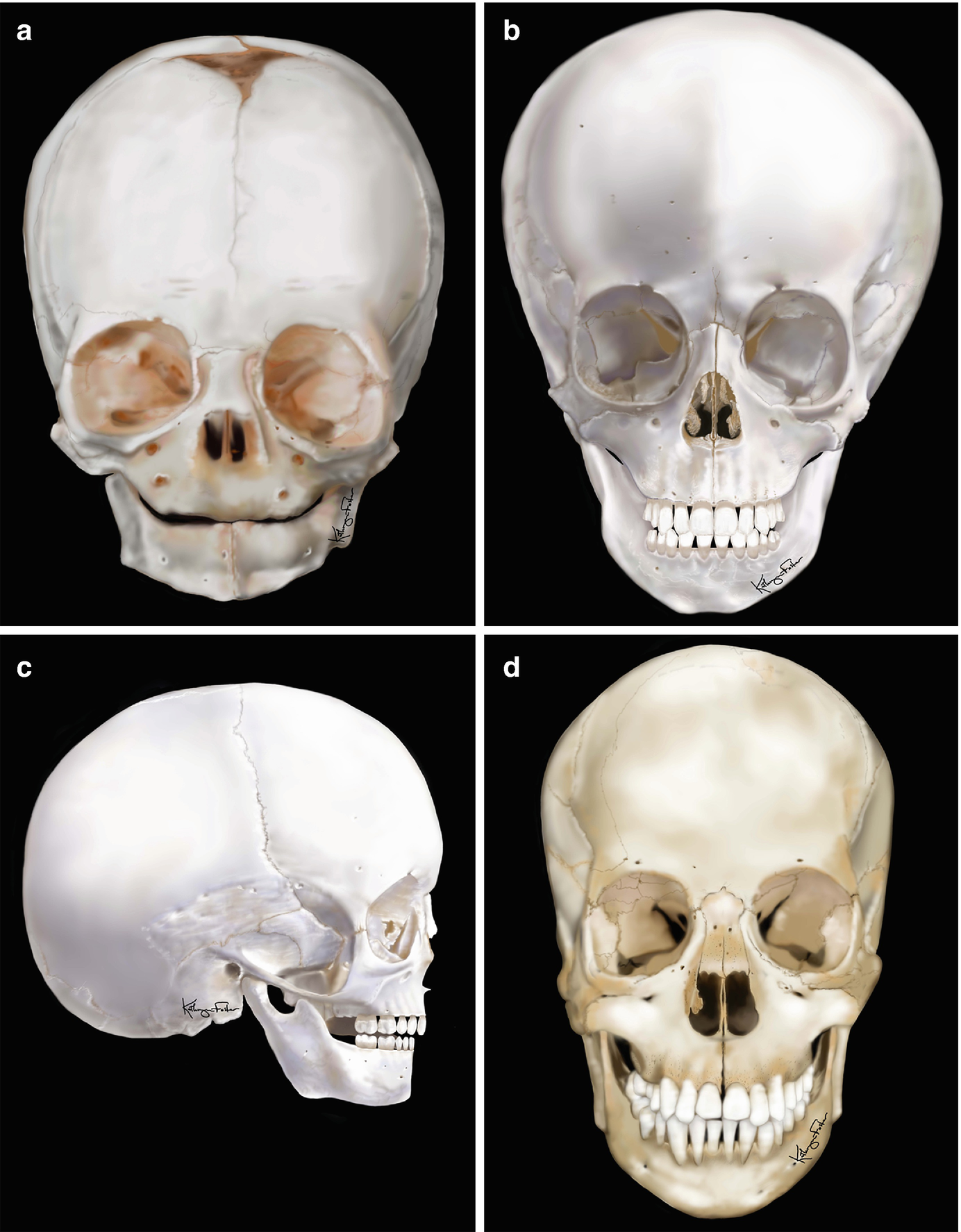
The relative prominence of the skull compared to the face in a (a) 6-month-old infant, (b, c) 5-year-old child, and (d) 12-year-old child
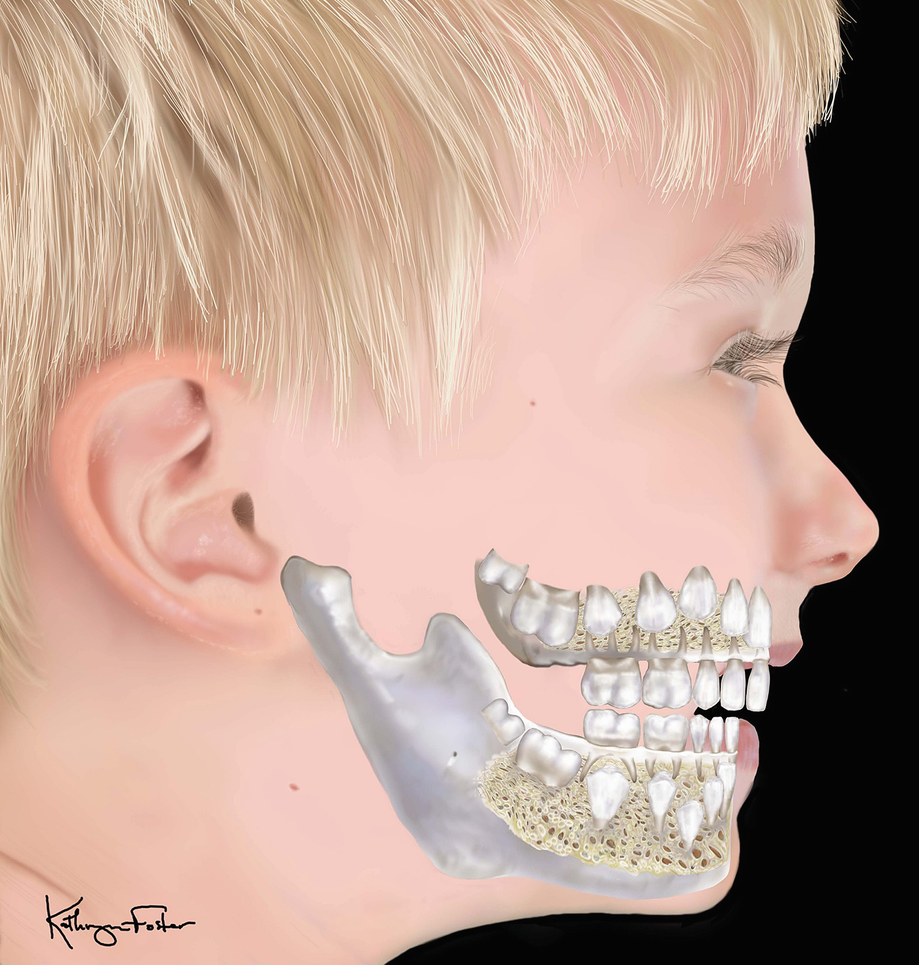
Representation of the developing dentition in a 5-year-old child
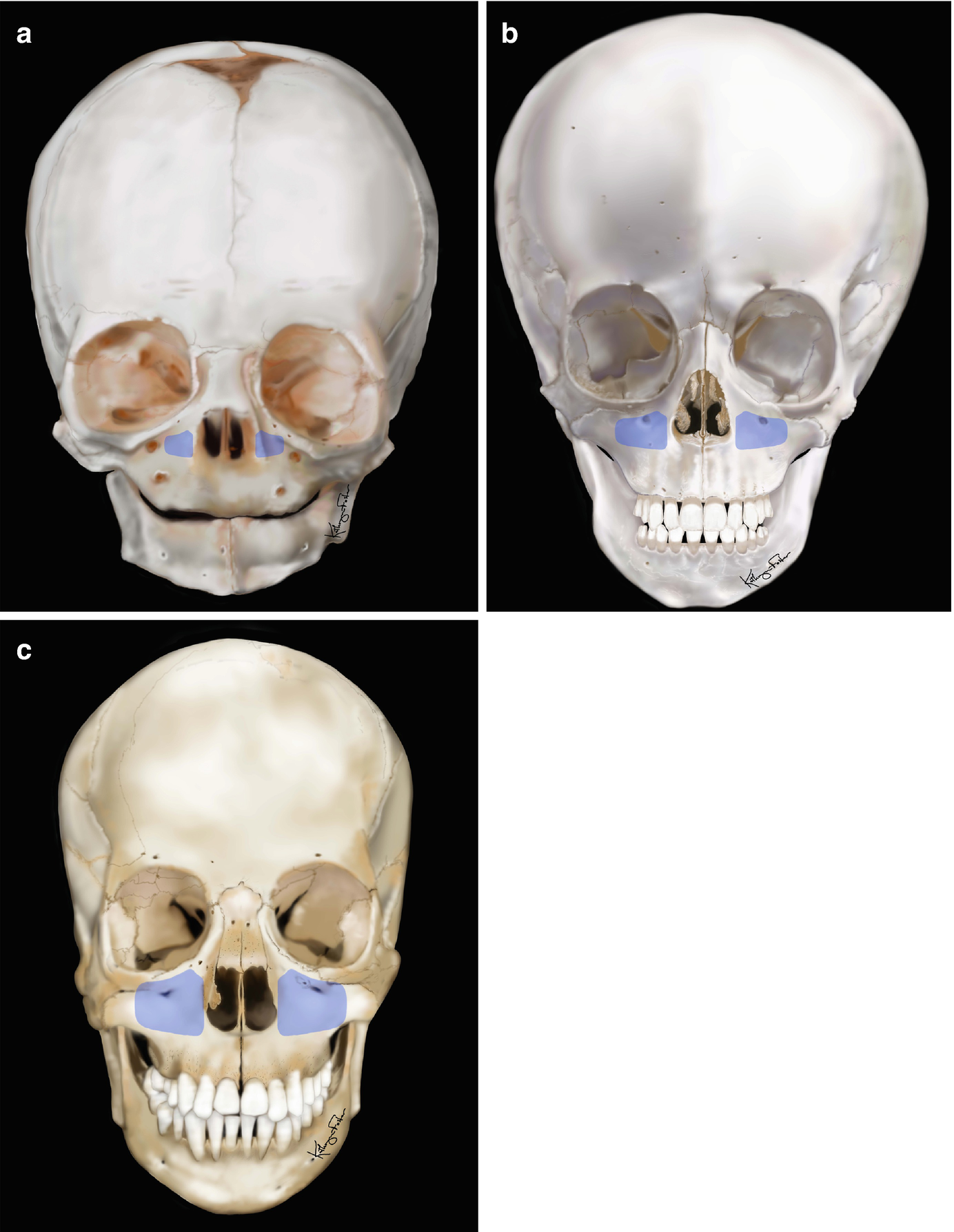
The size and pneumatization of the maxillary sinus in the growing child: (a) 6 months old, (b) 5 years old, (c) 12 years old
8.3 Maxillary Fractures
8.3.1 Background
8.3.1.1 Anatomy
Originating from neural crest cells, the maxilla forms via intramembranous ossification. The maxillary sinus is present at birth in a rudimentary form [7]. Growth of the maxilla is theorized to be in a downward and forward direction propelled by the apposition of bone at the cranial base and deep sutures of the maxilla, including the synchondroses of the sphenoid, ethmoid, and vomer [13]. Maxillary growth may also be directed by the overlying soft-tissue envelope applying forces on the developing bone (functional matrix hypothesis) [21].
The palate grows laterally from the midpalatal suture [22]. Some vertical maxillary growth occurs with the budding and eruption of the deciduous dentition during the first year of life [22]. The greatest transverse expansion of the palate occurs by the age of 2, when the majority of the cranial sutures are obliterated [10]. At approximately 6 years of age, when a child enters the mixed dentition phase, permanent tooth buds stimulate more maxillary growth and continued maxillary antral expansion occurs. The presence of the tooth buds increases the tooth-to-bone ratio, which increases the strength of the maxilla. As these permanent teeth erupt, the palate becomes more arched as additional alveolar bone forms around the teeth. Between the ages of 8 and 12 years, the palatal, premaxillary, and midline maxillary sutures are obliterated [10]. By the ages of 12–13 years, the adult dentition is erupted, except for the third molars [10]. While the maxillary sinuses can be appreciated on radiograph by 6 months of age, they do not reach their full size until after puberty [13]. Most of the growth of the maxillary sinus begins after the age of 5. The gradual pneumatization of the paranasal sinuses leads to denser, more fracture-resistant bone in the pediatric maxilla [5, 7, 17].
8.3.2 Diagnosis
8.3.2.1 History and Physical Exam
Initial evaluation and stabilization of the pediatric trauma patient should be in accordance with ATLS and PALS protocols [11, 14]. The airway should be assessed and secured while protecting the cervical spine. The oral cavity should be suctioned of blood and saliva and cleared of any debris [23]. Due to the higher probability of moderate-to-severe hemorrhage from a midface fracture, orotracheal airway placement should be considered to protect and secure the airway. While orotracheal intubation is preferred in situations where the airway is compromised or is at risk for compromise, a surgical airway such as a tracheotomy should be considered for severe panfacial fractures presenting with associated intracranial, thoracic, or abdominal injuries or when prolonged ventilatory support is anticipated. While nasotracheal intubation has the advantage of maintaining unobstructed access to the oral cavity during the repair of facial fractures, it is not typically attempted during stabilization of the pediatric trauma patient. If required for fracture repair, this can be attempted under more controlled conditions in the operating room with fiber-optic guidance after imaging has ruled out cranial base fractures that may place the patient at risk for inadvertent intracranial injury during intubation.
As always, a complete history and physical should be obtained as soon as feasible after the patient is stable. The surgeon should gain as much information as possible regarding the pediatric patient from accompanying adults. Most importantly, the surgeon should ascertain the mechanism of injury, including the etiology, force, and direction of impact [2]. The patient’s age, weight, last meal and fluid intake, medical and surgical history, current medications, allergies, family, and social history should be elicited.
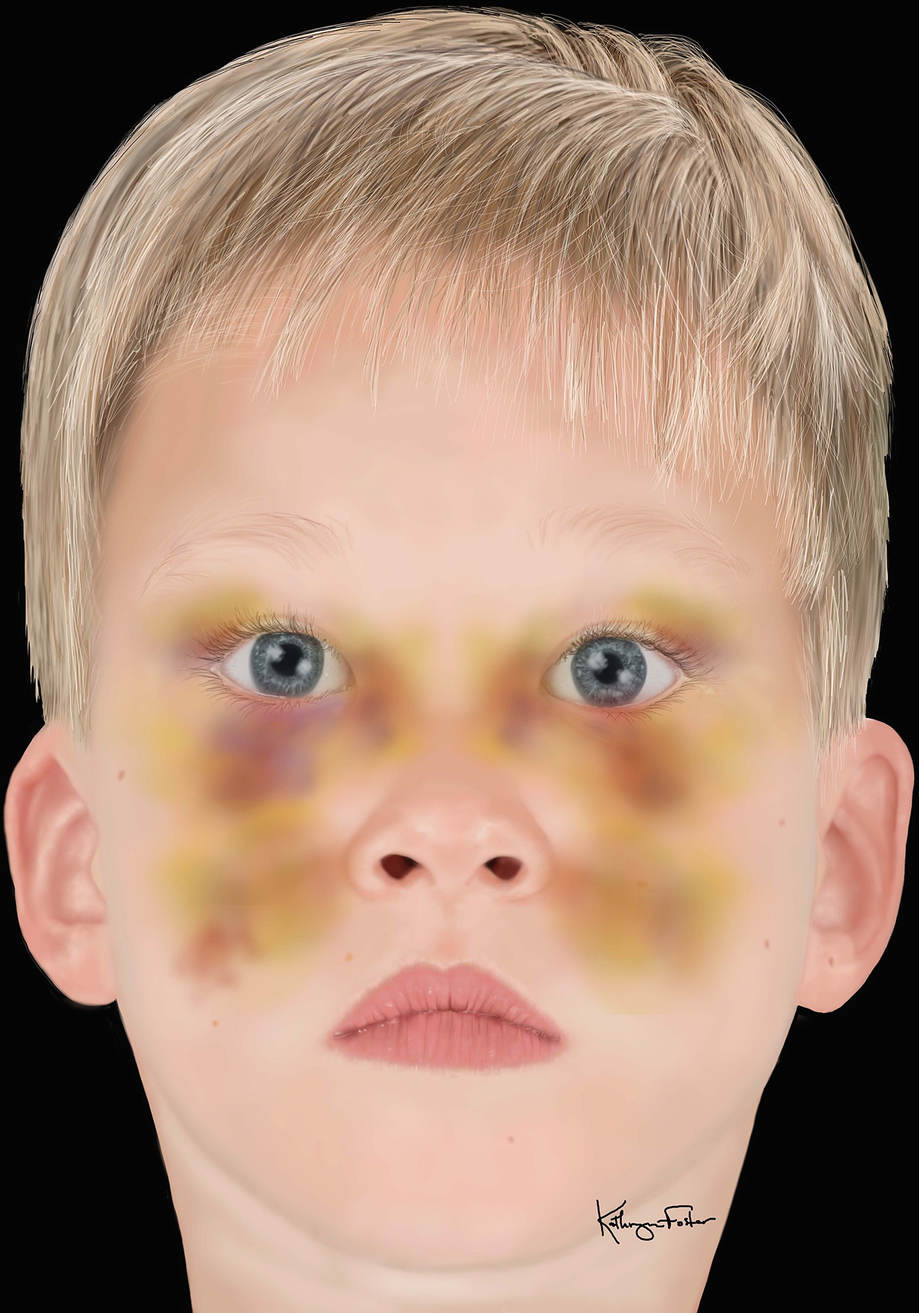
Midface ecchymosis and edema seen in child with midface trauma
A review of symptoms and an orderly palpation of the face are performed from the cranial vault to the mandible. Findings may include facial edema, orbital and midface ecchymosis, epistaxis, pain on palpation, bony step-offs, crepitation, pain on ocular movement, diplopia, blurred vision, and sensory abnormalities along the infraorbital nerve distribution [4, 11]. Mobility of bony segments at the level of the maxilla, at the infraorbital rim and nasofrontal suture, or at the frontozygomatic and nasofrontal sutures is highly indicative of maxillary fractures [4]. Intraoral exam findings may include malocclusion, trismus, palatal or vestibular ecchymosis and edema, gingival or mucosal lacerations, malposition or mobility of teeth, and fractures of teeth. Be aware that maxillary fractures combined with other cranial fractures may lead to cerebrospinal fluid (CSF) rhinorrhea. If a leak is suspected, the beta-2 transferrin test is highly sensitive for identifying the presence of CSF. If beta-2 transferrin is not available, a ring test may be performed. Here, the nasal fluid in question is placed on a piece of filter paper. If CSF is present it will separate from the blood and other fluids forming a “ring” or “halo.” This test may yield a false positive because water, saliva, and nasal discharge may also separate from blood [11].
8.3.2.2 Imaging
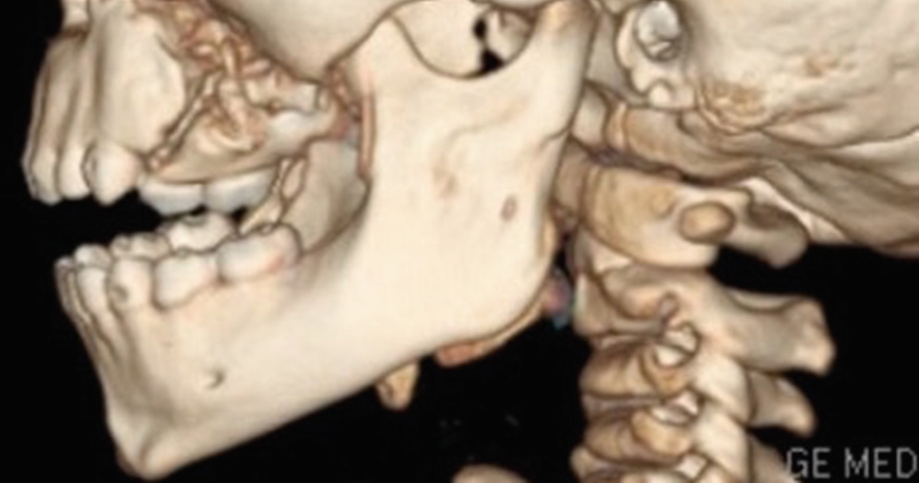
Computed tomography 3D reconstruction showing fracture of left posterior maxilla in a 5-year-old patient
Imaging modalities such as plain-film or panoramic radiographs, ultrasonography, and magnetic resonance imaging (MRI) are generally not indicated for maxillary fractures, even though they reduce radiation exposure to the patient [6]. Standard radiographs will not likely reveal all fracture lines of a complex fracture. Ultrasonography may be a good screening tool to determine the extent of injuries because it is safe and inexpensive and limits amounts of exposure. However, if edema, tissue emphysema, open wounds, or painful injuries are present, this method is ineffective and may cause the child pain on exam. Bony fractures are difficult to appreciate on ultrasound unless they are displaced. MRI is reliable for imaging soft-tissue injuries, but does not reveal the detail of bone fractures. Further, MRI, like CT, may require sedation for pediatric patient compliance. Therefore, these imaging techniques are not included in a standard workup for maxillary fractures in children [6].
8.3.2.3 Classification
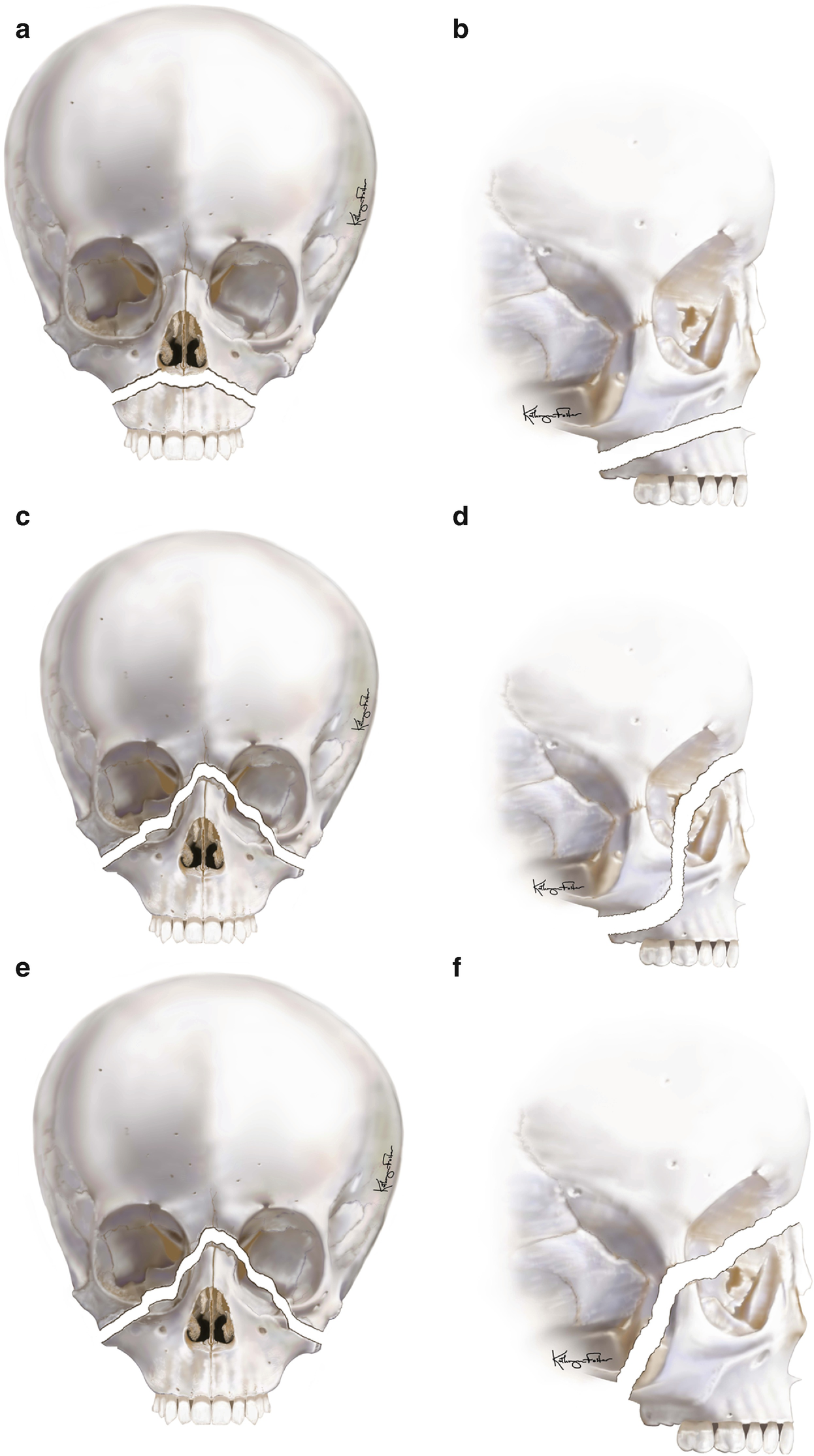
Le Fort fracture classification: (a, b) Le Fort I, (c, d) Le Fort II, (e, f) Le Fort III
The Le Fort I fracture occurs in a transmaxillary plane superior to the dentition and extends from the pyriform rims, through the anterior, lateral, and posterior walls of the maxillary sinus, and through the pterygoid plates of the sphenoid bone. The nasal septum is often fractured with this injury. This fracture results from trauma forces directed above the level of the maxillary teeth.
The Le Fort II fracture is pyramidal in shape and extends from the maxillary tuberosity through the medial aspect of the inferior orbital rim near the zygomaticomaxillary suture, through the lacrimal bone, and up to the nasofrontal suture. Again, the nasal septum is often fractured, and the nasal bones may become displaced. This fracture separates the central midface from the cranium with trauma forces directed at the nasal bones.
The Le Fort III fracture is the most severe and involves complete separation of the face from the cranium. This fracture extends from the zygomaticofrontal and zygomaticotemporal sutures, along the lateral orbit to the inferior orbital fissure and medial orbit, and then to the nasofrontal suture. With the involvement of the zygoma, orbit, and maxillary, this fracture represents a true craniofacial dissociation. The forces required to cause this injury are directed at the level of the orbits.
Although the Le Fort classification exists, pediatric maxillary fractures are rarely associated with a single classification [9]. Often, multiple patterns can occur in the same injury with varying degrees of displacement. The elasticity of the developing bone also often leads to incomplete or “greenstick” fractures [5, 23]. Pediatric maxillary fractures will also commonly occur in combination with dentoalveolar fractures.
8.3.3 Treatment
The goal in the treatment of pediatric maxillary fractures is to restore the patient’s preinjury facial form and function while minimizing disturbances to normal growth [7]. Re-establishment of the patient’s preinjury occlusion, facial height, facial width, facial projection, and facial symmetry should all be considered when planning treatment for cases involving maxillary fractures [23]. The treatment of pediatric maxillary fractures can be broadly categorized into surgical and nonsurgical options.
8.3.3.1 Nonsurgical Management
If the fracture is non-displaced or minimally displaced, as determined by physical exam and CT imaging, and there are no functional deficits or esthetic abnormalities, the pediatric patient can be treated conservatively with observation only. The patient should be followed at close intervals during the healing phase. The authors recommend weekly follow-up to assess for changes in occlusion and facial form as edema resolves. Bony healing in the pediatric population occurs more rapidly than in adults [4]. Complete fracture healing can be expected in 2–4 weeks in most cases. During the healing period, the patient must adhere to a non-chew diet, and physical activity should be limited. Compliance in young children may be difficult, so close parental supervision during the initial healing period is essential. Patients should refrain from any activity that could result in a fall or trauma to the facial skeleton (i.e., contact sports). In the active adolescent population this may be more difficult to maintain.
If a malocclusion exists, but facial form is unaffected, closed reduction may be attempted and the patient can be placed into maxillomandibular fixation (MMF). Closed reduction may not permit total anatomic reduction of the fracture, but is advantageous because of the less invasive nature of the procedure. In most cases, 2–3 weeks of MMF is an appropriate amount of time for the healing of minimally displaced fractures [4, 10]. Caution must be exercised, though, when choosing this method of treatment. The maxilla can be difficult to mobilize without surgical exposure. The mandible may be brought into occlusion with a malpositioned maxillary dentition by distracting the mandibular condyles out of the mandibular fossae. If this occurs, the mandible will fall back into its normal anatomic position when released from maxillomandibular fixation and a malocclusion will result. Therefore, the surgeon must ensure that the mandibular condyles are seated in their most anterior and superior position when reducing the maxilla and placing the patient into maxillomandibular fixation.

Application of Risdon cable for maxillomandibular fixation in pediatric patient: (a) Interdental wire loops are placed around a 25-gauge twisted wire that has been secured to the distal molars, (b) appearance of Risdon cable at completion of wire placement
8.3.3.2 Surgical Management
When a child experiences a displaced maxillary fracture after sustaining facial trauma, the surgeon must consider reconstruction over all three dimensions to restore occlusion and facial contours. In cases where the maxillary fracture is a component of panfacial fractures, several treatment approaches to reconstruction have been advocated. Some advocate realigning the external frame of the face, and then working inward while emphasizing facial width and projection. Others use the anterior cranial base as a reference to first restore occlusion. After occlusion is restored, reconstruction proceeds from the inside-out starting with the frontal bone, the nasoethmoid region, the orbit, and then the outer facial frame. The maxilla is usually fixated as the last step of this technique [23].
Open reduction and internal fixation (ORIF) is indicated for displaced maxillary fractures that result in a functional or esthetic abnormality [4]. When operating in the growing patient there are always concerns that normal growth may be altered. The effects of open reduction and internal fixation on craniofacial growth remain incompletely understood and several theories exist for why abnormalities occur [4]. These include bony changes from the trauma itself, alterations due to damage to growth plates and suture lines, and growth disturbance related to the disruption of the periosteum and subsequent scarring after surgical manipulation. The growth consequence of maxillary surgery in the pediatric patient has been extensively studied in the cleft population [27, 28]. Shetye and Evans found that the growth potential of craniofacial structures in unoperated cleft patients was more favorable than those who had undergone surgical repair [28]. While the effects of surgery in the cleft patient do not translate perfectly to the trauma patient, these studies give us some insight into the effects of surgical intervention in the growing patient. However, advocates of open surgical techniques note that many pediatric craniofacial deformities are corrected without adverse growth affects [5]. The fact remains, though, that controlled growth studies in the pediatric facial trauma patient are lacking. While open reduction and internal fixation techniques remain a viable option for treatment of displaced maxillary fractures, minimizing the amount of surgical manipulation to the pediatric facial soft-tissue envelope and skeleton should always be considered.
The osteogenic potential of children and rapid healing that occurs in pediatric bone make the early reduction and fixation of facial fractures an important principle to follow [4, 23]. Ideally maxillary fractures should be surgically treated within 4 days, but no later than 10 days following injury [9, 10, 23]. The longer the interval of time elapsed between injury and treatment, the more difficult the mobilization and reduction will be [10]. Open reduction allows for the direct visualization of the injured anatomy and allows the surgeon to achieve a more anatomic reduction. By exposing the bone, aggressive mobilization of the maxilla can be achieved, if necessary, and bleeding can be controlled under direct visualization. Internal fixation offers the advantage of eliminating or minimizing the length of maxillomandibular fixation needed. This improves the patient’s nutritional status and compliance with treatment [9]. In the authors’ experience, bone grafting is rarely needed in the pediatric facial trauma patient. However, in cases in which good bone contact cannot be maintained at the vertical and horizontal buttresses of the face or in highly comminuted cases, primary grafting may be necessary. Split-thickness calvarial grafts taken from the parietal bone are an excellent option in patients with an adequate calvarial thickness. These grafts are relatively easy to harvest, have low morbidity, and provide a large volume of cortical bone for reconstruction.
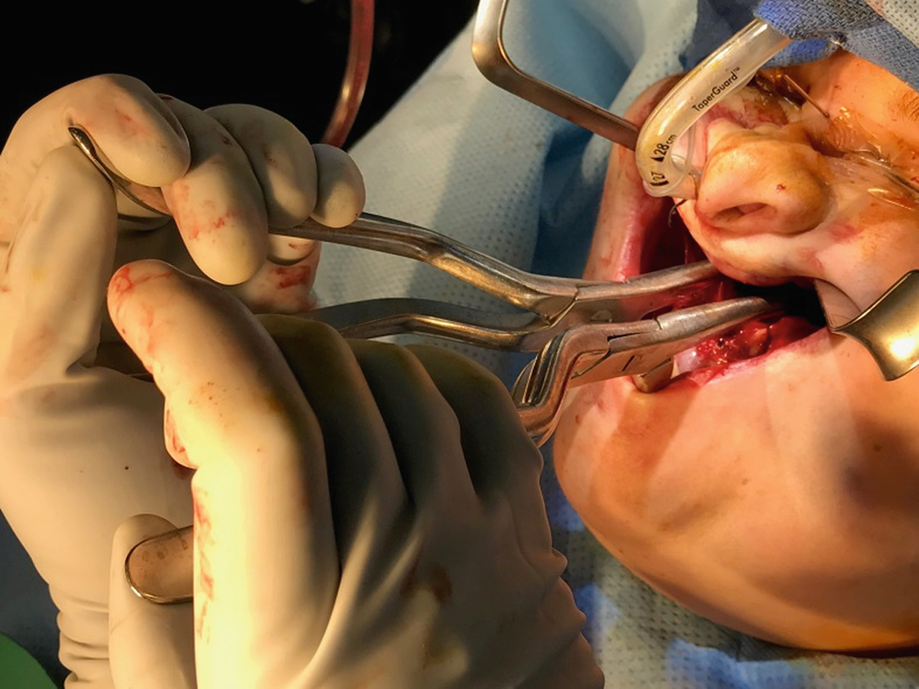
Intraoperative placement of Rowe’s disimpaction forceps

Pediatric maxillary fracture and treatment: (a) Le Fort I maxillary fracture with impaction in a 5-year-old patient, (b) reduction and fixation of maxillary fracture with biodegradable fixation system, (c) clinical photo of fixation of maxillary fracture with biodegradable hardware (photo courtesy of Nima Khorassani, DDS)
8.3.3.3 Fixation Systems
The choice between titanium and biodegradable fixation options has received considerable attention when treating pediatric facial trauma [4, 6, 10]. Non-resorbable systems made of titanium have greater strength with lower profile plates and have self-tapping screws available for use. These low-profile plates are not usually visible or palpable through the thin soft-tissue envelope of the pediatric patient. Most surgeons agree that if titanium plates are used for fixation in the growing patient, they should be removed when bone healing is complete, but the topic remains controversial [25]. While the ideal time to remove hardware varies among surgeons, most agree that hardware should be removed 2–3 months after placement [4, 10, 25]. Although hardware removal introduces the trauma of a second surgery, which may increase scarring and restrict growth further, it prevents plate migration, eliminates imaging artifact, and simplifies future surgical interventions that may be necessary at skeletal maturity (i.e., orthognathic surgery) [10].
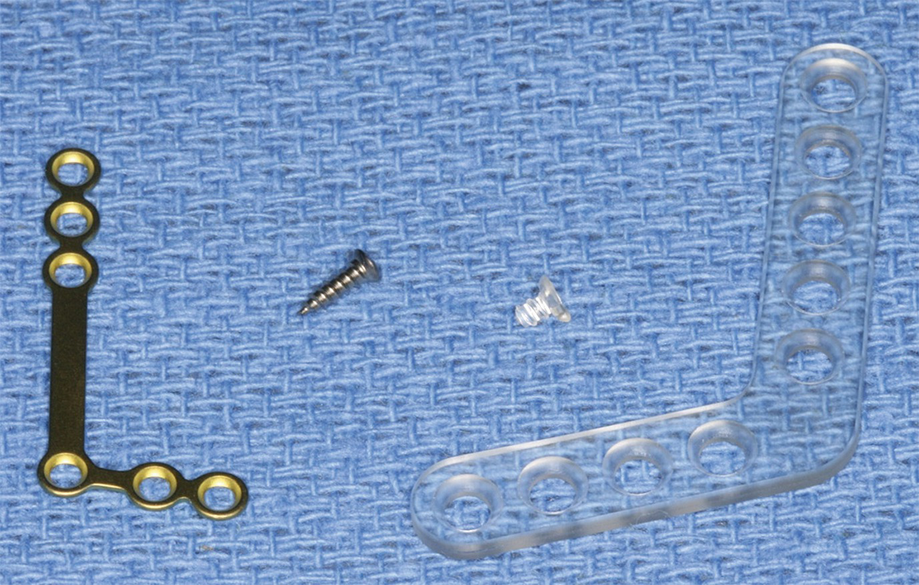
Comparison of 1.7 mm titanium L-plate and screw with 2.2 mm biodegradable L-plate and screw
8.3.4 Complications
Children with maxillary fractures are at high risk of concomitant, multiorgan, intracranial, and neural injuries due to the high-energy trauma sustained [4, 23]. Hospitalizations may create stress for the pediatric patient and the supporting family which results in changes in feeding patterns, circadian rhythms, and behavioral disturbances [26].
Postoperative infections, malunion, and nonunion are rare due to the healing capability and osteogenic potential in the pediatric population [4, 22, 25]. There is potential for either iatrogenic or traumatic nerve injury at the infraorbital nerve [25, 29]. One study demonstrated that infraorbital nerve sensory disturbances were present in approximately 50% of zygomaticomaxillary complex fractures [30]. The surgeon should also be aware of the potential for significant blood loss if major vessels are compromised; however, hemorrhage resulting in hemodynamic instability does not usually occur in conjunction with these fractures [31].
As mentioned above, the surgical management of maxillary fractures may reduce the osteogenic potential of the bone [10, 32]. The soft-tissue scarring that occurs following open reduction may further restrict growth [19]. The resultant growth disturbances to the facial skeleton may lead to maxillary hypoplasia, asymmetries, and malocclusion [25]. In younger children with post-trauma malocclusions, spontaneous correction may be seen as deciduous teeth exfoliation and permanent teeth eruption [4]. Developing teeth and roots may also be damaged [4, 22]. If any permanent teeth are lost during injury or subsequent treatment, they may be addressed when skeletal growth is complete with endosseous implants or prosthetic rehabilitation.
If malocclusion or midface asymmetry exists after initial treatments, orthodontics combined with orthognathic surgery may be indicated. Surgery in the post-trauma patient may be more challenging since osteotomies may need to be created outside the original fracture lines and soft-tissue scarring may limit mobilization and advancement of the maxilla. As with all types of trauma to the growing skeleton, children treated for maxillary fractures should be followed longitudinally by a dental provider or surgeon to assess growth at standard intervals and intervene with growth modification techniques in late childhood/early adolescence or surgery when growth is complete [25].
8.4 Zygomatic Fractures
8.4.1 Background
8.4.1.1 Anatomy
The zygoma establishes facial width and prominence of the cheek. It also makes up the lateral wall, inferior rim, and lateral floor of the orbit [33]. The zygoma has four articulations: the zygomaticomaxillary, the frontozygomatic, the zygomaticotemporal, and the sphenozygomatic sutures. The masseter, zygomaticus major, and zygomaticus minor have origins at the zygoma. The lateral canthal tendon and Lockwood’s suspensory ligament attach to the zygoma. The zygomatic nerve, a branch of V2, enters the orbit at the inferior orbital fissure and divides into the zygomaticofacial and the zygomaticotemporal nerve. The zygomaticofacial nerve exits through the zygomaticofacial foramen of the zygoma and provides sensory innervation to the skin of the cheek. The zygomaticotemporal nerve exits the zygomaticotemporal foramen of the zygoma, passes through the temporal fossa, and provides sensory innervation to the skin of the temple [34].
The infraorbital nerve is commonly injured in conjunction with zygoma fractures [35]. The infraorbital nerve, a branch of V2, gives off two branches prior to exiting through the infraorbital foramen of the zygoma: the middle superior alveolar and anterior superior alveolar nerves. The middle superior alveolar nerve innervates the sensory fibers of the maxillary premolars (occasionally the mesiobuccal cusp of the maxillary first molar), the associated gingiva and buccal mucosa, and the maxillary sinus. The anterior superior alveolar nerve innervates the sensory fibers from the maxillary incisors and canines, associated gingiva, palatal mucosa, and maxillary sinus. The infraorbital nerve also gives off nasal branches that provide sensory innervation to anterior portions of the nasal wall, floor, and septum. Once the infraorbital nerve exits the infraorbital foramen, it divides into three terminal branches: the nasal branch, which supplies the ala of the nose; the inferior palpebral branch, which supplies the skin of the lower eyelid; and the superior labial branch, which supplies the skin of the upper lip [34].
8.4.1.2 Classification
In 1961, Knight and North developed a classification system for zygoma fractures based on their study of zygoma fractures over an 8-year period. Their classification system used the anatomy of the fracture on the occipitomental/Waters view radiograph and included six groups: (1) fractures with no displacement, (2) isolated zygomatic fractures, (3) un-rotated depressed zygomatic body fractures, (4) depressed and medially rotated body fractures, (5) depressed and laterally rotated body fractures, and (6) complex fractures with increased comminution. In this study they found that isolated zygomatic fractures were associated with the highest incidence of trismus and type 4 fractures were associated with the highest incidence of diplopia [36]. This classification system has become obsolete as CT imaging has become the gold standard for diagnosis of zygoma fractures [18].
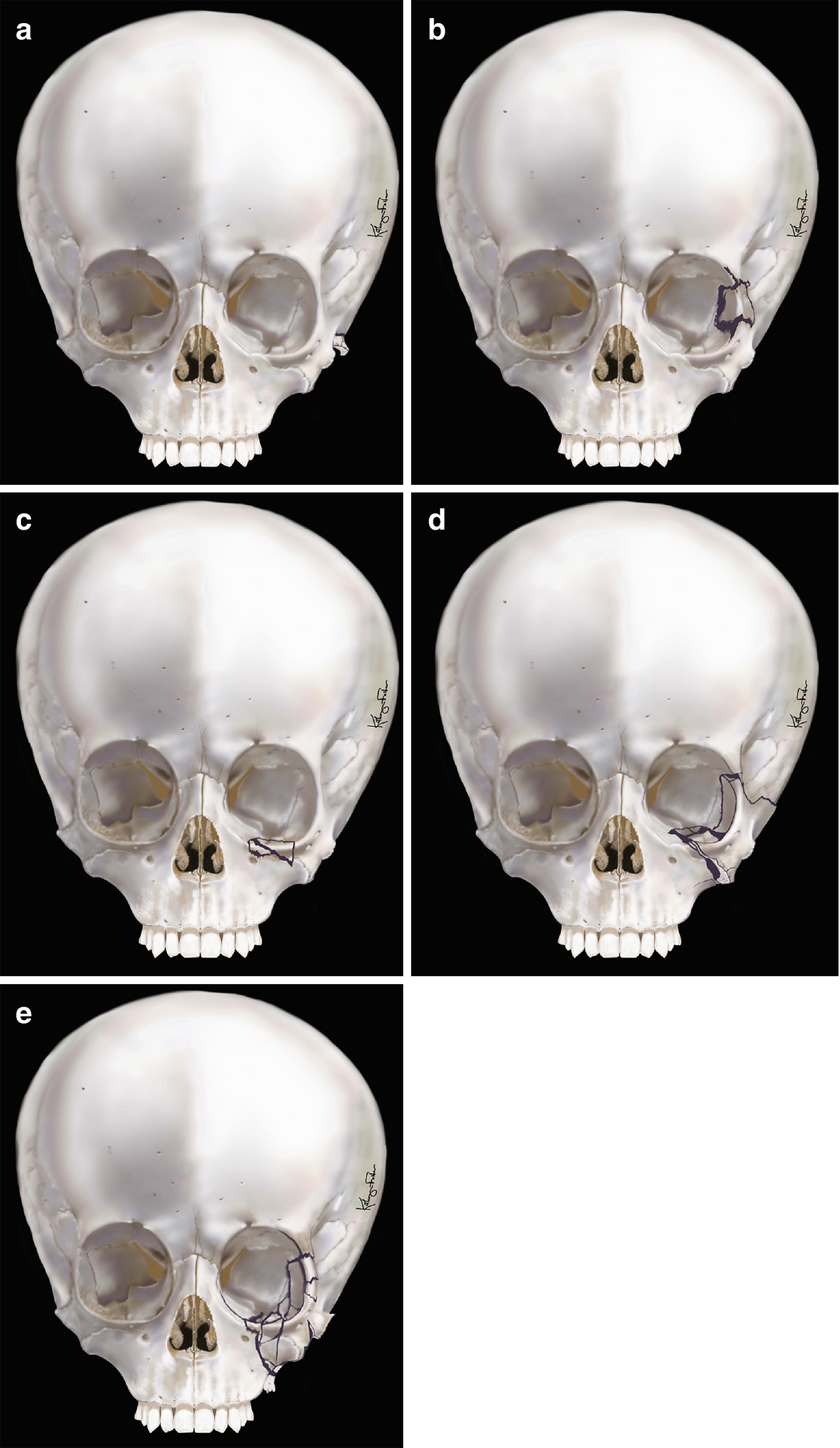
Zingg classification for zygomatic fractures: (a) type A1—isolated zygomatic arch fracture, (b) type A2—lateral orbital wall fracture, (c) type A3—infraorbital rim fracture, (d) type B—complete monofragment zygomatic fracture, (e) type C—multifragment zygomatic fracture (the same as type B, but with fragmentation, including the body of the zygoma)
8.4.2 Diagnosis
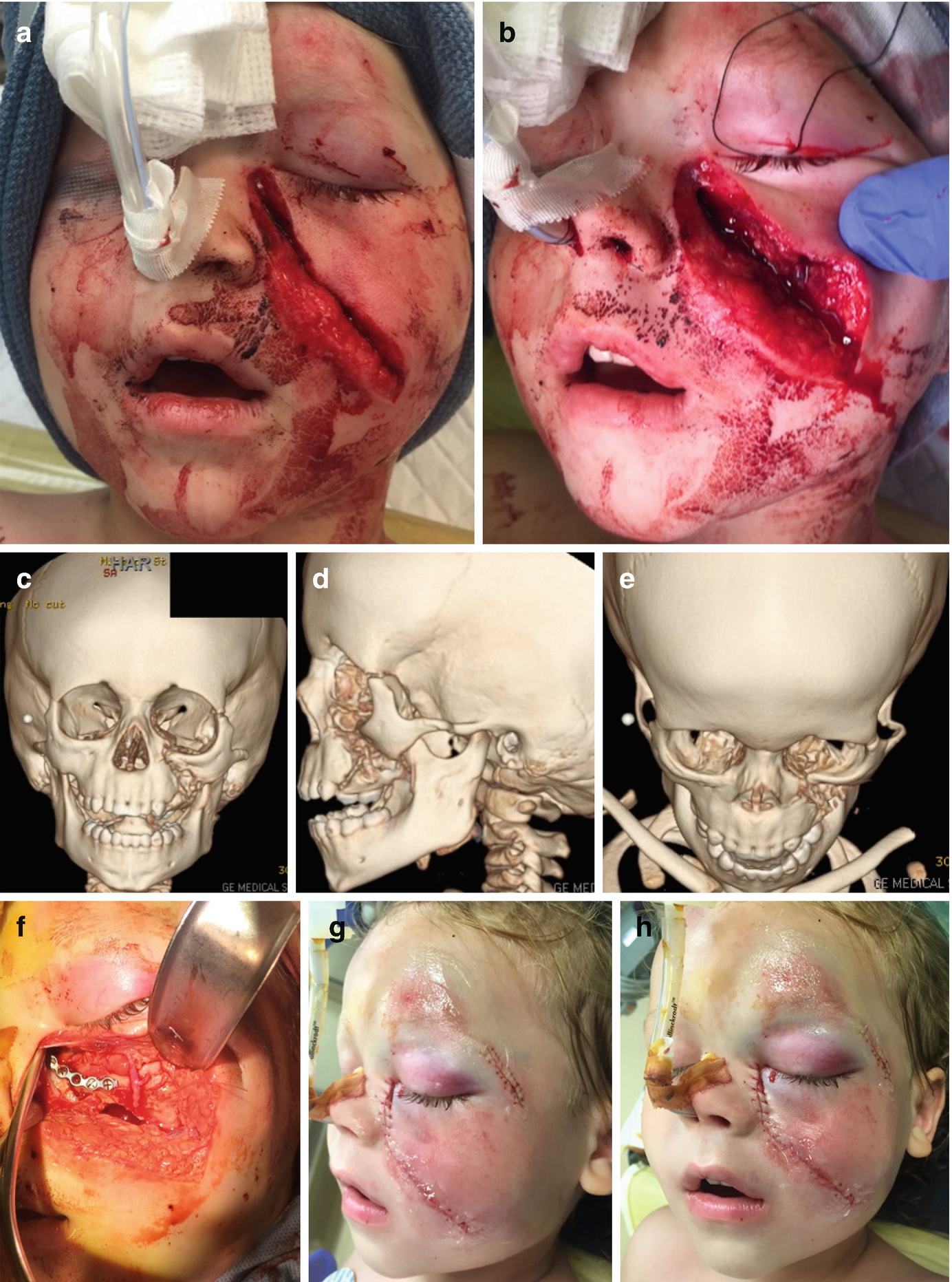
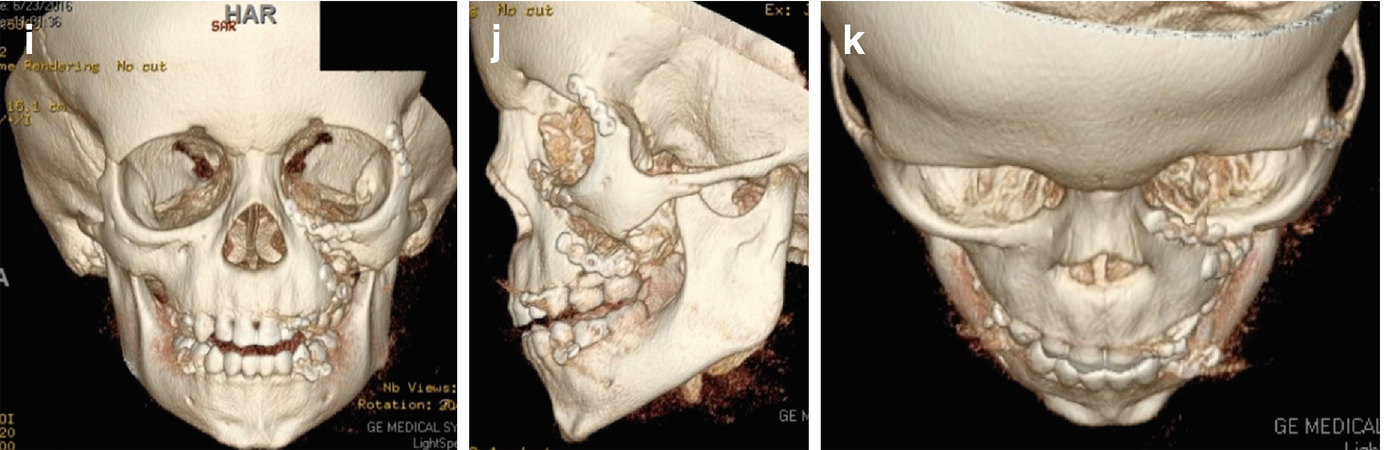
(a, b) Initial presentation of a 5-year-old child sustaining a horse kick to the face with a resultant zygoma and maxillary fracture (photos courtesy of Daniel Fisher, DDS, and Larry Parworth, DDS). (c–e) 3D computed tomography reconstruction of a 5-year-old patient with left maxillary and zygomaticomaxillary complex fractures. (f) Approach through existing laceration for repair of left zygomaticomaxillary complex fracture and titanium fixation at the level of the infraorbital rim. (g, h) Postoperative photos of a 5-year-old following open reduction internal fixation of left maxillary and zygomaticomaxillary complex fractures. (i–k) Postoperative 3D computed tomography reconstructions following open reduction internal fixation of left maxillary and zygomaticomaxillary complex fractures
8.4.2.1 History and Physical
Obtaining an accurate history will depend on the patient’s age and communication with family members or caretakers who witnessed the trauma. The mechanism of injury gives important clues as to the likelihood of a zygoma fracture. Additionally, when obtaining the history of the pediatric patient it is important to keep in mind thatvthe fracture can be the outcome of child abuse, although the documented percentage of occurrence is extremely low [12]. The physical exam can be challenging in children due to anxiety and compliance. The exam begins with evaluation of facial symmetry followed by a top-down assessment. Starting with the eyes, findings common to zygoma fractures include periorbital hematoma, subconjunctival hematoma (occurs in 64% of zygomatic fractures), lateral displacement of the eye, and binocular diplopia. Binocular diplopia is found in approximately 30% of comminuted zygoma fractures, 22% of noncomminuted displaced fractures, and 8% of minimally or nondisplaced zygomaticomaxillary complex fractures [18]. Entrapment of periorbital soft tissue is a medical emergency in the pediatric population and should ideally be treated within 24 h of injury [38]. There should be a low threshold for ophthalmologic consultation. The zygoma and its articulations are palpated for any step-offs or areas of tenderness. The nose is examined for ipsilateral epistaxis. The mandibular range of motion must be evaluated, as impingement of the zygomatic arch on the coronoid process can produce a painful limitation in mouth opening. A complete neurological examination is essential and should assess for disturbances in facial sensation. This is especially true along the infraorbital nerve distribution which has a high incidence of injury with zygomatic fractures. Neurosurgery consultation should be sought in cases with associated head or cranial injuries [18].
8.4.2.2 Imaging
Facial computed tomographic (CT) imaging is the standard of care for the diagnosis and evaluation of pediatric midface fractures (Fig. 8.13c–e). Plain facial radiographs have been found to provide limited diagnostic information for facial fractures in the pediatric population and facial CT can reveal previously undiagnosed fractures [39]. Considering the desire to limit radiation exposure in the pediatric population, post-reduction radiographs are not indicated if the postoperative physical exam is normal [5].
8.4.3 Treatment
The goal in treatment of pediatric zygoma factures is to restore preinjury form, function, and esthetics. The categories of treatment include conservative (nonsurgical), open reduction without fixation and open reduction with internal fixation.
8.4.3.1 Conservative (Nonsurgical) Treatment
The increased elasticity of the pediatric facial skeleton, increased flexibility at suture lines, and increased cancellous-to-cortical bone ratio result in a greater likelihood of greenstick or minimally displaced fractures in the pediatric population [33]. Nondisplaced or minimally displaced fractures not large enough to produce functional or esthetic deformities may be treated conservatively with soft diet, pain medication, and limitation of physical activity [9]. The length of time for activity restriction is dependent on the patient’s age and the severity of the injury. The pediatric facial skeleton has an increased osteogenic potential resulting in a more rapid rate of healing [40]. In general, the authors recommend approximately 3–4 weeks of limited activity to allow for adequate bone healing.
8.4.3.2 Fracture Reduction Without Fixation
Isolated zygomatic arch fractures can be treated with reduction without fixation via the Keen or Gillies approach. Alternative methods for fracture reduction without fixation include use of a Carroll-Girard screw or zygoma hook. These methods are used in adults but are not mentioned in the pediatric literature. The Keen approach is an intraoral approach utilizing a mucosal incision from the maxillary canine to the first or second molar. This incision is made approximately 3–5 mm above the mucogingival junction. A full-thickness mucoperiosteal flap is then reflected and an elevator can then be placed under the body of the zygoma or the zygomatic arch. Reduction is accomplished by using anterolateral force.
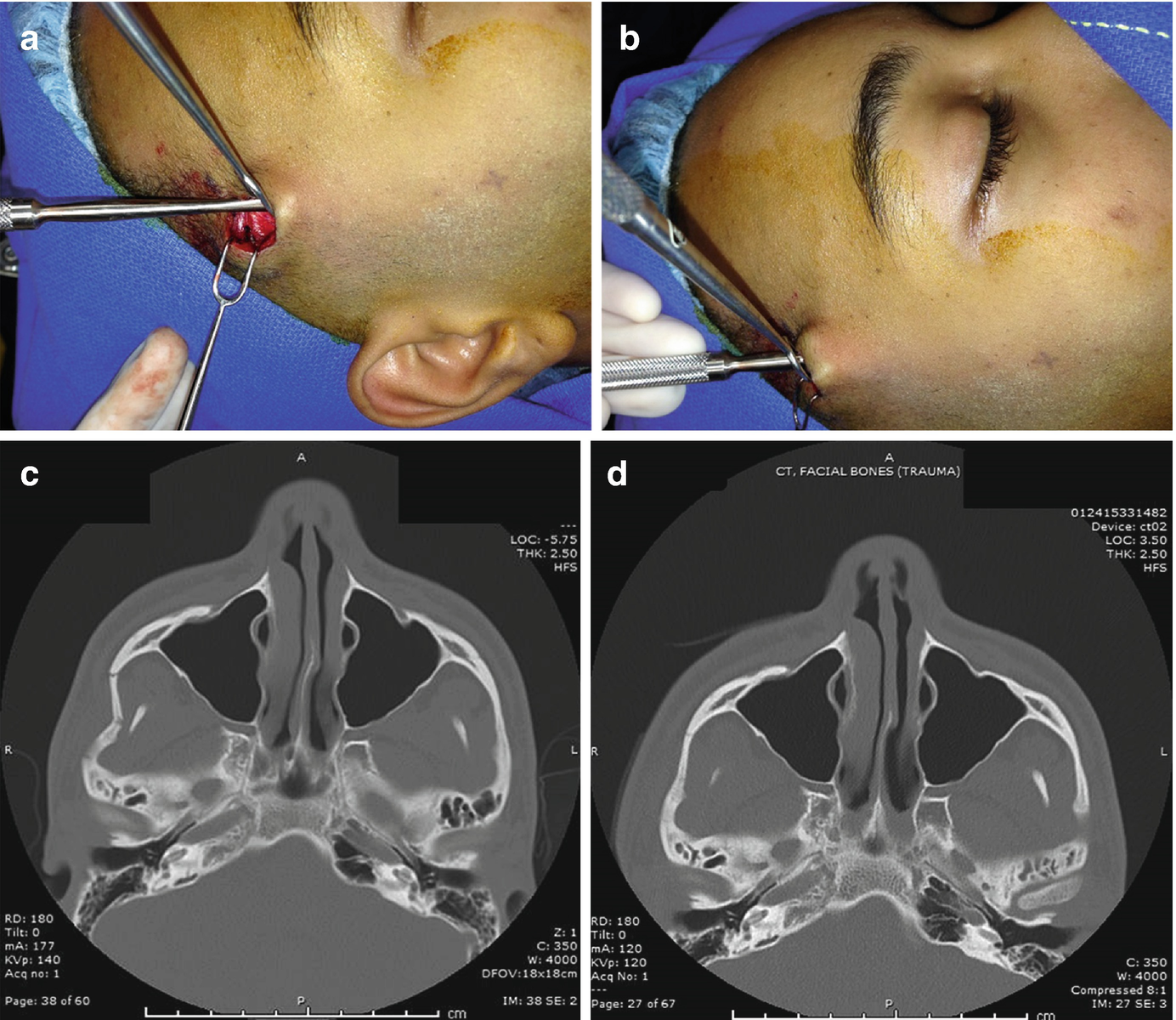
Gillies approach for reduction of depressed right zygomatic arch fracture—(a) incision and dissection down through superficial temporal fascia, (b) lateral force is applied after elevator is placed deep to the depressed zygomatic arch, (c, d) pre- and post-reduction axial computed tomography images for open reduction of depressed right zygomatic arch fracture utilizing Gillies approach
8.4.3.3 Open Reduction Internal Fixation
Open reduction with internal fixation (ORIF) is indicated for comminuted fractures, displaced fractures, and fractures that impact esthetics and function that cannot be treated conservatively.
There are several approaches for open reduction with internal fixation of zygoma fractures. If the fracture does not necessitate access to the orbital floor, a lateral eyebrow or upper blepharoplasty approach can be utilized. The lateral eyebrow incision allows for access to the frontozygomatic suture and lateral orbit [41]. The incision can be made above, through or below the eyebrow. An incision through the eyebrow can make the scar of the skin less conspicuous but may result in noticeable hair loss. The upper eyelid blepharoplasty approach involves making an incision parallel to the superior palpebral sulcus in a naturally occurring skin line. It also allows access to the frontozygomatic suture. An additional approach through the upper buccal sulcus gives access to the zygomatic buttress and zygomaticomaxillary suture [41]. If access to the orbital floor is necessary, a transconjunctival, subciliary, or subtarsal approach can be utilized. The authors prefer a transconjunctival approach to most fractures involving the infraorbital rim and orbital floor. This avoids a skin incision and allows for adequate exposure in most pediatric cases. After placing a corneal protector and injecting local anesthetic, an incision is made in the palpebral conjunctiva below the tarsal plate. Blunt dissection is carried to the orbital septum, which is then continued down to the periosteum of the orbital rim. Care is taken to remain superficial to the orbital septum. This pre-septal approach avoids the orbital fat deep to the septum that can obstruct the field and cause unnecessary bleeding. The periosteum is then incised and a subperiosteal dissection exposes the orbital rim and floor. Accessing the orbital floor allows for inspection of the sphenozygomatic suture, which has been shown to be the most reliable landmark for accurate zygoma fracture reduction [31]. Additionally, fractures may be approached through existing lacerations (Fig. 8.13f).
Open reduction with internal fixation allows for fixation in three dimensions. The fractured segment is anatomically reduced, and then secured with internal fixation to prevent displacement [42]. Stabilization of zygoma fractures can be at any of the zygomaticomaxillary complex articulations [18]. Common patterns for zygomatic fracture fixation include one-point stabilization at the frontozygomatic suture, two-point stabilization at the frontozygomatic suture and infraorbital rim, and three-point stabilization at the frontozygomatic suture, infraorbital rim, and zygomaticomaxillary buttress [18]. In particular, one-point fixation of noncomminuted zygomaticomaxillary complex fractures has been shown to be adequate for the fixation of zygomatic fractures in children [19]. Care must be taken to avoid the placement of screws into developing tooth buds. This is especially true when placing hardware at the zygomaticomaxillary buttress [2].
The choice between titanium plate fixation and biodegradable plate fixation for pediatric midface trauma was discussed earlier. One of the first clinical applications of biodegradable material in maxillofacial trauma was for the treatment of zygomatic fractures [43]. The lower functional stresses at the zygoma make it an attractive option for biodegradable fixation systems [44]. In 2010, Iatrou published his 9-year experience treating pediatric maxillofacial fractures [32]. He described the use of biodegradables mainly at the frontozygomatic suture and zygomaticomaxillary buttress for the treatment of zygoma fractures. In his protocol, when titanium plates were used, they were removed after fracture healing to prevent facial growth disturbance [32]. There are currently no published comparisons between biodegradable and titanium plates for zygomatic fracture fixation in the pediatric population. Wittwer et al., at the Medical University of Vienna, completed a study that compared clinical outcomes of zygomatic fracture fixation in the adult population using three biodegradable systems and a titanium osteosynthesis system between 2001 and 2003. A total of 64 patients were followed for 24 months. They found no significant difference between biodegradable materials and titanium fixation with respect to fracture healing and postoperative complications. Postoperative complications were minor and resolved spontaneously or with local therapy [41]. The use of biodegradable plates for fixation of pediatric zygoma fractures eliminates the need for a second surgery to remove hardware. Even though biodegradables have lower strength than their titanium counterparts, the rapid bone healing in children and decreased functional stresses on the pediatric zygoma make them an excellent choice when performing open reduction with internal fixation in this population.
8.4.4 Complications
Complications of pediatric zygoma fractures include persistent hypoesthesia along the infraorbital nerve distribution, enophthalmos, facial widening, and flattening of the malar region [44]. Surgical complications of open reduction with internal fixation include infection, hardware failure, asymmetry, soft-tissue injury, poor scarring, entropion, ectropion, fracture malposition, malunion, and nonunion [9, 45, 46]. Ferreira et al. found facial scarring to be the most common complication resulting from midfacial fractures in children [47]. Lizuka et al., with a mean follow-up of 14.2 months, showed no evidence of growth inhibition in pediatric patients with surgically treated maxillary and zygomatic fractures [48]. While zygoma fractures may cross bony suture lines, they do not disturb the main growth centers of the pediatric skull (the cranial base and nasal septum), making growth disturbances less likely. The fact remains, though, that controlled longitudinal studies are lacking and more evidence is needed in this area.
8.5 Conclusions
Pediatric maxillary and zygomatic fractures are rare and typically result from the high-energy trauma of motor vehicle collisions. As such, they are most commonly seen with concomitant injuries. After stabilization of life-threatening injuries, a thorough history of the inciting injury and careful physical exam of the facial skeleton must be completed. Computed tomographic imaging with three-dimensional analysis of the maxilla and zygomaticomaxillary complex is essential to an accurate diagnosis and proper treatment plan. Conservative, nonsurgical management is reserved for non-displaced or minimally displaced midface fractures with no functional or esthetic deficits. When fractures of the maxilla occur and result in a malocclusion, closed reduction utilizing maxillomandibular fixation can be considered. When the maxilla or zygoma is displaced and more significant functional or esthetic deficits result, open reduction with or without fixation may be necessary. The benefit of restoring form and function must always be weighed against the consequences of operating on the pediatric patient. The soft-tissue scarring and bony trauma created during surgical access, reduction, and fixation of the facial skeleton may lead to growth disturbances in the growing child. The use of biodegradable fixation systems avoids the second surgery required to remove hardware when titanium fixation is used. However, more data is needed to assess whether this benefit carries any growth advantage. In general, bone healing is accelerated in pediatric populations and therefore the timing of conservative treatments and closed reduction techniques is shorter in comparison to adults. Long-term complications from pediatric maxillary and zygomatic fractures include hypoplasia of the affected bones and bony asymmetry. Close longitudinal follow-up of these patients with early recognition of growth disturbance may allow for growth modification techniques prior to skeletal maturity and alleviate the need for further surgical intervention in the adult years.