CHAPTER 4 ECOLOGICAL PHARMACY: FROM GAIA TO PHARMACOLOGY
First, the world of life, taken as a whole, forms a single system bound to the surface of the earth; a system whose elements, in whatever order of association they may be considered, are not simply thrown together and moulded upon one another like grains of sand, but are organically interdependent like . . . molecules caught in a capillary surface.
THE GAIA HYPOTHESIS: STARTING WITH THE WHOLE, THE INTERDEPENDENCE OF ORGANISM AND ENVIRONMENT
Pierre Teilhard de Chardin, a visionary French Jesuit, paleontologist, biologist, and philosopher who was fascinated with evolution and its connection with spirituality, seems to have recognized the limitations of reductionism. He insisted that life must be studied in its totality on a large scale as a single system. As he contemplated the Earth and its life forms in the 1940s, he suggested the term geobiology to embrace all life systems and their environment as a self-organizing whole (Galleni, 1995).
Within two decades of de Chardin’s writing, a significant paradigm shift took place from the quantitative clockworks view to an exploration of the qualitative emergent view of self-organizing systems and even life itself. A system’s approach was starting to be supported in other sciences. For example, in Germany Hermann Haken developed his nonlinear laser theory and Manfred Eigen experimented on catalytic cycles (Capra, 1996); in the United States Heinz von Foerster focused his interdisciplinary team on self-organization; in Belgium Ilya Prigogine grasped the now understood connection between nonequilibrium systems and nonlinearity; meanwhile in Chile Humberto Maturana was postulating autopoiesis and life.
These paradigms that all converged on systems as a whole, coupled with Teilhard de Chardin’s insistence that the Earth must be studied as a unity, with both geological and biological points of view (Galleni, 1995), perhaps led the British atmospheric chemist James Lovelock to one of the biggest revolutions in viewing our planet that possibly we will ever know (Lovelock et al, 1974).
Lovelock worked as a consultant to the U.S. National Aeronautics and Space Administration (NASA) in the early 1960s. NASA contracted Lovelock to aid in the quest to detect life on Mars. Lovelock, contemplating the chemistry of the atmosphere, found that an atmosphere out of chemical equilibrium was the signature of life on a planet. An atmosphere rich in oxygen and methane, for instance, indicates the presence of organisms that are responsible for the uneven mix. Earth has just such an atmosphere (Lovelock, 1979). Through this investigation Lovelock had a flash of insight one day: the planet Earth as a whole is a self-organizing system. He saw the unity of the biosphere—a global organism (Turney, 2005).
Just a few years later, the further study of these features of the Earth resulted in the idea that life vigorously regulated terrestrial conditions, a sort of planetary homeostasis (Turney, 2005). By observing the geological evidence and paleoclimatic evidence, Lovelock, influenced by ecology, physiology, cybernetics, and systems analysis, hypothesized that the ocean’s salinity, the gaseous atmospheric concentration, and the surface temperatures were maintained within narrow ranges by feedback loops of organisms responding to variations in their environment (Lovelock, 1979).
Lovelock postulated that the climate and chemical composition of the Earth’s surface are kept in homeostasis at an optimum by and for the biosphere (Lovelock et al, 1982). The notion was that the biosphere adaptively regulated the Earth (Lovelock et al, 1974). In time, Lovelock (1995) viewed self-organization as emerging from the ensemble of biota and environment. He saw the flow of sunlight on the planet and feedback systems from living organisms to automatically generate comfortable life conditions that synchronistically evolved with the needs of the organisms on the planet. He called this idea of a living planet the Gaia hypothesis.
A model as whole and beautiful as Gaia was predictable. Chemists and physicists probing matter were finding that nature did not consist of isolated components, but rather appeared as a complex web of relations between the various parts of a unified whole. As Heisenberg expressed decades before the Gaia hypothesis was formulated, “the world thus appears as a complicated tissue of events, in which connections of different kinds alternate or overlap or combine and thereby determine the texture of the whole” (Capra, 1982).
Life has existed on Earth for over 3.8 billion years (Lenton, 2002). During this time, the Earth’s surface has been subject to increasing solar luminosity and declining volcanic and tectonic activity, and to such perturbations as massive asteroid impacts (Lenton, 2002; Lovelock et al, 1974; Watson et al, 1978). For example, despite the fact that the heat of the sun has increased by 25% over the last 4 billion years, the Earth’s surface temperature has remained constant, which creates an agreeable environment for life (Lovelock, 1987). The Gaia hypothesis suggests that practically all metabolisms are intimately connected to the flow of chemical compounds (Lovelock, 1989). For instance, the greenhouse gases of carbon dioxide, methane, and sulphur compounds can produce highly reflective clouds, thus affecting the temperature of the Earth’s surface and, in turn, influencing the metabolism of life on the planet (due to temperature change), which again changes the flow of chemical compounds to the surface and atmosphere (Kleidon, 2004).
It seems that life affects the Earth’s surface environment at a planetary level, significantly increasing the cycling of free energy, essential elements, and water; inducing extreme thermodynamic disequilibrium of the atmosphere; and altering the chemistry of the atmosphere, oceans, land surface, and crust (Lovelock, 1987). In turn, the state of the environment influences life, creating feedback loops between life and its environment (Lenton, 2002). These circular processes are organized through feedback loops that are found in every living system. The unusual aspect of the Earth’s hypothesized feedback loops is that they link together living and nonliving systems. For instance, the Gaia theory weaves together plants, microorganisms, and animals with rocks, oceans, and the atmosphere (Capra, 1996).
In this construct, life and the environment evolve together as one system, so that not only does the species that leaves the most progeny inherit the environment, but the environment that favors the most progeny is itself sustained (Kirchner, 2003). This life-enhancing dance of environment and organism can be understood as an emergent property of evolution, because life-enhancing effects would be favored by natural selection (Lenton, 1998). This relationship also requires a radical rethinking of the neo-Darwinist view of evolution. Lovelock deduced the following principles from his observations of the planet (Lovelock, 1989):
At its simplest, the idea that the entire ensemble of living organisms in its interaction with the environment—the biosphere—can be considered a single system has become the basis for a whole series of unfolding programs of research (Turney, 2005).
Now, some 40 years after Lovelock’s realization, a statement issued from a joint meeting in 2001 of the International Geosphere-Biosphere Programme, the International Human Dimensions Programme on Global Environmental Change, the World Climate Research Programme, and the International Biodiversity Programme in a meeting in Amsterdam to study our planet begins, “The Earth System behaves as a single, self-regulating system comprised of physical, chemical, biological and human components.” It seems the science of Gaia has become conventional wisdom (Turney, 2005).
Even some scientists who do not agree with the Gaia hypothesis acknowledge what Lovelock’s vision has added to the study of earth sciences (Turney, 2005; Volk, 2002). The very term earth science exists because of Lovelock’s work. Volk (2002), who does not embrace the implications of the Gaia hypothesis, says, “I was inspired by Lovelock’s early writings to move into issues about the effects of life on a global scale that led to technical work I would not otherwise have accomplished. . . . Gaia became a way of thinking, a mantra to be mindful of the biggest scale.” Many critics accept that it is essential to understand the Earth system as a unity, rather than as a set of disconnected components (Kirchner, 2003).
One of the issues is the defining feature of a complex system like Gaia that makes it extremely difficult to analyze, namely, that the planet is not a well-designed machine, but a complex ensemble of life that constantly rebuilds itself within a range of variable parameters, like all living organisms. This creates a model impossible to analyze from a reductionist perspective (Kirchner, 2003).
Yet a deeper reservation is that a living planet has all the hallmarks of scientific communities coming to grips with a major paradigm shift, a revolution in science (Kuhn, 1962). Whether or not one agrees with the enchanted vision of a biotic Earth, the issue is more than academic. Given the havoc created by our own species, it is vital to comprehend how our planet functions and how it is likely to respond to immature fostership (Lenton, 2002).
Capra (1996) points out that the conception of the universe as an interconnected web of relations is one of the major themes that recur throughout modern physics. The elucidation of the patterns and relationships between a system’s components may yield models that provide a more accurate depiction of reality. He goes on to suggest that Gaia is a mere realization of this line of reasoning. Moreover, such a systems approach may provide a wider perspective for understanding the process of evolution, inviting us to recognize that humans belong to a process that is much more grand than the human species.
COHERENT COUPLING, EXPANDING THE COEVOLUTION CONSTRUCT: ADAPTATION TO THE ENVIRONMENT
Isolating the organism from its environment has been a fundamental tenet of the study of biological processes. In many laboratories around the world this practice is still followed in hopes of gaining further insight into life processes. However, this may lead to incomplete conclusions. Maturana and Varela (1987) proposed that, because organisms are inexorably interwoven with their environments, it is impossible to speak of environment and organism as separate entities. They presented this interrelationship as structural coupling (later called “coherent coupling”) in their landmark book the Tree of Knowledge. They define coherent coupling as a history of recurrent interactions leading to the structural congruence between two (or more) systems (Maturana et al, 1987).
In other words, autopoietic (self-organizing) unities, such as organisms and environment, can undergo coupled histories of structural change due to their consistent and constant interactions. Coherent coupling recognizes the congruence between autopoietic systems. This congruence can include the system and its environment or systems affecting systems. In this paradigm, the environment is seen as a medium, which illustrates the interwoven nature of organism and environment. Development of the autopoietic systems involved thereby arises from transformations that each invokes in the other. This very much challenges the neo-Darwinist evolutionary theory, which in some authors’ opinions drastically underestimate the effects and inseparability of the environment and organism (Cairns, 1996; Scapini, 2001; Thaler, 1994). Such an interdependent relationship is considered unique and diachronic and is a defining principle of an organism and the environment (Scapini, 2001).
The construct of coherent coupling dictates that organism and environment are mutually enfolded in multiple ways, and what constitutes the world of a given organism is enabled by that organism’s history of coupling with its environment (Varela et al, 1991). Indeed, on a human level it is well accepted at this juncture that our intertwining with our environment provides constant perturbation requiring a systemic reorganization of physiological function (Schulkin, 2003).
Although some researchers are realizing the profound effect the environment has on physiological function, especially in regard to health and disease, other researchers have taken it a step further. Cairns’ group (Cairns et al, 1988) published an extremely controversial paper some years ago stating that mutations can be environmentally directed. Following up on Cairns’ work a few years later, Thaler (1994) came to the same conclusion, declaring that the environment can invoke genotypic change and postulating that both the environment and the organism’s perception of the environment can induce genetic engineering genes to rewrite themselves and thus rewrite sections of DNA code. Cairns and Thaler were suggesting a complex engagement of organism and environment. What they perhaps did not know was that they had just provided Maturana and Varela with molecular evidence for their coherent coupling construct. This greatly challenged the prevalent neo-Darwinists’ perspective that views mutations as random events, not specifically adaptive as suggested by Cairns and Thaler. Such a non-Darwinian response, well beyond haphazard natural selection, infers a primary form of intelligence that had developed billions of years ago (Pechere, 2004).
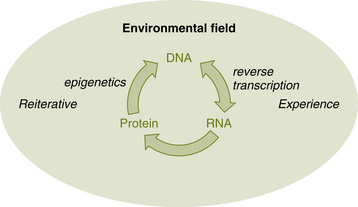
The construct of coherent coupling provides the understanding of an autopoietic system’s ability to be extensively shaped by interactions with its environment over time, and vice versa. Many may see this as the fitting of a system to its environment, but this is not what is meant by coherent coupling. Rather, this construct denotes congruence between an autopoietic system and environment due to changes prompted by each one. It is also important not to confuse this construct with the concept of coevolution, a subset of evolution that includes population genetics and theoretical ecology. Although coevolution accounts for species-species or species-environment interaction, it differs from the coherent coupling paradigm in that the species are still seen separately from their environment and surrounding species. Coevolution still follows the central dogma of biology: Information flows from DNA to RNA to protein and, by extension, to the cell and on to multicellular systems. Crick (1970) originally formulated this dogma as a negative hypothesis stating that information cannot flow from protein to DNA. What this central dogma of biology implies is that a cell’s experience has no effect on DNA sequence (Figure 4-1).
Maturana and Varela (1987) challenged the central dogma by implying that experience can have an effect on DNA. They pointed out that the confusion is in viewing DNA as “uniquely responsible” instead of as having an “essential participation.” Although the organisms and environment are recognized as autonomous in the coherent coupling model, they are also recognized as inseparably engaged in mutually affecting relationships. The result is ontogenic adaptation of the organism to its medium: the changes of state of the organism correspond to the change of state of the medium (Maturana, 1975). Thus organisms are seen as shaped due to historical recurrent interactions with their environment, just as the environment has been shaped by its interactions with the organism.
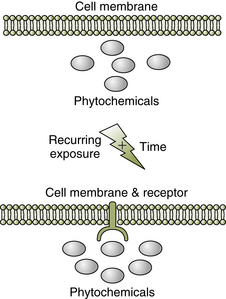
On a microcosmic scale, for instance, cellular membranes have coherently coupled with the abundance of sodium and calcium ions. This is seen through the specialization of proteins in the membrane to allow for active transport and the inclusion of metabolic processes that include sodium and calcium. This implies that the genome adapted to the reoccurring experience of the membrane with sodium and/or calcium. On a macrocosmic scale, the paradigm of coherent coupling leads to an easy realization of the Gaia hypothesis whereby the planetary environment (e.g., temperature, ocean salinity, and atmospheric gases) is modified by various species and, in turn, these species phenotypically and genotypically morph to the environment. It can be stated that all “evolution is coevolution” and that all “development is codevelopment.” Could it be that all evolution and all development are environmental coupling?
Ultimately interpreting Maturana and Varela’s work results in the idea that the coupling of organisms with a high capacity for adaptation goes beyond response to the physicochemical dimension; the morphological, physiological, and psychological plasticity of an organism firmly embeds that organism in its surroundings, creating a dynamic response to recursive perturbations. Put simply, the phenotype depends to a significant degree on the environment, and this is a necessary condition for integrating the developing organism into its particular habitat (Gilbert, 2002).
COUPLING OF HUMANS WITH PHYTOCHEMISTRY: PLANT-HUMAN COALITIONS
The constant interwoven nature of organism and environment requires some sort of exchange of information to account for species plasticity. Markos (1995) defines this exchange that allows species to read their environment, thus integrating into Gaia, as “informational flow.” The informational flow relevant to the discussion between plants and humans is, in its most basic form, chemistry—molecular messaging—although there are likely many other cues that are important to plant-human coalitions.
The secondary metabolites of plants are well known to modulate the interrelations—both positive (i.e., attractant) and negative (i.e. repellent)—among plants and their consumers. The presence of secondary compounds in plants provides information to other species, and due to a reiterative history of interactions, generates a mutual enfolding between plants and humans: plants have always provided oxygen, shelter, clothing, food, and medicine for humans. In turn, we transport, seed, cultivate, and, with our metabolic waste, fertilize plants and provide carbon dioxide.
Higher primates have been evolving and have been exposed to plant chemistry for about 88 million years. The higher primates, considered to be omnivores, are nevertheless primarily herbivores. Over such an evolutionary time scale, all higher primates relied on the predictability of vegetative parts of plants as food sources (Johns, 1996). This includes Homo sapiens, with 5 to 7 million years of exposure to phytochemistry. Of course, this contact with various plant parts exposed the consumer to thousands of secondary metabolites. Estimates of the number of plants in the early human diet range from 80 to 220. Clearly if Homo sapiens regularly consumed such a number and volume of plant foods, its members were exposed to a very high number of phytochemicals. A very conservative estimate would be in the range of 80,000 to 220,000, and the number is quite likely much higher. Ames et al (1990) makes an estimate of the number of secondary metabolites in the current human diet taking into account only those secondary metabolites that are also known to function as pesticides. He observes that even with the great reduction in variety in the human diet compared to our hunter-gatherer ancestors, the modern number of secondary metabolites in the diet is about 10,000 compounds. Thus, even now we are exposed to a great amount of “information” from plants.
If we have coherently coupled with plants, then by default this means that plants have shaped us through informational molecular exchange—and vice versa, we have shaped plants. This shaping, if the hypothesis is solid, should range from DNA to protein. It is easy to see that humans have shaped plants by looking at the cultivation of crops; the original species of any of the crop plants has changed drastically due to human intervention. It is not quite as easy to recognize that plants have shaped us, though one obvious, well-known example is the “shaping” of the cytochrome P-450 (CYP 450) genes. This ancient superfamily of enzymes consists mostly of microsomal and mitochondrial proteins and in humans represents about 75 different CYP 450 genes (Danielson, 2002) (Figure 4-2).
Danielson (2002) points out that CYP 450 genes allow animals to generate a metabolic resistance to plant compounds designed to dissuade grazers and also allow plants to generate new compounds to deter herbivory. He goes on to point out that these CYP 450 genes in plants and animals have been engaged in a cyclical process, generating novel compounds in plants and generating resistance in animals. Jackson (1991) discusses the observation that particular plant compounds, such as alkaloids, glycosides, phenolics, uncommon proteins, unusual free amino acids, steroids, essential oils, terpenes, and resins, are capable of altering the metabolism and potentially changing the biological fitness of humans as well as their domesticated animals, and even the obligate parasites of each species. She points out that detoxification of plant compounds represents an avenue of potentiating individual and group shifts in gastrointestinal function, structure, and endocrine metabolism. But this influence on physiology does not just stop with transient functional effects.
CYP 450 genes have an unusual ability to evolve rapidly, following a quick-paced, nonlinear time course (Danielson, 2002; Nelson et al, 1993). A large-scale expansion of the CYP 450 gene family is thought to have provided a cache of proteins from among which novel isoforms provided adaptive strategies for metabolizing plant compounds. The resulting diversity in these genes is believed to be due to the recurring exchange of molecular information between the secondary metabolites of plants and mammals needing new enzymes to detoxify these plant compounds (Gonzalez et al, 1990). Therefore, the rich exposure of humans to phytochemistry ultimately promoted human biological variability affecting our genes (Gonzalez et al, 1990; Jackson, 1991; Nelson et al, 1993). Was it haphazard mutations that led to such abilities? Or were genotypic changes environmentally directed, as Cairns’ and Thaler’s work suggest?
Another example of coherent coupling between plants and humans is the steroid receptors. Specifically, the estrogen receptor is the earliest member of the steroid receptor family (Hawkins et al, 2000; Wu et al, 2003). The gene structure and ligand-binding properties of the classical estrogen receptor (ER-α) are known to be highly conserved for 300 million years of vertebrate evolution. Thus, the binding of an estrogenic chemical to ER-α in fish, amphibians, reptiles, birds, and mammals (including humans) shows relatively little difference (Katzenellenbogen et al, 1979; Pakdel et al, 1989; Welshons et al, 2003; White et al, 1994). The orthodox view that this protein occurs only in vertebrates needs revision: The microbial organisms known as mycorrhiza, living on the roots of plants, have a receptor called “NodD,” which has a high amount of genetic homology with the human estrogen receptor. Plants also express a protein identical to the human 5α-reductase enzyme (Fox, 2004; Li et al, 1997). Steroids and flavonoids, produced by plants, bind these proteins (Baker, 1992; Gyorgypal et al, 1991). Thus, molecules that have a shape and electronegativity similar to that of the estrogens are used as a communication strategy between plants and fungi (Gyorgypal et al, 1991).
An evolutionary perspective suggests that the communication strategy of plants pertains to us as well. Phytochemical messenger molecules used by symbiotic soil fungi can be sequestered by humans, bind to estrogen receptors, and thereby influence gene expression. Fox (2004) attempts to explain the fact that the NodD and estrogen receptor share no common evolutionary ancestry by invoking the construct of convergent evolution—that is, these different species have responded to similar environmental signals, via natural selection, with the same adaptive traits. However, this leaves the homology between these proteins to mere chance. If we view this through the lens of the coherent coupling paradigm, it offers an example of interspecies plasticity in response to environmental context.
Through this lens, humans and plants would be seen to shape themselves to mutual signals. Wynne-Edwards (2001) postulates that plants chosen for domestication may have a higher occurrence of phytoestrogens. This could potentially enhance the ovulatory cyclicity in women, which might mean more humans to cultivate more crops, an arguable benefit for the particular plant species. Wynne-Edwards goes on to point out that humans have receptors in the nose and cheeks that bind native steroids and plant compounds, which in turn signal the brain. Studies have demonstrated that mammals will consume steroids in foods at some times and reject them at other times, depending on physiological and reproductive conditions (e.g., in pregnancy, rats will reject foods with steroids in them). Thus, true to the coherent coupling paradigm, there is a plasticity of response between animals and plants (Figure 4-3).
Of significance, the effects of flavonoids, nonsteroidal secondary metabolites of plants, share key similarities in mycorrhiza and mammals. Flavonoids can regulate gene transcription in both groups. Moreover, some of these flavonoids can modulate the endocrine system and regulate mammalian physiology through activity on steroid receptors and prostaglandin-synthesizing enzymes (Baker, 1995). In addition, humans express a protein, the 5α-reductase enzyme, that is homologous in sequence and identical in function (the reduction of steroid substrates) to a plant protein (Fox, 2004; Li et al, 1997). Hence, it should come as no surprise that plants have a long history of utilization in treating endocrine ailments; currently, phytochemistry is being explored for the regulation of human fertility. This leads Baker (1992) to suggest that flavonoids may have an evolutionary role in steroid hormone activity. More importantly, this is an obvious example of informational exchange between plants and humans.
That flavonoids are considered conditionally essential nutrients (Challem, 1999) adds to the intrigue. In other words, humans have “coupled” with these particular flavonoid “signals” to such a degree that they enhance our long-term health (Manthey et al, 1998; Martinez-Valverde et al, 2000). One wonders how many other plant compounds, with regular consumption, enhance human health. As research on plant metabolites continues, it is increasingly obvious that many phytochemicals are at least favorable to, if not necessary for, human health. Considering only the vitamins and minerals of plant origin makes it obvious that human physiological processes are dependent on the phytochemistry of plants. Moreover, the evolutionary history of humans’ ingesting plants with a multitude of phytochemicals suggests that the interface of myriad phytochemicals with animal systems may be informative about pharmacology.
HORMESIS AND XENOHORMESIS—ADAPTATION TO THE PHYTOCHEMICAL ENVIRONMENT
There are a number of reasons that the complex chemistry that is inherent in the ingestion of a plant produces different effects than does an isolated chemical. Of these reasons, pharmacokinetic potentiation, pharmacodynamic convergence, and hormesis and xenohormesis are the best known and the easiest to discuss in the existing framework of pharmacology. Pharmacokinetic potentiation involves processes related to absorption, distribution, metabolism, and excretion, whereas pharmacodynamic convergence involves modulation of multiple biochemical pathways, membrane dynamics, receptor binding cooperativity, and shifts in the degrees of freedom of proteins (enzymes and receptors). Although both of these modes of activity are unique to the ingestion of chemical mixtures, they are commonly put under the rubric of synergy, even though they would ideally be discussed separately. The last mode, hormesis, has been well established by the field of toxicology, and the concept is slowly encroaching into physiology and pharmacology. Regardless of the scientific discipline of origin, it is a useful construct for understanding how plant chemistry interfaces with living systems. Xenohormesis provides an overarching construct to encompass much of what has been previously discussed.
Hormesis Defined
The term hormesis is derived from the greek word hormon meaning “to excite.” In other words, on ingestion of a hormetin, physiological processes are stimulated into activity. Simply put, hormesis is a paradoxical effect of a toxic chemical or radiation at low dose (Trewavas et al, 2003). Stebbing (1982) defined hormesis as low-dose stimulation followed by higher-dose inhibition. A more complete definition by Calabrese, who has spent the last 15 years bringing hormesis back to the attention of physiologists and pharmacologists, is “an adaptive response characterized by biphasic dose responses of generally similar quantitative features with respect to amplitude and range of the stimulatory response that are either directly induced or the result of compensatory biological processes following an initial disruption in homeostasis” (Calabrese et al, 2002). The idea behind hormesis is a dose-response relationship, a beneficial physiological upregulation induced by small doses of a toxin. Many terms have been used to describe this effect (Table 4-1), including the common biphasic dose response.
TABLE 4-1 Previous Terms Applied to the Hormesis Dose Response
| Compensatory response | Bell shaped |
| Facilitation-inhibition | β curve |
| Intermediate disturbance hypothesis | Bidirectional |
| Paradoxical dose responses | Biphasic |
| Reverse response | J shaped |
| Stimulatory-inhibitory | U shaped |
| Subsidy-stress gradient | |
| Previous Laws Referring to Hormesis | |
| Hebb law | |
| Yerkes-Dobson law | |
| Arndt-Schulz law |
From Calabrese EJ, Baldwin LA: Defining hormesis, Hum Exp Toxicol 21(2):91, 2002.
Calabrese and Baldwin (2002) point out that not only have the hormesis dose-response phenomena been labeled with diverse terminology but there are also several biological “laws” referring to hormesis (see Table 4-1). Although this suggests that the phenomenon has repeatedly been “discovered” by different research groups, it is also a comment on the unfortunate lack of conceptual integration across scientific disciplines.
What the hormesis dose-response data suggest is that there is a common regulatory strategy for biological resource allocation and a plasticity of regulatory processes dependent on environmental perturbations due to a long history of coherent coupling (Spelman, 2006) or coevolution with phytochemistry.
History of Hormesis: Politically suspect but Scientifically Solid
As mentioned, the hormetic response is oriented toward dose-response effects of substance. Although Calabrese and coworkers have brought this construct back into acceptance in the sciences, it had long been recognized in ancient systems of pharmacology.
For example, in the 5000-year-old Ayurvedic system there is a tenet that everything, even poisons, can be used as medicine if properly utilized. Thus, very small amounts of heavy metals were used to rejuvenate the system in the weak, convalescing, and aging. In the sixteenth century Paracelsus (Philippus Aureolus Theophrastus Bombast von Hohenheim), a Swiss chemist, was known to use toxic substances, with particular attention to dose (Gurib-Fakim, 2006; Wood, 1992). Although there is much skepticism about this therapy, it is written that his results were particularly positive (Wood, 1992). Nonetheless, Paracelsus’s therapies included the use of heavy metals, and centuries later this led to use of the well-known term quack derived from the German word quacksalver or “quicksilver,” an old term for mercury. By the late 1800s published research had demonstrated that chemicals that were toxic to yeast could stimulate growth and respiration if used in lower dosages (Calabrese et al, 1999).
By the 1920s a researcher committed a blunder in the politics of science by associating the phenomena of hormesis with homeopathy (Calabrese, 2006). Considering that this was only a few years after issuance of the Flexner Report, an association with homeopathy was a death sentence to any scientific hypothesis, irregardless of the existence of reproducible evidence. Although the Flexner Report had resulted in the needed elimination of many of the illegitimate schools of medicine of the early twentieth century, it also, apparently by design, put on its hit list any school teaching a system of medicine other than allopathy. It did not help matters that there was also a paucity of explanations based on the biochemical understanding of hormesis at that time (Stebbing, 1982). However, laboratory observations of the hormesis phenomenon continued unbiased in other parts of the world, and a German journal, Zell-Stimulations Forschungen, was established to report hormetic effects (Calabrese et al, 1999). By 1943 the scientific method cut through the politics in the United States. Researchers at the University of Idaho reproducibly observed the phenomenon, calling it “hormesis,” unaware of its previous labels (Calabrese et al, 1999).
About 50 years later a newsletter of original research, Stimulation Newsletter, which lasted just over a decade, reported the enhancement of plant growth and yield by exposure to low-dose radiation (Calabrese et al, 1999). Upon the arrival of the 1980s, despite lingering skepticism about the hormesis phenomenon, a book providing a lengthy review of the research on radiation hormesis was published in the United States (Luckey, 1980). By the end of the twentieth century, through the work of the Calabrese group, a substantial database of dose-response studies demonstrating hormesis to be common and reproducible had caught the attention of physiologists and pharmacologists (Calabrese, 2006). Hormesis as a scientific principle is solid. But is it here to stay?
Utility of Hormesis: Understanding Humans and Their Relations to the Environment
Hormesis is not just relevant to poisons such as heavy metals, synthetic pesticides, radiation, and pollutants (Calabrese et al, 1999). Needed substances such as vitamins, minerals, and oxygen are also toxic at excessive doses (Calabrese et al, 1999). Nor is this principle only observed with natural compounds. This principle applies to endogenous compounds as well. Biosynthetic compounds moving through the human system, such as the adrenalines, adenosine, androgens, estrogens, nitric oxide, opioids, many peptides, and prostaglandins, all may have beneficial effects at low concentrations but detrimental effects at high concentrations (Calabrese, 2006). Some pharmaceuticals are also known to conform to this principle. For example, low doses of antibiotics may actually enhance reproduction of pathogenic bacteria, whereas higher doses are toxic to these microbes (Calabrese et al, 1999). Probably the best known and most commonly consumed hormetin is alcohol. Alcohol, a solvent, can clearly be toxic. However, at low doses alcohol is known to be beneficial to health and protective against cardiovascular diseases and some cancers.
Calabrese and Baldwin (2002) suggest that the hormesis response provides a biological buffering response to protect against environmental and endogenous insults. The observation of this response in so many different organisms and cell types against such diverse chemical groups (and radiation) suggests a systemwide feedback response resulting in upregulation of many regulatory processes (Calabrese et al, 1999) and an evolution-wide biological strategy (Calabrese et al, 2002). By overcompensating to an initial disruption caused by an environmental stressor, an organism is protected against the possibility of further exposures (Calabrese et al, 1999).
The hormetic dose-response effects seen for so many phytochemicals also suggests that the mode of activity for health enhancement by fruits, vegetables, and spices may be due, at least partially, to the evolutionary protective response previously mentioned. Furthermore, it argues against a strictly antioxidant mode for plant-based foods, which has become a common assumption among many clinicians and researchers. Like exercise and caloric restriction, many phytochemicals may act as mild stressors to induce an adaptive response via upregulation of multiple genes producing a protective effect (Calabrese, 2005; Mattson et al, 2006) (Box 4-1).
BOX 4-1 Well-Researched Plant Compounds That Are Beneficial at Low Doses but Detrimental at High Doses
From Trewavas A, Stewart D: Paradoxical effects of chemicals in the diet on health, Curr Opin Plant Biol 6(2):185, 2003; Mattson MP, Cheng AW: Neurohormetic phytochemicals: low-dose toxins that induce adaptive neuronal stress responses, Trends Neurosci 29(11):632, 2006.
At this juncture, some researchers thinking outside the box are applying the hormesis construct to grasp the relationship between the natural pesticides occurring in plants and human health. Many of the secondary compounds plants produce are antifeedants, antimicrobials, and insecticides for the plants’ protection (Poitrineau at al, 2003). The well-respected researcher Bruce Ames points out that of all the pesticides in the diet, both those that are naturally produced by the plant itself and synthetic pesticides applied to the plant by farmers, 99.9% are naturally occurring (Ames et al, 1990). This becomes particularly relevant to human health in that many of these natural “pesticides,” such as flavonoids (Baker, 1998) and coumarins (Zangerl et al, 2004), are known to be beneficial to human health in multiple ways, including through anticancer activity and beneficial cardiovascular effects (Baba et al, 2002; Hamer et al, 2006; Hollman et al, 1997; Hoult et al, 1994; Knekt et al, 1996; Lin et al, 2001; Nijveldt et al, 2001). At low doses these compounds appear to activate adaptive cellular stress-response pathways (Mattson et al, 2006). At high doses, however, many of these compounds can become carcinogenic (Trewavas et al, 2003).
What this also suggests is that diets high in animal-based foods and processed foods may lack the protective effect of diets high in phytochemicals (Johns, 1996). Ames and Gold (2000) point out that about 80% of U.S. and 75% of U.K. citizens eat insufficient fruit and vegetables to provide minimal protection against cancer. After summarizing 200 epidemiological studies, Block et al (1992) reported that consumption of a diet rich in phytochemicals from fruits and vegetables reduced cancer risks by about 50%. Knoops et al (2004) found that in individuals aged 70 to 90 years, adherence to a phytochemical-rich diet was associated with a more than 50% lower rate of all-cause and cause-specific mortality. Norris et al (2003) showed that good health habits, one of which includes consumption of a phytochemical-rich diet, are associated with a 10- to 20-year delayed progression of morbidity. What might seem paradoxical to the casual observer is that many phytochemicals, evolutionarily derived to protect plants from predators, are detrimental to health at high doses (Figure 4-4).
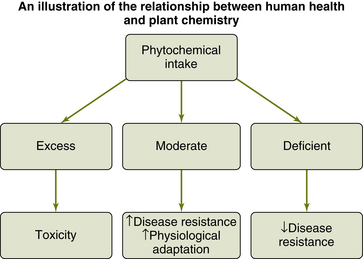
Figure 4-4 Exposure to secondary plant metabolites, many of which were selected to protect plants against bacteria, insects, and herbivores, can be protective to human health. In excess doses, however, many of these natural microbicides and insecticides are toxic. In insufficient doses, human health may be compromised. At ideal doses human health may be enhanced due to the hormesis principle, and the individual may therefore be more resistant to disease processes and better able to respond to changing environmental circumstances.
(Adapted from Johns T: The origins of human diet and medicine: chemical ecology, Tucson, 1996, University of Arizona Press, p 213.)
Xenohormesis
The majority of life forms on the planet either feed on, or live in, close proximity to photosynthesizing organisms (photoautotrophs). Thus there is a long-term evolutionary relationship between photoautotrophs and heterotrophs (fungi and animals). Much of this relationship is based on the secondary metabolites from plants. Plants are known to synthesize secondary metabolites in response to environmental conditions. These phytochemicals, in turn, may be utilized by their surrounding heterotrophic neighbors as cues to impending environmental changes. Thus, when ingested or absorbed by coexisting life forms, such as bacteria, fungi, animals, or humans, certain phytochemicals may provide a chemical signature of the state of the environment (Howitz et al, 2008).
The polyphenols are one example of phytochemical compounds carrying information about environmental conditions. This class of compounds includes the anthocyanidins, catechins, chalcones, flavanones, flavones, isoflavones, and tannins. Spelman et al (2006) suggested that the metabolic expense of generating such molecules would generate a “chemical economy,” an efficient and multiple use of one molecule. Indeed, these molecules are known to be multifunctional in that they are antioxidants, antibiotics and fungicides, herbivory deterrents, and ultraviolet radiation protectants. However, they are also known to play a role as signaling molecules, carrying environmental information to heterotrophs. Stafford (1991) proposed that the original role of the polyphenols was as signaling molecules and that their other properties evolved later. Flavonoids do provide cues to plant development (Taylor et al, 2005), and it has also been proposed that flavonoids were the original steroid signaling molecules (Baker, 1992).
Accumulating evidence does suggest that mammals sense stress-signaling molecules. The mammal that could respond to molecules such as the polyphenols would have an advantage over those competitors that could not interpret these environmental cues. A possible explanation for this phenomenon is based in evolutionary biology. Kushiro et al (2003) proposed that the biosynthetic pathways for signaling compounds originated in a common ancestor of plants and animals. As the phyla diverged, the heterotrophs eventually lost their ability to synthesize polyphenols, but retained the ability to respond to these messenger molecules. The retention of the ability to respond to these molecular cues likely allowed for an anticipatory adaptation to environmental changes (Howitz et al, 2008).
At the least, recurring interactions between phytochemicals and heterotrophic proteins on an evolutionary time scale may have generated conditional requirements for some phytochemicals (Spelman et al, 2006). The consumers of these molecules have been shown to respond by inducing cellular defenses and resource conservation (Howitz et al, 2003). For example, it is well known that the polyphenols butein, fisetin, and the well-publicized resveratrol extend life in fungi, nematodes, flies, fish, and mice (Westphal et al, 2007). In addition, concentrations of polyphenols required to extend life span in the laboratory (approximately 10 μM) are detectable in the leaves and fruits of stressed plants (Howitz et al, 2008). This interspecies hormesis, a mode of interpreting stress signals from surrounding organisms to improve survival potential, has been termed xenohormesis (Howitz et al, 2003).
The xenohormesis hypothesis varies from the hormesis model in that the stress occurs in one organism and the coexisting species, which have evolved to sense the surrounding chemical ecology, are the beneficiaries (Howitz et al, 2008). In regard to the age-old game of adaptation to the environment, it is sensible to propose that absorbed phytochemicals carry information about the status of the environment and imminent changes in an animal’s food supply. Moreover, considering the evidence that stress-induced plant compounds upregulate pathways that provide stress resistance in animals and humans and an evolutionary imperative for anticipatory adaptation, plant consumers would sensibly have modes to perceive these chemical cues and react to them in ways that are beneficial.
The hormesis and the xenohormesis hypotheses are not mutually exclusive. Responses to absorbed toxins (hormesis) and the ability to respond to molecules of environmental origin as molecular signals (xenohormesis) were likely concurrent developments in evolution. Howitz and Sinclair (2008) point out that in an animal’s response to complex mixtures of phytochemicals both responses are likely at play.
This environmental coupling may have resulted in a conditional dependence on phytochemicals for the modulation of particular proteins. For example, the nucleotide-binding sites of protein kinases appear to bind the polyphenolic flavonoids and stilbenes with reasonable affinity. The evidence indicates that these polyphenols do not compete with the enzyme’s nucleotide substrates; rather they bind elsewhere (Gledhill et al, 2007; Howitz et al, 2003). Molecules such as resveratrol and quercetin have been found to bind not to conserved domains but to hydrophobic pockets (Gledhill et al, 2007). This may partially explain their ability to modulate multiple proteins. Howitz and Sinclair (2008) suggested that this is consistent with the driving of these polyphenol-protein interactions by selective pressures rather than by coincidental binding. This also suggests that these interactions are likely potentiating with one another and with endogenous regulators.
There are also data demonstrating that many of these compounds bind to the same binding pockets as endogenous regulators (Baker, 1992). Both types of interactions open the door to the claims of synergy so often cited for multicomponent extracts from medicinal plants (Spelman et al, 2006). At the least, the ingestion of phytochemical cocktails likely involved multiple interactions that go well beyond ligand binding and involve subtler molecular dynamics (Spelman, 2005) (see Molecular Modes of Activity later in the chapter).
The xenohormesis hypothesis makes a number of predictions that rest squarely on organisms’ relationship of coupling with their environment (Lamming et al, 2004). First, there is likely a substantial cache of medicinal molecules that are upregulated in stressed plants that can benefit the user. Second, xenohormetic phytochemicals serve as messenger molecules by interacting with a variety of enzymes involved in regulating stress responses and survival. Third, these molecules should be relatively safe for human consumption. Fourth, there may be conserved domains in enzymes and receptors that do not interact with endogenous molecules (Howitz et al, 2008). Last, many phytochemicals, due to a history of recurrent interactions with heterotrophic proteins, may have developed a structural congruence that potentiates the effects of endogenous regulatory molecules. If the aforementioned predictions hold true, then xenohormesis may provide the philosophical underpinnings explaining why many phytochemicals have been documented to enhance health.
The xenohormesis hypothesis, when fully recognized, has implications for the foundations of pharmacology. Although classical pharmacology is based on high affinity and selectivity, many physiologically active phytochemicals are known to function with broad specificity and low affinity (Ágoston et al, 2005). This creates a quandary for the pharmacological paradigm, because many phytochemicals are known to affect multiple proteins. Although polyvalent binding (binding of a small molecule to multiple proteins) is considered an inferior pharmacological strategy by pharmaceutical standards, it may have been the original mode of upregulation of defensive physiological responses and provide distinct evolutionary and pharmacological advantages.
Danger of a Second Rejection from the Political Halls of Science
Although the argument just described is based on solid science, a logical extension of this argument is the use of food and medicinal plants for enhancement of health. Many opponents of the view that plants have therapeutic value claim that there is not enough of any one chemical in plants to make them therapeutically useful (Spinella, 2002), Although this argument explains why medicinal plants are sometimes called “crude drugs,” it also demonstrates a gross misunderstanding of the pharmacology of complex mixtures. Furthermore, it completely misses the hormesis phenomenon.
Thus the argument that food and medicinal plants are not effective for inducing physiological change because they are too dilute to have activity contradicts the entire database of hormesis studies demonstrating that minute doses of substance do induce a general and reproducible biological response. At the same time, the hormesis principle should call into question the practice of concentrating an active constituent from a plant by standardization. Although the quality and identity of medicinal plant preparations must be ensured, concentration of one constituent in a medicinal plant preparation may, in some cases, breach the dose for beneficial activity and move toward a detrimental dose, particularly if the compound operates through a hormetic mode of activity. Nonetheless, understanding hormesis can help the allopathic community appreciate one of the possible modes of activity for medicinal plants. The hormesis principle was dropped like a hot potato in the early part of the twentieth century because of the association with homeopathy. Will hormesis again be shunned because it is invoked as an explanation to understand the action of medicinal plants? One would hope that science would cut through petty politics in the interest of human health.
When hormesis is viewed in an ecosystem context, hormetic responses as measured by the effects on growth can turn out to be a result of altered competition between species. If a competitor, parasite, or disease of a species is more susceptible to a certain chemical than the species itself, then the species will experience a relief from a resource-demanding stress factor and hence increase growth at low concentrations of that chemical. This is the basic principle behind the beneficial effect of pharmaceuticals such as penicillin on vertebrates and leads to the xenohormesis hypothesis.
ECOLOGICAL PHARMACY: THE UNDERPINNINGS OF PHARMACOLOGY
The aforementioned evidence logically leads to the end point of a discussion on pharmacology; that is, how has adaptation to phytochemical exchange influenced the physiological processes of organisms consuming plants? A key point is that ingestion of plants, a process that has been going on for 300 million years for vertebrates, 88 million years for higher primates, and 7 to 10 million years for humans, leads to exposure to an array of plant compounds in every swallowed bolus. Never has the consumption of edible foodstuffs involved a single, isolated compound. This is of pharmacological significance: our current model in pharmacology attempts to induce physiological change through the ingestion of one chemical at a time.
In an unspoken oversight of the medical sciences, the rationale for the approach of isolation and purification of active constituents from “crude drugs” has never been made explicit. The general conclusion drawn from a century of research on isolation of active constituents from medicinal plants is that medicinal plants typically contain numerous active compounds (Gilbert et al, 2003; Singer et al, 1962; Spelman, 2005; Spelman et al, 2006; Williamson, 2001). A key point regarding the politics surrounding the use of food and medicinal plants to promote human health is that multiconstituent plant medicines were not forsaken because of research that demonstrated harmful or ineffective activity, but because they were too complex to study in their multiconstituent form (Vickers, 2002). Nevertheless, pharmacological modeling has used isolation as a fundamental tenet of inducing physiological shifts in humans. Unfortunately, this methodology is deficient in revealing the mode of activity of the bulk of food and medicinal plants because it neglects the possibility of synergic, additive, or antagonist activity of multiconstituent remedies (Cech, 2003). Moreover, it grossly simplifies human health to only those parameters observed in reductionist models.
According to a pharmacological paradigm supported by a foundation of human adaptation to the informational input from plants, our physiological processes, down to the level of our genes, have undergone a history of recurring biochemical interactions with complex phytochemistry that has lead to the structural congruence of humans and plants. The previously discussed shifts in DNA, the homology of proteins, and the ligand-receptor relationship between humans and plants are examples of structural congruence. Humans have integrated with plants so that multiple concurrent biochemical perturbations are ordinary. Reiterative exposure to minute doses of numerous plant metabolites provides constant stimuli for biological adaptation (Jackson, 1991). In turn, this adaptation has had profound effects on human health.
Jackson (1991) aptly calls attention to system stability, writing that system diversity is proportional to system stability. Another way of expressing this in regard to human health is that the stability of health may be seen as a function of exposure to phytochemical diversity. Keith and Zimmermann (2004) suggest that many genes might need complementary action to modify disease processes. In other words, therapy could be more effective if pharmacological agents engaged with more than one biochemical site. Quite likely, the majority of the multitude of plant constituents that ancient humans regularly consumed throughout their evolution had a positive affect on many of the health-modifying genes because of the millions of years of history of recurring exposure to multicomponent phytochemical mixtures. Observations do indicate that people who consume a phytochemical-rich diet have a significantly better health status than those who have a diet low in phytochemicals (McCarty, 2004). It is quite likely that phytochemicals interface with a large percentage of the estimated 10,000 health-modifying genes (Keith et al, 2004). Unfortunately the current number of pharmacological targets, approximately 300 to 400, is anemic compared with the phytochemical-gene interface that occurs in diets rich in plant-based foods.
A pharmacological model that accounts for millions of years of exposure to arrays of phytochemicals provides not only a recognition of plants as sources of medicines but also a multitarget approach that single-chemical, stand-alone interventions cannot offer (Keith et al, 2004). And it returns us to the origins of pharmacology, in which what humans regularly ingested, somewhere between 80 and 220 plants with an estimated 80,000 to 220,000 secondary metabolites, modified multiple physiological processes in a concerted manner. The understanding of the translational response of numerous proteins to multiple perturbations, such as provided through phytochemistry, holds promise for the fields of medicine and biology not because it is a new insight, but because it is an ancient process that shaped human physiology. Such a paradigm shift would also advance the understanding of biological molecular networks and open up further therapeutic strategies.
MOLECULAR MODES OF ACTIVITY
Cellular Membrane and Signal Transduction
Cellular morphology is the result of a nonlinear and dynamic molecular flux, especially related to the cell membrane. Although the membrane has been described as a system driven by thermodynamic equilibrium (Aon et al, 1996), it is more accurately seen as an emergent structure consisting of highly asymmetrical structures and phase transitions (Perillo, 2002).
Typically, mammalian cellular plasma membranes consist of about eight major classes of lipids (Simons et al, 2004) and includes embedded proteins in its bilipid structure. Because signal transduction and the complex behavior of chemical reactions is coupled to the dynamics of membranes, the membrane has been closely scrutinized in hopes of further understanding the cell’s ability to receive, process, and respond to information. Unfortunately there has been (and still is) an epistemological divide between the analysis of the complex behavior involved in biochemical events and the structural aspects of the membrane involved in signaling phenomena, especially in relation to signal transduction involving exogenous molecules (Perillo, 2002).
Until very recently, explanations of signal transduction were based on a linear model that defined successive steps in the decoding process and focused on compounds with high affinity and selectivity. However, the membrane, key in its interactions with the ensemble of phytochemicals to which early humans were consistently and constantly exposed, may also respond to compounds that do not exhibit high affinity and high selectivity for a particular receptor species. Ignoring these interactions may lead to erroneous conclusions in the basic sciences.
Significantly, systems properties of heterogenous molecular ensembles could induce minute difference in the strength of attractive forces among molecules and increased degrees of freedom within a pharmacological system (Buehler, 2003). Just as phase separations and self-assembly processes are systems properties of molecular ensembles, a pharmacological systems approach is required to understanding a phytochemical matrix interacting with another biological system (Spelman, 2005). The author proposes three modes of activity based on recently elucidated behaviors of the cell membrane, two that involve the bilipid membrane and one that is based on concerted activity.
1. Cooperative Binding by Receptors: Receptor Mosaics
The discovery of direct receptor-receptor interactions rigorously challenges the historical belief that the receptor is the minimal unit for drug recognition and activity and therefore that high-affinity, high-specificity compounds are superior ligands (Kenakin, 2004). The existence of various types of receptor mosaics, clusters of receptors functioning as a unit that demonstrate cooperative binding, suggests a plasticity of the steric conformation of receptors (Agnati et al, 2005). In the receptor mosaic model each receptor is seen as a subunit of a multimeric protein.
Recall the cooperative binding of oxygen to hemoglobin. After one oxygen molecule binds to hemoglobin, the affinity of the other binding pockets for oxygen increases. Thus, the likelihood of subsequent binding of oxygen molecules is increased.
Cooperativity is considered a mode of self-regulation by multimeric proteins (Koshland et al, 2002) and is hypothesized to be so for receptor systems as well (Agnati et al, 2005). In receptor mosaics the conformational change caused by the binding of the first ligand is transmitted to adjacent receptors with reciprocal contact to change the affinity for subsequent ligand binding. The change in affinity is due to the conformational change induced by the first bound ligand, which induces sequential changes of the multimeric protein’s neighboring subunits. This change in protein conformation may make subsequent binding easier (positive cooperativity) or more difficult (negative cooperativity).
Because a phytochemical matrix consists of hundreds of compounds, including groups of constituents that vary slightly in their structure but are based on a common backbone (Yong et al, 2004), there may be both high-affinity ligands and low-affinity ligands for a given receptor. Once the high-affinity ligand binds to a species of receptor, other receptors, because of intramolecular transfer of the conformational change to the adjacent peptides, may be able to bind the lower-affinity ligands and play a role in cellular messaging. Accordingly, the search for only high-affinity compounds within such a matrix may miss lower-affinity compounds that could bind within receptor mosaics due to cooperative binding. This suggests one possible molecular explanation of the synergistic effects so often invoked by phytotherapists to suggest that plant medicines and foods cannot be reduced to an “active” constituent. The receptor mosaic model also suggests the need for an expansion of the traditional pharmacological methodology of searching for only high-affinity ligands within plant chemistry (Figure 4-5).
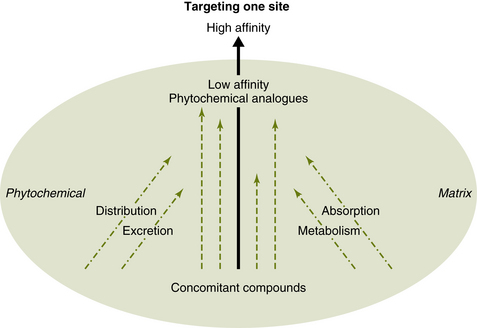
Figure 4-5 Under the current pharmacological model, only high-affinity and high-selectivity compounds are sought, and compounds with lower affinity for receptor (and enzyme) binding are overlooked. In contrast, the receptor mosaic model holds that the low-affinity compounds typically accompanying high-affinity compounds in plant extracts may cooperatively bind, affecting signal transduction. In addition, the concomitant compounds commonly improve the pharmacokinetics (absorption, distribution, metabolism, and excretion) of the low-high affinity compounds.
2. Shifts in Membrane Electronics and/or Shape: Nonspecific Membrane Interactions by Exogenous Molecules
Many components of signal transduction, such as receptors, are anchored in the plasma membrane and therefore are subject to the biochemical milieu of the plasma membrane. Of the four basic receptor signaling modes—gated ion channels, metabotropic receptors, receptor enzymes, and the steroid receptor—three are directly linked to plasma membrane processes. This lipid-rich two-dimensional environment allows for hydrophobic interactions that lead to alterations in component access, orientation, and effective concentration (Weng et al, 1999). Hence, modulation of the molecular organization of the membrane may have an effect on signal transduction.
Many drugs are amphiphilic or hydrophobic molecules, and a common site of action for these compounds is the plasma membrane (Perillo, 2002). Among the amphiphilic compounds, many of the central nervous system depressants (Goodman et al, 2001) will, because of their molecular properties, self-aggregate into micelles (Perillo, 2002). Despite significant molecular investigation into modes of activity for some of the hydrophobic drugs (e.g., the local anesthetics) no specific receptors for them have been revealed (Franks et al, 1984; Schreier et al, 2000). Rather, these compounds demonstrate activity at the plasma membrane surface (Perillo, 2002).
Hydrophobic and amphiphilic compounds, and the resulting micelles, may induce shape changes, membrane disruption, vesiculation, and solubilization (Schreier et al, 2000). Consequently, exogenous molecules may generate membrane asymmetries that result in membrane tensions (Garcia et al, 2000; Perillo et al, 2001). As expected given the thermodynamics of open systems far from equilibrium, the membrane perturbations caused by curvature tensions and the flux of molecular movements from one monolayer to the other shift the resting state of the membrane and reorganize cellular shape (Perillo, 2002). Changes in the curvature of the membrane, as well as its composition, lead to demonstrated changes in the function of the membrane when it interfaces with an exogenous molecule (Farge et al, 1993; Garcia et al, 2002; Mui et al, 1993). Given that protein conformation is dependent on molecular interactions, structural change may also induce alterations in protein conformation (Simons et al, 2004). This could result in signal transduction.
Notably, many of the secondary compounds of plants are amphiphilic or hydrophobic (e.g., hyperforin in St. John’s wort, the curcuminoids in Curcuma longa [turmeric], alkylamides in Echinacea species) and would accordingly likely display similar behavior. Given the evolutionary history of plant ingestion by humans, membrane interactions with “nonactive” compounds in plants were likely routine. Consumption of a plant led to ingestion of active constituents and other phytochemicals that influenced membrane dynamics. Consequently, with recognition of evolutionary precedent, the combination of compounds affecting the membrane with active compounds binding to receptors was part of routine physiology. This may be a partial explanation of why many isolated plant constituents do not appear to function in the same way as they do when given in a whole-plant extract.
3. Polyvalent Activity: Biochemical Convergence
The last two modes of activity were discussed in relation to the realm of an isolated cell. However, signal transduction involves networks of cells, tissues, and organs. Following the science of physics, molecular biology is slowly moving from study of the components of signaling to investigation of the context in which the signaling occurs. Study at the molecular level of components alone will not advance the understanding of when and why cells interact in their typically nonlinear, nonlocal, multiple feedback loops (Maini, 2002).
Physiology does not run in linear, sequential processes involving one chemical at a time. Robust systems, like living organisms, are likely quite responsive to numerous but subtle chemical perturbations (Ágoston et al, 2005). Thus multisystem analysis will probably be found to be essential to understanding signaling networks (Plavec et al, 2004). Allowing for models that include multitarget and multipathway assaying could clearly elucidate the informational connectivity of networks. Aon et al (1996) refer to the network of interactions established between dynamic subsystems through common intermediates or effectors (hormones and second messengers) as dynamic coupling.
It is well established that the overall combination of nonnutritive phytochemicals appears to be key to plants’ positive effects on health, that the health-giving effects of plants are not always related to the nutrient content (McCarty, 2004), and that significant consumption of secondary compounds from plants plays an important role in the prevention of chronic diseases (Liu, 2003). Although some constituents are interfacing with receptors and membranes, others are influencing pharmacokinetics. For example, concomitant compounds, frequently considered excipient nonactive constituents, can affect absorption, distribution, metabolism, or excretion of other constituents, enhancing (or antagonizing) their bioavailability (Eder et al, 2000). Moreover, as the xenohormesis hypothesis suggests, many of the excipients removed from our foodstuffs and medicinal preparations may upregulate beneficial physiological processes.
Recognition of such subtle perturbation would eventually allow the understanding of a disease-modifying molecular network and further pharmacological target potential. Monitoring of targets tripped by polyvalent groups of compounds will almost certainly lead to the recognition of yet further biochemical connectivity. Moreover, as our knowledge of the range of perturbable sites improves, proteins expressed by what are now considered mere “housekeeping” genes will likely be recognized as disease modifying. The outcome could be an expansion of the understanding of the disease-modifying gene network and further therapeutic targets (Keith et al, 2004). Such a perspective will likely lead to the acknowledgement that a multitarget perturbation, as happens with the consumption of any plant product, holds the potential for significantly more therapeutic activity than single-chemical, stand-alone interventions.
The ingestion of plants leads not only to the potential for multiple compounds to interface with multiple targets, but also for single compounds, due to their broad specificity, to engage multiple targets. Generally, the pharmacological sciences consider these molecules “dirty” because of their lack of selectivity. Such molecules are thought to have more potential for producing adverse events because of “off-target” effects than does a highly selective chemical. However, dozens, if not hundreds, of multifunctional compounds have been identified in natural products chemistry that are known to be quite safe (Corson et al, 2007). For example, the well-known phytochemical group of the salicylates are known to interact with multiple proteins. The ubiquitous catechins, such as epigallocatechin-3-gallate, have demonstrated considerable chemopreventative activity via induction of apoptosis, inhibition of multidrug resistance pumps, promotion of cell cycle arrest, and inhibition of cyclooxygenase-2 (Khan et al, 2006). The curcuminoids are documented to engage over 60 molecular targets to protect against cancer and regulate the expression of inflammatory enzymes, cytokines, adhesion molecules. and cell survival proteins (Goel et al, 2008). The not uncommon resveratrol modulates the function of over two dozen enzymes and receptors, leading to protection against cancer, atherosclerosis, and diabetes while promoting endurance (Howitz et al, 2008).
Csermely et al (2005) have found using network models of pharmacology that the partial inhibition of multiple targets offered by a mixture of chemicals is often more efficient than the complete inhibition of a single target. For example, Wald and Law (2003) suggest that a combination of six drugs at subclinical doses—a baby aspirin, three blood pressure drugs (at half the standard dose), a statin, and 800 mcg of folic acid—could extend life by 11 years (Figure 4-6).
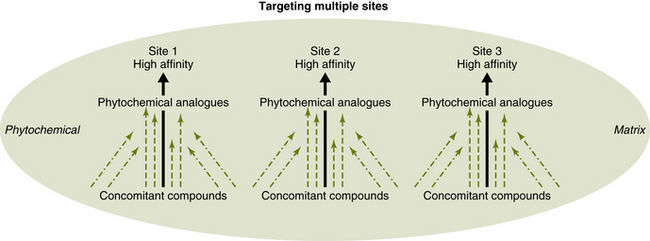
Figure 4-6 Physiology is a complex process that operates in a symphonic manner with multiple receptors, enzymes, and genes being affected at any given moment. When a plant extract or food is ingested, the phytochemicals triggers many sites concurrently, which can then converge on a positive outcome.
In addition, in a meta-analysis encompassing 56,000 patients with hypertension, Law et al (2003) concluded that combinations of two or three drugs at half the standard dose delivered therapeutic effects comparable to those of one or two full-dose antihypertensive medications. Not surprisingly, the multiple low-dose drug combination was preferable because of the reduction in side effects. Clinicians have historically overcome single-target insufficiency by using combination drug therapy such as seen in today’s clinical protocols for treatment of human immunodeficiency virus infection, tuberculosis, and cancer. Csermely et al (2005) propose that partial drug inhibition by multiple drugs could prove to be a superior pharmacological strategy to strong inhibition by one drug at a single target. This is likely due to the need for complementary action on multiple targets to modify disease processes (Keith et al, 2004).
When combinations of various pharmacological compounds are screened, the natural outcome will almost certainly necessitate further exploration of the connectivity of physiological pathways. Borisy et al (2003) discuss the unexpected but beneficial interactions that a systematic screening of combinations of small molecules reveals. They report, for example, that an antipsychotic agent coupled with an antiprotozoal drug demonstrates antineoplastic activity and that a fungistatic agent coupled with an analgesic produces antifungal activity against resistant strains of Candida albicans. In these instances, however, these ensemble properties never would have been realized if the effects had been broken apart and studied in isolation.
If the ensemble properties of a chemical matrix are necessary for physiological and pharmacological effects, and it appears that they are, then the purification process from whole plant to isolated compound is inadequate for the elucidation of pharmacological activity (Wagner, 1999; Wang et al, 2004). Moreover, the phytochemical matrix, rather than the phytochemical isolate, offers an opportunity for an enhanced perspective. The study of phytochemical matrices interfacing with mammalian systems, with the addition of improved technology, will almost certainly elucidate biochemical pathway connectivity that has been unattainable with previous methodology. The medical sciences would do well to heed Etxeberria (2004), who suggests that the properties of a unity cannot be accounted for by accounting for the properties of its components. Here again it remains true that the whole is greater than the sum of the parts.
 Chapter References can be found on the Evolve website at http://evolve.elsevier.com/Micozzi/complementary/
Chapter References can be found on the Evolve website at http://evolve.elsevier.com/Micozzi/complementary/