FOUR
The Body-Mind and Health

Think with your whole body.
—Taisen Deshimaru
Anna and Michael had been married for seventeen years when both began to complain that the other no longer focused on the rest of the family. They spent an inordinate amount of time ruminating resentments about each other. Meanwhile, their two children were entering the first few years of high school and presenting new challenges for their parents. Both Anna and Michael felt they didn’t have the energy to keep up with the constant attention needed to maintain clear limits and expectations for the kids. Though they did not want to admit it, they felt relieved when their kids began to spend more time on their computers, playing video games and on social media. This meant less monitoring was necessary because the kids left the house less, but they began to match their parents in obesity, fatigue, and dysphoric moods.
Perplexed by everyone’s loss of energy, Anna asked their physician whether the entire family had contracted Lyme disease. They felt ill and did not know why. He ordered blood tests for each of the family members. Though there was no evidence of Lyme disease, he expressed concern that they all had become significantly overweight. He also reported that both Anna and Michael had high levels of C-reactive protein (a measure of inflammation), blood glucose, and LDL (bad) cholesterol. Anna had developed type 2 diabetes, and Michael had metabolic syndrome (a cluster of conditions—increased blood pressure, high blood sugar, excess body fat around the waist, and abnormal cholesterol or triglyceride levels that occur together, increasing the risk of heart disease, stroke and diabetes). Anna responded by saying, “We were already very depressed! Now you are telling us we have bad genes? That makes me even feel worse.” Michael agreed. In response, their physician prescribed Prozac for both of them. With this medication Band-Aid their physician missed the opportunity to offer comprehensive health care and refer them to therapists to avert disastrous long-term mental and physical health. Though he started the consultation constructively by warning the entire family about their weight and both parents of their looming illnesses, the integrative approach they needed was compromised by the quick fix of “mismanaged” care.
What role should psychotherapy play in helping this family? Psychotherapy in the twenty-first century could be renamed “behavioral health,” because self-care behaviors have major effects on the immune system, the brain, and the body in general. These interactions have a profound effect on mental health. It is this relationship that is explored in this chapter.
BEHAVIORAL HEALTH
Anna and Michael’s family has become the new norm. There are now overwhelming numbers of people like them throughout the developed world. Plagued with health problems brought on by poor physical and emotional self-care, they suffer bidirectional causal pathways between acquired physical and psychological impairments.
The Centers for Disease Control estimates that health behaviors account for 50 percent of adverse health outcomes in the United States—as much as genetics (20 percent), the environment (20 percent), and access to health care (10 percent) combined. These statistics suggest that half of all health conditions are preventable by changes in self-care behavior (Amara et al., 2003). Research has found that 40 percent of medical patients have a comorbid psychological disorder, while 75 percent of patients with a psychological disorder also suffer with a comorbid physical disorder (Kessler, Ormel, Demler, & Stang, 2003). Essentially, health behaviors represent the interwoven natures of physical and mental health.
The number of Americans suffering at least one physical illness is predicted to increase to 157 million by the year 2020. This is a mental health crisis that can no longer be overlooked. Though these statistics are ominous, integrated health care providers, including psychotherapists, can work to avert disaster to the health of millions of people through a better understanding of the bidirectional causal interactions among the mind, brain, and the immune system found in the field of psychoneuroimmunology, and providing approaches consistent with it. Since its emergence as a rigorous field of research, psychoneuroimmunology has identified many interrelated mental and physical health dysregulations. Not so coincidentally, this field of inquiry the emerged with the surge in numbers of people, like Anna and Michael, with chronic and acquired illnesses that dysregulate the immune system who also suffer from psychological disorders.
Because over half the population of the United States unknowingly suffers from self-inflicted immune system dysregulation, psychotherapy in the twenty-first century must promote lifestyle and behavioral health changes. Not doing so is like building a house on a sandbar of a hurricane-swept beach.
THE IMMUNE SYSTEM
To get a better idea how chronic health conditions develop and cause psychological disorders, it is useful to put the immune system in perspective. Just as we are optimally endowed with a stress response system to deal with external danger, such as fighting off or fleeing from a predator, our bodies are protected from pathogens by a dynamic immune system. Whether the threat is from foreign bacteria, a contagious virus, or simply a cut on the finger, your body marshals internal resources to protect its cells and maintain homeostasis. Like the police, fire, and ambulance services combined, the immune system protects the body from external and internal threat. When working optimally it can save your life. When activated inappropriately it can cripple your life.
As a diffuse sense organ scattered throughout the body, the immune system communicates to the brain by both neural (fast) and hormone (slower) subsystems, influencing mood, cognition, and behavior. It comprises two main components: specialized cells that carry out protective functions and chemical messengers that allow those cells to communicate with one another and the rest of the body. The cells and the chemical messengers interact to mediate the location and intensity of inflammatory responses to protect the body from harm.
Chronic stress combined with poor self-care, such as inadequate sleep, impoverished diet, lack of exercise, and extra weight, inappropriately activates the immune system, with damaging effects. Anna and Michael, like millions of other people, acquired chronic conditions that turned their dysregulated immune systems into threats. Their immune systems switched from protectors to overactive enemies triggering autoimmune disorders and a downward spiral of significantly compromised physical and emotional health. Whether in response to adverse childhood experiences or chronic stress, or simply because of poor self-care, autoimmune disorders result in a variety psychological disorders, which then further exacerbate existing autoimmune disorders. To understand how this occurs and how psychotherapists can intervene, it is useful to highlight the multiple feedback loops that make up the immune system.
Specialized cells that carry out protective functions include the lymphocytes, which come in a wide variety of cell types, including B and T cells, produced in the bone marrow and thymus gland, respectively. Macrophages are the general foot soldiers that gobble up threatening bacteria, memory B cells are the snipers trained to attack specific targets, and helper T cells are communication officers, alerting other troops to an invasion. To maintain homeostasis in the body, these cells work together in a precise and coordinated dance choreographed by chemokines and cytokines, chemical messengers that allow those cells to communicate with one another and the rest of the body.
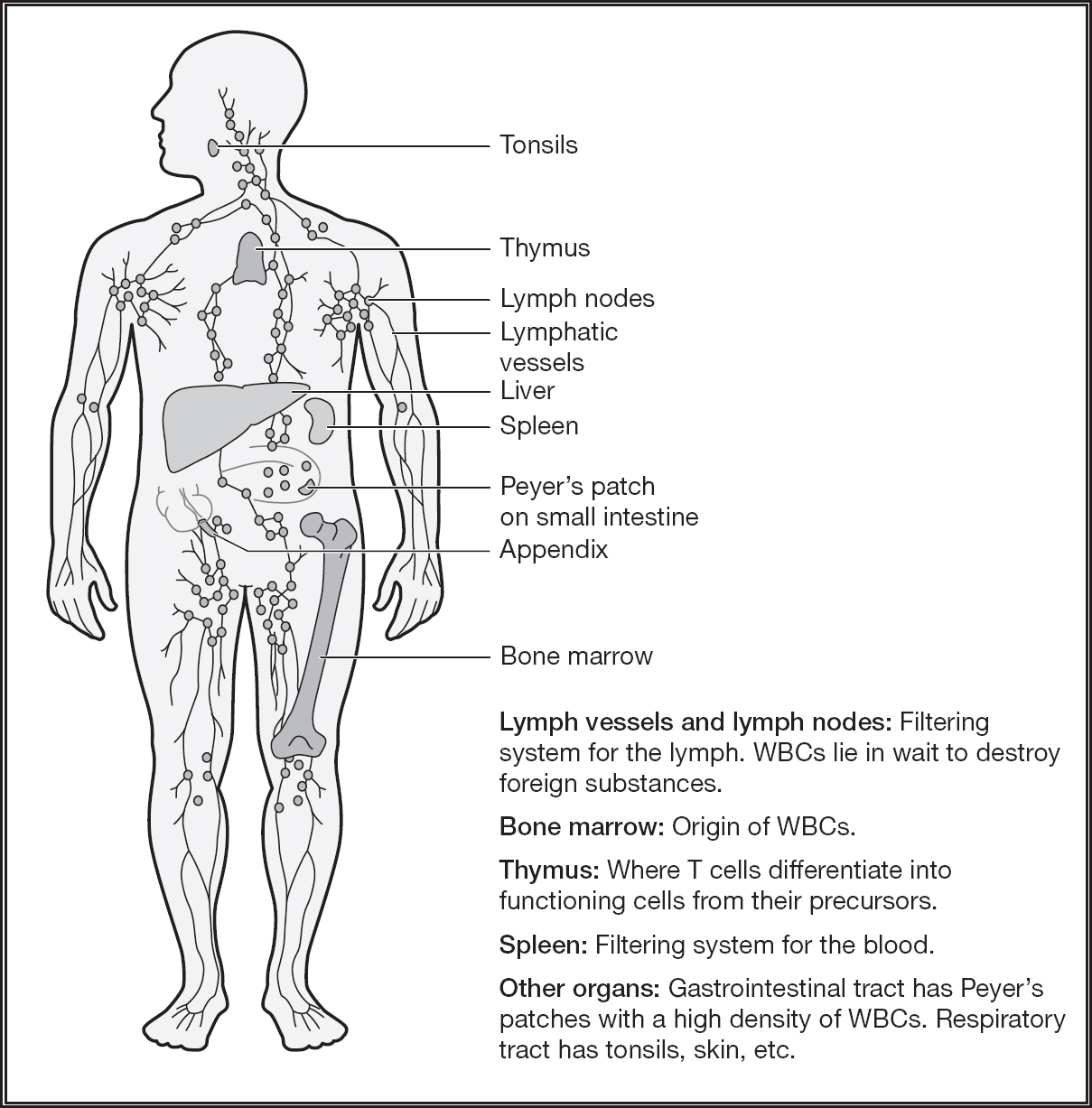
Figure 4.1: The components of the immune sytem.
After lymphocytes are born they are tested in the bone marrow and thymus to be exposed to molecules of your body so that they will not attack the body. The ones that bind strongly to “self” molecules are killed off or undergo “editing” in the genes that give rise to receptors. Autoimmune disorders develop when this process fails. Facilitated through cytokines, pathogens are killed while avoiding harm to the body.
When harmful substances enter our body, cells in our immune system called macrophages (big eaters) serve as an immediate defense. These Pac-Men of the immune system detect, destroy, and clear foreign substances from the body. This first response system also includes neutrophils (40–75 percent of white blood cells), which aid the macrophages in killing foreign substances while initiating the inflammatory response. The term inflammation comes from a Latin term meaning “set on fire”; inflammation serves as a protective mechanism so that the healing processes of tissue repair can begin. For local responses, like a bruise on the knee, inflammation also produces a physical barrier that prevents the spread of infection into the blood stream.
Chronic Inflammation
While short-term inflammation that responds to injury or illness represents a healthy process, chronic inflammation is not. Chronic inflammation represents a common factor among many psychological disorders and poor health. Because it is such a dominant feature, a better understanding of chronic inflammation will help our efforts to put it under control. In other words, to understand how a healthy system becomes unhealthy so that we can shift it back to healthy again, we need to understand how chronic inflammation gets turned on and off.
Normally, as part of the inflammatory response, infected or damaged cells send out alarm signals via chemical messengers called cytokines that attract and guide specific immune cells to the site of infection or damage. Cytokines (cyto means “cell” in Greek, and kinos means “movement between cells”) are communication substances, proteins released by immune cells that act on target cells to regulate immunity. Cytokines include the interleukins (meaning “between the white blood cells”) and tumor necrosis factor alpha. There are pro-inflammatory and anti-inflammatory cytokines. The pro-inflammatory cytokines (PICs) coordinate inflammatory responses. Anti-inflammatory cytokines, as their name implies, work to dampen the inflammatory response. With chronic inflammation the PICs dominate. Inadequate diet, poor-quality sleep, and lack of exercise (the topics of Chapter 5), as well as stress, depression, autoimmune disorders, and obesity, are associated with excess release of PICs, and thus chronic inflammation. As a common marker of inflammation, a fluid produced by the liver called C-reactive protein (CRP) can be measured in a standard blood panel. One of the findings from Anna’s and Michael’s blood panels was moderately high levels of CRP. While very high levels of CRP are caused by infections, moderately high levels are associated with autoimmune diseases, obesity, and depression (Kendall-Tackett, 2010). Chronic inflammation represents one of the common factors associated with autoimmune disorders, such as rheumatoid arthritis, lupus, type 2 diabetes, Addison’s disease, Crohn’s disease, and ulcerative colitis. Chronic inflammation is also associated with neurodegenerative diseases such as multiple sclerosis, Parkinson’s disease, and Alzheimer’s disease, and with psychological disorders.
Inflammation and Associated Psychological Disorders
- Depression
- Bipolar disorder
- Autism
- Posttraumatic stress disorder
- Cognitive decline
In addition, in children inflammation has been linked to:
- Tourette’s syndrome
- Obsessive compulsive disorder
- Attention deficit hyperactivity disorder
Anna and Michael, along with their kids, were unknowingly stoking up the amount of PICs and offering themselves few opportunities to generate anti-inflammatory cytokines through life style changes (described in Chapter 5). As a result, they were all suffering from chronic inflammation, with its associated fatigue, cognitive deficits, and depression.
When Anna returned to her family doctor for follow-up, complaining that she felt too little improvement, she was referred to me. Initially, she was quite perturbed that I addressed her poor self-care behaviors and associated them with her depression. Her resistance to these factors began to dissipate when I noted that both of her children began developing some of the same symptoms when their self-care matched hers.
The Brain’s Immune System
Chronic inflammation can lead to depression, cognitive impairments, fatigue, and achiness. Brain systems are affected by inflammation that can also perpetuate inflammation. These systems directly affect energy levels, pain, coping responses, and sense of self. So when Anna and Michael complained about lethargy, difficulty generating positive thoughts, depression, and cognitive deficits, inflammation likely played a significant role.
The brain has specialized cells with immune-like functions. Chief among them are the glial cells. Initially glial cells were thought of as the substance that holds neurons together (glia means “glue”) and were erroneously thought to function only as support cells. Glial cells include microglia and astrocytes, which are considered part of the brain’s resident immune cells.
Microglia make up 6–12 percent of all the cells in the central nervous system, where they constantly monitor for potential immune problems. They have many of the same receptors as peripheral macrophages and so can recognize bacteria and viruses. When they detect danger microglia release PICs such as interleukin-1 (IL-1) (Maier & Watkins, 2009). Chronic stress and compromised health “prime” microglia so that they make and release PICs more easily when they encounter danger again. In other words, once the immune cells in the brain have been activated, it is more likely that activation will occur in the future.
Astrocytes play a significant role in the immune system by providing a point of interaction between cytokines and neurons via genetic transcription and synaptic plasticity. Astrocytes exchange signals with neurons, detect and react to immune signals, and release PICs, which influence peripheral immune cells. Through this process of monitoring, reaction, and learning, astrocytes can play significant roles in the perpetuation of the inflammatory spiral.
Activation of the inflammatory pathways in the brain adversely affect memory and mood. The excessive release of PICs from microglia and astrocytes in the brain, as well as in the rest of the body, including from fat cells, causes wide-ranging detrimental psychological effects. Overexpressed PICs cause cognitive deficits that involve disturbances in synaptic strength. High concentrations of receptors for PICs are located in the prefrontal cortex and hippocampus, potentiating cognitive impairments, including poorer working memory, episodic memory, and executive functions (Lin & Marsland, 2014). This is why Annie and Michael had an executive network “brownout.” For example, excessive IL-1 in the hippocampus has been shown to impair memory by interfering with brain-derived neurotrophic factor (BDNF), which is involved in neural plasticity, neurogenesis, memory, energy balance, and mood.
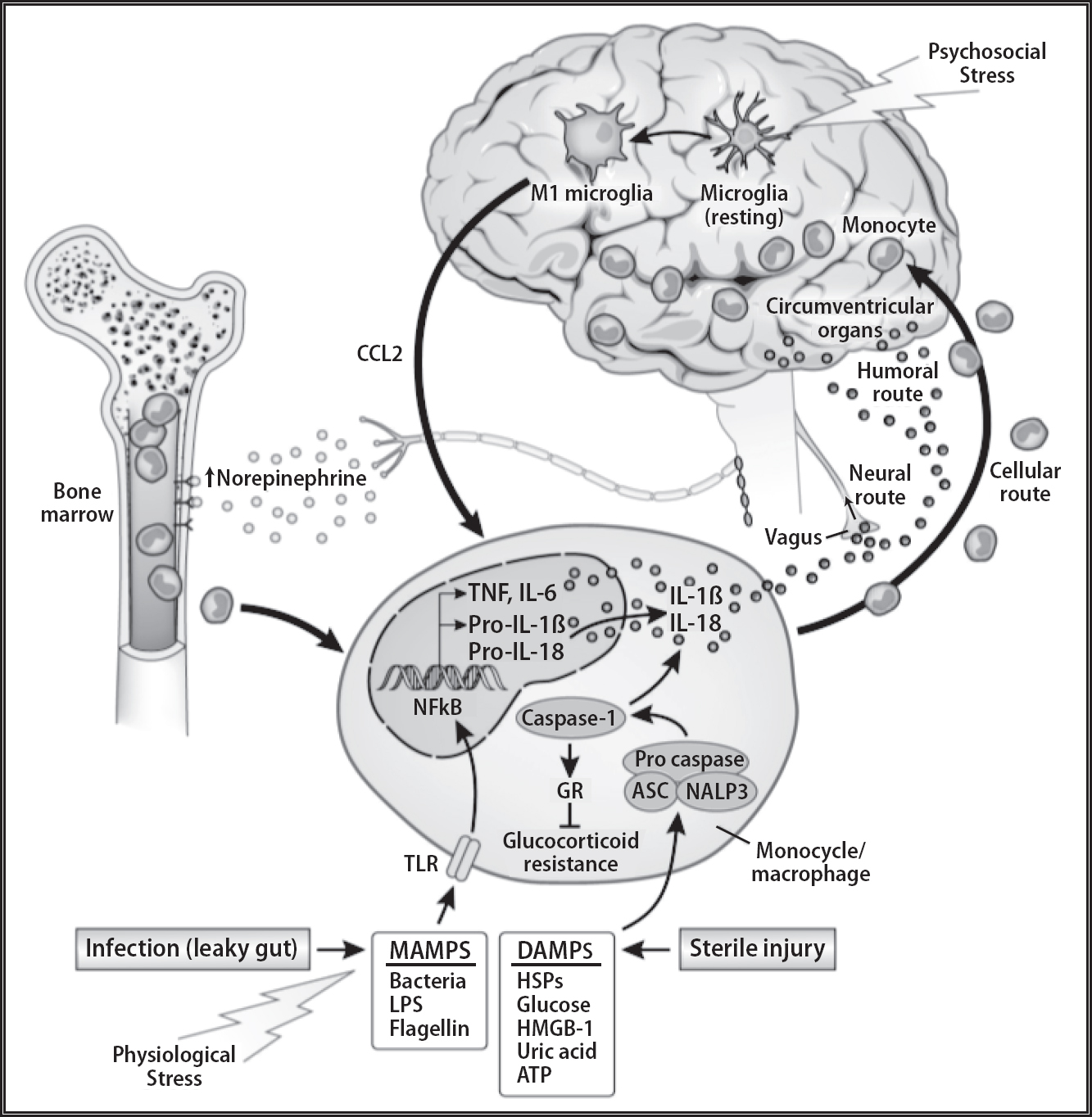
Figure 4.2: Immune system interactions with the brain. Note how the microglia cells in the brain function as the resident immune system of the brain. Microglial activation results in the production of pro-inflammatory cytokines such as interleukin (IL)-1β, (IL-6), and tumor necrosis factor-α (TNF-α) influencing the surrounding brain tissue.
“Figure 3.1”, “Figure 8.5”, “Figure 8.7”, “Figure 8.11”, from THE NEW MIND-BODY SCIENCE OF DEPRESSION by Vladimir Maletic and Charles Raison. Copyright © 2017 by Vladimir Maletic and Charles Raison. Used by permission of W. W. Norton & Company, Inc.
Because chronic inflammation deteriorates overall health and contributes to cognitive deficits and mood disorders, promoting lifestyle changes that lower inflammation should be a major goal of therapy. For example, given that extra fat cells contribute to chronic inflammation, weight loss should be a major goal—but this also represents a major challenge to building the therapeutic alliance!
The Mental Health Consequences of Excessive Fat Cells
According to the World Health Organization approximately 2 billion people are overweight. Approximately 68 percent of the population of the United States is overweight. As of 2016, 35 percent of men, 40 percent of women, and 17 percent of children and adolescents were obese—the United States leads the world with this pandemic. Obesity contributes to many other chronic health conditions strongly associated with psychological disorders. On average, obese people over 40 will die 6 to 7 years earlier than non-obese people. And those fewer years are marked by cognitive deficits and emotional dysregulation. Obesity shortens telomeres more than smoking.
Being overweight or obese causes a systemic feedforward breakdown in multiple homeostatic pathways, including a condition referred to as leptin resistance, further fueling obesity and rising cortisol levels (Newcomber et al., 1998). Obesity also causes inflammation, which in turn increases the risk of illnesses like autoimmune disorders, coronary heart disease, stroke, hypertension, sleep apnea, and type 2 diabetes. These physical illnesses are all associated with depression, anxiety, and cognitive impairments.
The Centers for Disease Control and Prevention defines obesity as a body mass index (BMI) over 30. Independent of age, BMI is inversely associated with a range of cognitive impairments and temporal lobe and global brain atrophy, cognitive decline, and incidence of dementia. A meta-analysis showed that people who are obese during midlife harbor a 1.35-fold increase risk for dementia late in life (Anstey, Cherbuin, Budge, & Young, 2011). A large study spanning thirty-six years found that those who were obese in midlife were three times more likely to develop Alzheimer’s disease than those who were not obese (Whitmer et al., 2005). The higher the BMI, the greater the circulating IL-1. And where the fat cells were located is critically significant. Central adiposity (belly fat) predicts dementia independent of BMI. In fact, people with normal weight who nonetheless had central adiposity were 89 percent more likely to develop dementia than people without it (Whitmer et al., 2008). In other words, the larger the belly, the greater the risk of dementia. These findings have led to the concept that an apple-shaped body places you at a greater risk than a pear shape. Because Michael carried an abnormally large belly, he was at greater risk for cognitive, mood problems, and dementia.
Obesity blocks BDNF, which is one of the many ways it contributes to dementia and depression. With the increase in PICs associated with obesity, there is a corresponding decrease in BDNF. Obesity also results in the dysregulation of energy intake and expenditure. Because BDNF plays an important role in energy balance, it can reduce inappropriate feeding while increasing energy output. Obesity increases feeding and decreases energy.
With the pandemic of overweight and obese people in the United States, the number of people with type 2 diabetes (90–95 percent of all those with diabetes) is well over 28 million (Nezu, Raggio, Evans, & Nezu, 2013). By far the most significant risk factor of type 2 diabetes is obesity, and especially fat above the hips. This makes excessive belly fat diagnostic of not only health problems but also cognitive and mood problems.
To understand the relationship between obesity and immune system dysregulation, it is useful to consider that obesity is regarded as a chronic subclinical inflammatory condition. Not only do adipose tissues (fat cells) swell up, but the dead cells are not cleared out efficiently in obese individuals, setting in motion inflammatory cells and macrophages to cluster around dead and degenerated cells to engulf and digest them. Like a stagnant pond with muck building up because there are no streams coming in or out, in fatty tissues fat cells decay without any clearing or nourishment. Chronic inflammation present in obese people is distinct from acute inflammation—it is no longer involved in tissue repair. Fat cells leach out PICs, producing 10–35 percent more circulating PICs, such as IL-6, than in nonobese people. This makes these cells function as agents of mood instability and cognitive impairment.
In addition to contributing to depression and cognitive deficits, IL-6 is associated with coronary heart disease and insulin resistance and is increased in people who are obese (Brunn et al., 2003). The higher the IL-6, the greater the risk of developing the type 2 diabetes. And with less glucose getting into cells, the brain is starved of fuel. In fact, some neurologists are calling Alzheimer’s disease “diabetes type 3.”
Autoimmune and Psychological Disorders
Inflammation represents the common denominator for metabolic syndrome, obesity, and depression. These conditions break down many of the homeostatic processes important to maintain general health and mental health. Though the periodic inflammatory response is critical for tissue repair and homeostasis, chronic inflammation dysregulates the immune system feedback loops, leading to a variety of pathological conditions, such as autoimmune disorders.
Like Michael, one in five Americans likely have metabolic syndrome. The percentage of people increases with age, reaching more than 40 percent among people in their sixties and seventies (Resnick & Howard, 2002). Metabolic syndrome is marked by insulin resistance, dyslipidemia (abnormally elevated cholesterol fats in the blood), and elevated blood pressure. All these factors are strongly predictive of cardiovascular disease and accelerated neurocognitive deficits.
Both a symptom and cause of major health problems and psychological disorders, autoimmune diseases represent bidirectional causal interactions with psychological disorders. For example, it is now well documented not only that people who develop type 2 diabetes are prone to depression. Once the inflammatory spiral begins, it is difficult to put on the brakes. Anna would have been best served had their family physician recommended significant lifestyle changes to pull out of the self-perpetuating nose dive into inflammation, diabetes, depression, and cognitive deficits.
People with type 2 diabetes are twice as likely to experience depression as their counterparts who do not suffer from diabetes. Large meta-analytic studies have shown that there is a bidirectional causal relationship between type 2 diabetes and depression across diverse populations (Nezu et al., 2013). In other words, type 2 diabetes increases the likelihood of becoming depressed, and depression increases the likelihood of developing type 2 diabetes.
The Stress-Inflammation Connection
Michael complained that when he began to feel overwhelmed with stress he seemed to pick up any virus going around. Eventually, he transitioned into a phase of feeling like he had the mild case of the flu all the time: achy and tired, with a queasy stomach, but never breaking out into a full blown flu with a fever and its associated symptoms.
It has long been the folklore of psychology that stress dampens the immune system. Short-term stress, in fact, has been shown to suppress T cell formation and natural killer cell function and to decrease the ability to repair broken DNA. But stress can enhance inflammation, too. Stress can activate PICs, including within the glial cells in the brain.
Michael’s chronic stress felt like an odd mix of feeling nervous and fatigued at the same time. Chronic stress can cause epigenetic changes in the expression of PIC genes in immune cells. With more peripheral inflammation Michael experienced, the more neuroinflammation he had. With mood and cognition compromised, his self-care deteriorated. He joined Anna in eating more and moving less. With significant weight gain, he also joined her in malaise and obesity.
Addressing Anna’s poor self-care, associated health problems, and depression was only the beginning of therapy. There was a backstory that Anna did not tell her physician: she had experienced increasing stress during the previous few years. Her boss demoted her after her job performance had begun to suffer because of low energy, and her new position required that she work swing shifts. Because her employer did not provide adequate parking, finding her car on a dark street at midnight presented a stressful challenge. Because the neighborhood was crime infested, she asked that the security guards provide an escort to her car. After her boss told the security department not to allow any of the guards to leave company property, one night she was physically assaulted and robbed. In response to her union grievance, her boss relented and allowed a security guard to escort to her car, but the residual symptoms of the trauma persisted. She found little support at home during the year before visiting her primary care physician. The only semblance of comfort she found was “vegging out” on the couch watching movies with Michael.
Anna’s chronic stress, exacerbated by trauma and poor self-care, combined to trigger an autoimmune disorder. Multiple physiological pathways contributed to her increased inflammation and decreased stress tolerance. The chronic and acute stress disrupted the insulin balance in her body and led to insulin resistance, which paved the way for type 2 diabetes through a series of metabolic changes. When stressed, her body assumed that more fuel was needed, and to accommodate, genes activated in cells to increase glucose uptake. During and following the assault, her body released surges of norepinephrine, adrenaline, and cortisol, which also increased blood glucose. The increases in cortisol triggered the breakdown of protein and its conversion in her liver to glucose. The excessive cortisol resulted in too much glucose floating around, which increased risk of insulin resistance. It was in the year prior to visiting her primary care physician that she developed type 2 diabetes.
We see the same dysregulating spiral with illnesses like arthritis, chronic pain, fibromyalgia, and chronic fatigue syndrome. As people become more depressed, their physical illness increases, which leads to greater depression. Decreased stress tolerance, increased anxiety, and depression associated with these chronic diseases combine to potentiate all of them together.
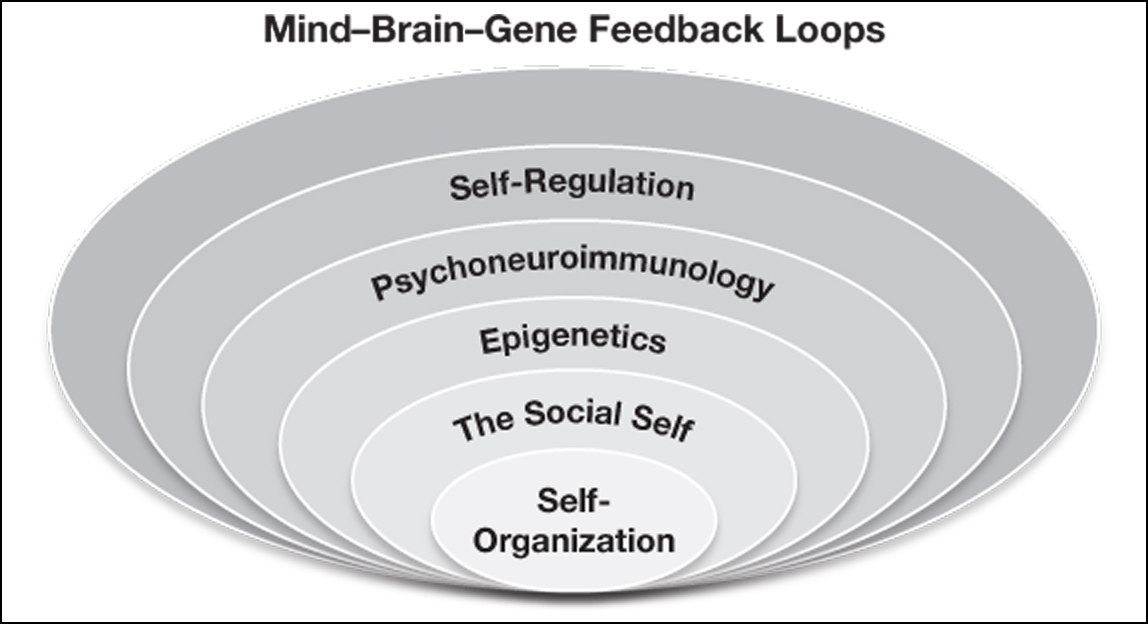
It is difficult to calculate how many people are depressed, suffer from cognitive deficits, and do not know that they are afflicted with spiraling dysregulations of their immune system, but it could be in the many millions. PICs tend to rise when physical health is compromised by autoimmune disorders, excess weight, and increases in response to stress. Stress and poor health impact multiple systems to create a spiral of decompensation within all levels of the mind-brain-gene feedback loops. The nonlinear interactions of all these factors put the person at greater risk for more serious physical and psychological disorders.
Psychotherapy in the twenty-first century must address the pandemic of acquired inflammatory diseases that have a devastating effect on mood and cognition. Lifestyle changes such as diet, exercise, and sleep (all addressed in Chapter 5) can affect the immune system. But before turning to those self-maintenance factors, we need to better understand a major part of the immune system, the gut.
GUT FEELINGS, THE ENTERIC NERVOUS SYSTEM, AND IMMUNE INTERACTIONS
Sylvia ambivalently sought psychotherapy at the suggestion of her primary care physician because of her vague complaints about intestinal discomfort and periodic sinus infections. He prescribed an ongoing “maintenance dose” of antibiotics to keep the sinus infection at bay. Willing to try anything to get over these physical problems, nevertheless at her first session she was more like a window shopper than someone seriously interested in psychotherapy.
Despite her nagging health problems and the time invested in trying to get well, she was devoted to her new business, a not yet financially successful bakery café. She ate breakfast there every morning, consisting of many new recipes for croissants and a “tall skinny latte.” Her lunch included other “yummy” pastries. Because of the constant problems with her gastrointestinal tract, she consumed herbs that were advertised as being “gentle for the stomach.” She also wondered if the fatigue, mild depression, and stress she experienced were related to “gut feelings” that her business was going to fail. Though her central concern revolved around her business, how was her gut communicating with her brain?
The gastrointestinal system, or gut, has been referred to as the “second brain” in the media and as the enteric nervous system (ENS) in the scientific literature. It includes a large group of neurons wrapped around the walls of the gastrointestinal system, which extends down from the esophagus to the distant colon, comprising approximately 500 hundred million nerve cells. The ENS maintains connections with the parasympathetic and sympathetic branches of the autonomic nervous system. It receives signals to stimulate digestive activity from the parasympathetic branch through the vagus nerve and to inhibit activity from the sympathetic branch. In other words, when a person is in fight-or-flight mode, the sympathetic branch acts to suspend digestive activity so that the person can devote all available energy to the challenges ahead. On the other hand, when stress subsides, the parasympathetic system promotes the rest-and-digest response.
Roughly 90 percent of the signals conveyed through the vagus nerve travel from the gut to the brain, while only 10 percent travel in the opposite direction. In other words, signals coming from the gut to the brain are more prevalent than those coming from the brain to the gut. The ENS is endowed with so-called dendritic cells that have tentacles that extend into the gut’s interior. They can communicate with gut microbes that live near the gut wall. If they detect the presence of potentially dangerous bacteria, they can trigger a cascade of inflammatory reactions in the gut wall in an effort to control the pathogens, as in food poisoning.
Considering that our ancestors on occasion unknowingly ate contaminated food, a responsive immune system in the gut evolved to protect them from infection, illness, or even death. The connectivity between the gut and the brain became fine-tuned and responsive so that our ancestors would know that something was wrong. From this perspective, it is understandable that the gut makes up the largest component of the body’s immune system: about 60 percent of the immune system is located around the intestines. with more dedicated cells than the thyroid, pituitary, gonads, adrenals, and circulating blood combined.
Many of the immune cells living in our gut are located in clusters in the small intestines in the Peyer’s patches, as well as scattered throughout the walls of both the small and large intestines. A major part of the gut’s immune system is located in a pouch-like area called the cecum and, dangling from it, the appendix. Overall, the cecum, appendix, and Peyer’s patches represent a significant amount of immune activity.
The gut’s abundant immune cells play a major role in signaling between the ENS nerves and the brain through the vagus nerve. These ongoing reports about the state of the gut and body in general comprise important interoceptive information. When inflammation occurs in the gut, many of the sensors become more sensitive to normal stimuli, especially in response to stress. As inflammation occurred in Sylvia’s gut, her interoceptive information told her something was wrong, but not what. She interpreted these gut feelings as intuition that her business was in jeopardy. One of her responses was to consume excessive amounts of simple carbohydrates, through which she found temporary relief. Unfortunately, that temporary relief led to a long-term, out-of-control cycle of chronic inflammation. Over a lifetime, Sylvia’s ENS adapted to stress that she encountered and to her diet by adding chemical tags to stress-response genes. As an adult she may have tended to sensitize gut reactions to stress, often complaining of “nervous gut feelings.”
The increased production and release of pro-inflammatory cytokines (PICs) can contribute to a wide range of gut illnesses, including the aptly named inflammatory bowel disease. PICs travel to the brain either through binding to receptors on the vagus or through spilling into the bloodstream and traveling up to the brain like a hormone, traversing the blood-brain barrier and activating the microglia in the brain, which respond to and release more PICs.
“Gut feelings,” or interceptive information, may be misinterpreted as “intuition.” Because so many factors contribute to inflammation in the gut, including poor diet, stress, and excessive antibiotic use, normalizing gut health optimizes mental health.
The Colony Within
The gut contains more than 100 trillion microorganisms, including bacteria, fungi, and archaea. If gathered together, they would weigh between two and six pounds, close to the weight of the brain. Outnumbering people on the planet by 100,000 times, the microbes in one person’s gut perform many life-sustaining functions. The 1,000 bacterial species that make up what is called the gut microbiota contain 7 million genes, which means there are 360 bacterial genes for every human gene. Only a small fraction of our body’s genetic content is of human origin, the reason many refer to us as superorganisms.
Approximately 90 percent of the 1,000 species of bacteria in the colon fall in either of two broad categories, Firmicutes or Bacteroidetes. Whereas the Firmicutes family increases fat absorption, because they are quite efficient at extracting calories from carbohydrates, the Bacteroidetes do not depend on carbohydrates and are more dominant in lean people. The ratio between Firmicutes and Bacteroidetes in the general public in the Western world has shifted in the direction of Firmicutes, turning on genes that add fat cells, increasing the risk for obesity, diabetes, and cardiovascular disease. Because people who are overweight tend to have a significantly higher ratio of Firmicutes to Bacteroidetes, they also tend to have abnormally high levels of leptin, because their extra fat cells produce more than lean people. Whereas with a lean person leptin signals the brain to decrease appetite, an obese person becomes resistant to its effects, and this critical feedback mechanism breaks down.
These microbes are so important that in 2008 the US National Institutes of Health launched an extension of the Human Genome Project to investigate the microbiome. The project has identified the complex interrelationships between a person’s microbiota and overall health. It is now clear that the microbes in our gut participate in a wide variety of roles in our body, including the immune system, inflammation, detoxification, neurotransmitter signaling, vitamin production, and nutrient absorption. Together, the microbes, peptides, cytokines, and neurotransmitters interact with the brain. Approximately twenty different types of hormones are available to be released into the bloodstream at a moment’s notice if needed. Neurotransmitters are synthesized in the gut, and up to 95 percent of the body’s serotonin is stored there.
To understand why we must take gut bacteria into consideration for mental and physical health, it is important to keep in mind that gut microbiota differ widely among people, even identical twins. And within one individual, the diversity and abundance of microbes also vary over a lifetime. The diversity of microbes is low during the first three years of life while a stable microbiome is being established. Diversity reaches its peak during adulthood and wanes as we enter our senior years. Low diversity during the developmental years coincides with the vulnerability to neurodevelopmental disorders such as autism, while low diversity later in life coincides with the development of neurodegenerative disorders such as Parkinson’s and Alzheimer’s diseases.
Dysbiosis
Gut microbes constantly adapt to shifts in digestive fluids, acidity, available nutrients, and time intervals between meals. As the owner and head baker, Sylvia experienced stress that slowed down digestion. Microbes sensed changes and so activated genes that helped adapt to changing conditions. When her gut-microbiota-brain axis became unbalanced, she entered a state called dysbiosis, a microbial imbalance.
In a treatment meeting that included her primary care physician, we focused on the role of antibiotics in her dysbiosis. Since World War II, antibiotics have saved millions of lives. However, the more recent overuse of antibiotics has contributed to dysbiosis, and Sylvia’s prescription of antibiotics for her sinus infection resulted in dysbiosis for her. This condition has been associated with a wide range of health and mental health disorders. We decided to phase out her prescription.
For healthy people, the lining of the gut is tight, so that microbes and other substances are prevented from passing into the abdominal cavity. However, the thickness and integrity of the thin mucus lining of the gut can vary depending on genetics, stress, and diet. Permeability of the lining, often referred to as “leakiness,” can increase through stress and through excessive consumption of simple carbohydrates, saturated fat, and various food additives. When the combination of these factors occurred with Sylvia, her gut became leaky, and the inflammatory process spread throughout her body. This condition, referred to as metabolic toxemia, reduced her energy level while increasing her fatigue, pain sensitivity, anxiety, and depression.
A molecule referred to as lipopolysaccharide (LPS) is localized in the outer layer of gram-negative bacteria, which include Firmicutes and Proteobacteria. LPS levels increase in response to a high-animal-fat diet, and it is considered an endotoxin because it is a toxin that comes with bacteria and triggers a strong immune response. The resulting inflammation leads to a chain of events that increases the leakiness of the gut by allowing LPS to sneak between the cells in the lining of the gut and into the blood supply, where it interferes with the hormone insulin and promotes type 2 diabetes and heart disease. A regular diet of simple carbohydrates and saturated fat results in high levels of LPS, which stimulates cells that release PICs to make the gut even leakier, further spreading inflammation throughout the body, including the brain. The glial cells in the brain respond to the PICs by producing yet more inflammation, resulting in impaired cognition and dysphoric moods. Meanwhile, the hypothalamus becomes less responsive to satiety signals in the form of leptin from the gut, promoting overeating and further exacerbating the gut-brain system.
Contributors of Leaky Gut
A diet high in simple carbohydrates and saturated fats feeds the Firmicutes and lipopolysaccharides, leading to gut permeability.
Of significance to mental health, LPS is involved in a chain of events that increase depression (Maes et al., 2008). Because LPS makes the gut more permeable, PICs are more likely to gain access to and stimulate more PICs in the brain, increasing the risk for neurodegenerative disorders such as Alzheimer’s disorder, lupus, multiple sclerosis, and autism, as well as depression. LPS has been shown to decrease the production of BDNF (Guan & Fang, 2006). This means that the brain-enhancing process of neurogenesis is blocked from making new cells in the hippocampus, and the person becomes prone to memory deficits and neurocognitive impairments. It is not surprising that up to three times as much LPS has been found in the plasma of Alzheimer’s disease patients as in healthy adults (Zhang et al., 2009). Obese people tend to have much higher levels of LPS than do lean people, and it appears to trigger inflammation in their fat cells. Obesity is not simply layer upon layer of fat cells but fat tissue that has malfunctioned and become inflamed.
Lean people tend to have a high level of a type of gut bacteria referred to as Akkermansia. Though at best only representing 3–5 percent of gut bacteria, Akkermansia plays a significant role in supporting gut lining and regulating mucus production. The health of the mucus layer is critical for keeping other bacteria a safe distance from our gut’s epithelial and immune cells. Despite the relatively low numbers of Akkermansia compared to other gut bacteria, if they are damaged or reduced in number the critical function they play in gut mucus lining maintenance is lost, resulting in inflammation. Not surprisingly, the lower the Akkermansia level, the higher the BMI. Another way to look at the effect of the relationship between the bacterium and the gut lining is that a higher level of Akkermansia acts to turn on genes to make more mucus, helping promote a healthy gut lining and preventing a leaky gut. A diet higher in saturated fat lowers Akkermansia, while a high-fiber diet promotes it. Akkermansia seems to thrive with the consumption of a type of fiber known as oligofructose, which is especially found in foods such as onions, bananas, leeks, and asparagus.
Mind, Brain, and Gut
Michael, introduced at the beginning of this chapter, felt that he had a “nervous stomach.” The gut communicates with the brain through multiple channels. Microbes coat the razor-thin layer of mucus and cells of the inner lining of our intestines. This puts them in very close proximity to the gut’s immune cells and cellular sensors that encode our gut sensations. Many of the brain-gut interactions occur here, ensuring that signals generated by the microbes are received by the brain and, in turn, those sent back to the gut are influenced by our emotions. Gut feelings are bidirectional, linking cells in the brain with those in the gut.
Interactions among the mind, brain, and gut can occur through top-down or bottom-up pathways that are emotionally laden via both branches of the autonomic nervous system. However, Michael primed his sympathetic branch and his neuroendocrine system so that he responded to assumed threats that were benign. For example, stress primed his hypothalamus to release corticotropin-releasing hormone and dampen the activity of gamma-aminobutyric acid, the principle inhibiting neurotransmitter in the body. This means that his stress tolerance and his ability to self-sooth tended to be low. In addition to the heightened tendency to easily trigger the release of stress hormones, such as norepinephrine and cortisol, which increased his hypervigilance and anxiety, corticotropin-releasing hormone stimulated his pituitary to release adrenocorticotropic hormone, which traveled through his bloodstream to the adrenal glands, which in turn released adrenalin, more norepinephrine, and cortisol. Simultaneously, the stress induced a gut reaction that impacted the composition and activity of the gut microbiota. He became prone to a wide range of sensations, including belly pain and gut contractions that resulted in frequent diarrhea. His stomach slowed down and even reversed itself, so he often felt like throwing up. Not only does the elevation in cortisol contribute to changes in the mix of gut bacteria, but it also increases the gut’s permeability and triggers the release of PICs, which further increases the gut’s permeability (Vanuytsel et al., 2014).
Early stress is also associated with alterations in microbiota and their metabolites, as well as with the stress circuits in the brain (Bercik et al., 2011). The neurodevelopmental changes resulting from early stress occur through multiple pathways, including epigenetic modifications of the brain-gut axis and stress-induced changes in gut microbiota and their products, which can further impair the brain. These abnormalities can begin to develop in utero and soon after birth. Interference in the infant’s gut microbiome and a variety of challenges, including stress, nonvaginal delivery, unnecessary use of antibiotics, and unhealthy dietary habits during pre- and postnatal periods, can lay the groundwork for brain-gut disorders (Mayer, 2016). The pathophysiological syndromes that result from early stress and deprivation include the elevation of pro-inflammatory processes, as measured by C-reactive protein levels, decades later in life.
Throughout life chronic stress can stimulate the growth of many pathogens in a person’s gut, making those pathogens more aggressive. Stress signals can also reduce the thickness of the mucus layer lining the colon, making it leakier, allowing microbes greater access to the gut’s immune system, circumventing many of the gut’s defensive mechanisms. Norepinephrine can stimulate the growth of bacterial pathogens that can cause serious stomach ulcers, gut infections, and sepsis. It can also activate genes in the pathogens that make them more aggressive and increase their odds of survival in the intestines. Certain gut microbes can modify norepinephrine into a more powerful form, intensifying its effect on other microbes (Mayer, 2016). Overall the combination of chronic stress, poor diet, and dysbiosis can increase the leakiness of the gut leading to greater metabolic toxemia.
In the epigraph to this chapter, Taisen Deshimaru’s quote, “Think with your whole body,” referred to being consciously present with every ounce of your being. Based on the concepts described in this chapter, we actually do think and feel with our whole body. The interactions between the immune system, including within our gut, and the brain are dynamic. Dysregulation within the gene-immune-brain-mind feedback loops can have confusing and devastating effects on mood and cognition.
Psychotherapy by necessity must focus on the behavioral health of clients. This means that self-maintenance factors such as diet, exercise, and sleep, the focus of Chapter 5, need to be addressed as foundational factors to mental health.