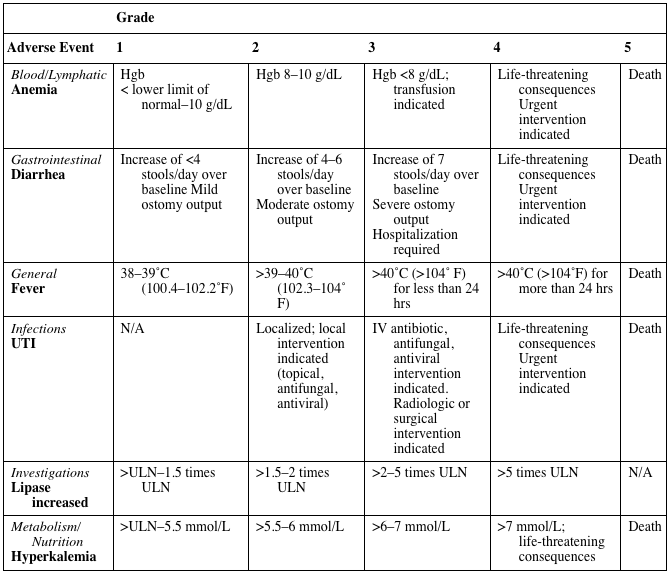
CTCAE EXAMPLES
| Grade | |||||
| Adverse Event | 1 | 2 | 3 | 4 | 5 |
| Blood/Lymphatic
Anemia | Hgb
< lower limit of normal–10 g/dL | Hgb 8–10 g/dL | Hgb <8 g/dL; transfusion indicated | Life-threatening consequences Urgent intervention indicated | Death |
| Gastrointestinal
Diarrhea | Increase of <4 stools/day over baseline Mild ostomy output | Increase of 4–6 stools/day over baseline
Moderate ostomy output | Increase of 7 stools/day over baseline
Severe ostomy output Hospitalization required | Life-threatening consequences Urgent intervention indicated | Death |
| General
Fever | 38–39˚C (100.4–102.2˚F) | >39–40˚C (102.3–104˚ F) | >40˚C (>104˚ F) for less than 24 hrs | >40˚C (>104˚F) for more than 24 hrs | Death |
| Infections
UTI | N/A | Localized; local intervention indicated (topical, antifungal, antiviral) | IV antibiotic, antifungal, antiviral intervention indicated. Radiologic or surgical intervention indicated | Life-threatening consequences Urgent intervention indicated | Death |
| Investigations
Lipase increased | >ULN–1.5 times ULN | >1.5–2 times ULN | >2–5 times ULN | >5 times ULN | N/A |
| Metabolism/ Nutrition
Hyperkalemia | >ULN–5.5 mmol/L | >5.5–6 mmol/L | >6–7 mmol/L | >7 mmol/L; life-threatening consequences | Death |