CHAPTER 9
Pain, Pain Control, and Sedation
Gro Haukali, Stefan Lundeberg, Birthe Høgsbro Østergaard, and Dorte Haubek
In clinical dentistry and medicine, pain is synonymous with significant discomfort. However, it is important to remember that pain has a necessary and purposeful function. Pain signals tissue damage, and thereby alerts the individual to take action in alleviating such damage.
Pain may be acute or chronic and associated with trauma, diseases, postoperative healing and treatment. In this chapter, the main focus will be on acute pain associated with dental procedures (procedural pain). Painful procedures experienced during childhood have proved to be one of the most important factors behind fear, anxiety and behavior management problems in connection with dental treatment (see also Chapter 6).
Pain is defined as a subjective sensory and emotional experience (Box 9.1), which may or may not be associated with tissue damage. This implies that only the child patient himself or herself can decide whether a clinical procedure is painful or not [1]. This may be difficult to understand for the dentist who will tend to relate the level of pain to the level of tissue damage. There are several reasons for this discrepancy between pain perception and tissue damage that we observe [2].
During the past 25 years, more focus has been given on children’s perception of pain in different health‐care situations, based on the fact that children’s pain has been underestimated and not sufficiently treated. It was believed that the newborn child’s central nervous system (CNS) was immature and not able to register pain as in adults. Research has, however, shown that both neonates and small children are at least as pain sensible as the adult based on less developed functions that inhibit or modify pain responses in their CNS [3]. It has also been shown that children may be hypersensitive for pain when exposed to painful stimuli in early life, particularly children with chronic diseases and those who have been critically sick. Such children may show signs of hyperalgesia, either general or local, and their behavior may be affected by it. After a painful event or trauma, a pain memory frequently develops. The pain memory has two different properties, a physiologic and an affective component, both of which have long‐term effects.
Evidence also exists that children who have experienced painful dental procedures, particularly at young ages, are more pain sensitive and display more behavioral problems during dental treatment than those who have not. The risk for this increases potentially if the painful stimuli have been experienced in combination with a feeling of lack of control. A typical clinical situation is when the child is being exposed to painful procedures under restraint (behavioral control), or when the painful stimulus comes suddenly without having prepared the child for it (informational control).
Another reason that children may perceive pain in situations without tissue damage is based on classical conditioning. If a child has undergone a painful procedure based on tissue damage (e.g., drilling into dentin) during a dental procedure, and this stimulus (unconditioned stimulus) is combined with other stimuli, such as the sound and the water spray of the drill (conditioned stimuli), it may result in pain perception when the conditioned stimuli are presented alone (Figure 9.1).

Figure 9.1 Pain perception based on classical conditioning: a painful procedure based on an unconditioned stimulus (e.g., tissue damage when drilling into dentin), combined with other stimuli such as the sound and the water spray of the drill (conditioned stimuli), may result in pain perception when the conditioned stimuli are presented alone.
There are a number of additional factors known to affect the individual perception of pain, such as peripheral and central sensitization, biological variation, previous pain experience, context, and a variety of psychological factors. The complexity of pain perception can explain the lack of correspondence between the degree of noxious stimuli and the intensity of pain experience. From the neurophysiological point of view a noxious stimulus creates both a local (peripheral) sensitization in the damaged area and changes within the CNS (central sensitization) which amplify the ingoing signaling to pain areas. This plasticity within the nervous system can alter the pain signaling for an extended time, long after healing has occurred in the affected area (physiological pain memory) [2,4]. The transmission of pain impulses does not only target the sensory cortex. A complex system with signaling from the sensory cortex as well as direct impulses from lower brain structures reaches the limbic system. The limbic system accounts for the affective reaction to a pain stimulus and plays a major part in pain perception. Previous pain experiences (psychological pain memory) and fear are two of the most important factors contributing to the affective component of pain perception.
A simplified illustration of the frequently observed lack of correspondence between the degree of tissue damage and pain reaction is illustrated in Figure 9.2.

Figure 9.2 The perception of procedural pain due to tissue damage (nociceptive pain) depends on a variety of factors, owing to the fact that the stimuli are modulated in the central part of the brain before reaching the sensory cortex. Previous pain experiences (psychological pain memory) and fear are the most important factors contributing to the affective component of pain perception.
Methods of pain control
The complexity of pain perception is a challenge for the dental practitioner when treating children. However, the prevention and alleviation of pain is a basic human right that exists regardless of age, which demands some basic principles for good clinical pain practice to be maintained in pediatric dentistry (Box 9.2).
Measuring pain is an important factor to improve care in children. The aim is to provide acceptable pain levels for the child undergoing dental treatment and therefore we as caregivers have to find ways to assess the pain experience in the individual child. Pain assessment puts pain on the treatment agenda, and can help us to identify pain under treatment and to evaluate new pain treatment strategies. Since pain is personal and subjective, age‐appropriate self‐report scales should be used when possible, e.g., visual analogue scale, faces pain scale and colored analogue scale (Box 9.3). In children with limited communication skills indirect measurement can be used as observation of behavior (e.g., behavior rating scales) and physiologic reactions (e.g., heart rate, sweating). In clinical practice, these methods must be adapted to the cognitive and linguistic skill of the individual child and be easy to use.
Both the prevention and treatment of procedural pain should be based on a preoperative judgment of factors that may be assumed to affect the child’s perception of pain. These factors may be divided into child‐centered approaches and a disease‐centered approach, and examples are given in Box 9.4.
The child‐centered approaches are based on the great plasticity of pain perception in children, meaning that environmental and psychological factors may be powerful in influencing their perception of pain. The most basic techniques are described in Chapter 6. The selection of methods should be based on a clinical judgment of how vulnerable the child may be, which must be done in collaboration with the child itself and its parents. Among factors to be taken into consideration are age and maturity, temperament, previous pain experiences, and family/social network.
The need for disease‐centered approaches must be estimated based on both the nature and extent of tissue damage and the vulnerability of the child. Robust and experienced children with ability of coping and control are able to undergo considerable dental treatment with minimal use of pharmaceutical agents. On the other hand, the vulnerable child with low coping ability and previous negative experiences with painful procedures must be taken care of by extensive use of both psychological and pharmaceutical methods.
Local analgesia
Local analgesia is an efficient and safe method for controlling pain in pediatric dental care. The rate of success, however, depends on both the technique and the operator. Some methods of injection produce minimal discomfort, as will be described in the following. Furthermore, the operator’s attitude and confidence in administrating local analgesia influence the success rate, and it has been shown that some dentists find it stressing to give a mandibular block to children below school age [5,6].
In addition to pain control, local analgesia can be used as a diagnostic tool and in the control of hemorrhage.
Preparation of the patient
Children of all ages should be given some kind of preparation before an oral injection, adapted to their age and maturity. The preparation must be preceded by establishing a good relationship between the operator and the patient and consists of giving the child a feeling of control during the situation (see Chapter 6). If the child shows signs of fear this should be dealt with before the start of the procedure. Ask the child why they experience fear and try to find solutions to their worries. It may be advisable to mention the injection in a session prior to the treatment session. In young children of 3–6 years, a simple statement that local analgesia will be used should be made along with an adjusted explanation. A more elaborated introduction can be given just prior to the injection, taking into account that the child should only be given information that he or she can handle. A young child can become more nervous when faced with too much detailed information. Whether the needle should be shown to the child before injection should also be considered from the same point of view. When the child is older (6–7 years old), he or she will be able to handle more detailed information, and the dentist has to give the child more control. It may also be necessary to teach different coping techniques, e.g., relaxation and paced breathing. However, children vary quite considerably in their coping abilities; some benefit greatly from watching and viewing every step, in other cases it is better to distract the child at the actual moment of the injection. When children develop the ability of abstract thinking at about 12 years, their reaction to pain is more like that of an adult. Most children at this age will also be able to take full responsibility for whether local analgesia should be used or not. Before that age, the dentist has the responsibility to make the decision.
Intraoral topical analgesics
Topical local analgesic agents are extremely important in reducing pain during intraoral injections and should be used routinely. They are available as spray, gel, ointment, and solution. The most commonly used are lidocaine (5 or 20%) and benzocaine (20%) as ointment or gel. Topical analgesic agents will anesthetize the surface tissue to a depth of 2–3 mm, if used properly. The ointment or gel is applied on dry mucosa for 2–5 minutes depending on the concentration of the agent. For many children the use of topical analgesics will be connected with their first experience of intraoral pain control, and it is of great importance that it is given sufficient time to be effective. The child must also be informed about the strong taste of the agent. The application is most practically made with a cotton bud over a limited area. It is important to limit the amount used (Figure 9.3a). The uptake from the mucosa is rapid, and it is important to remember that the concentration of the active agent in the topical solution often is high.

Figure 9.3 (a) Topical application of local analgesia ointment on a cotton bud at the injection spot. (b) Infiltration analgesia followed by (c) a transpapillary injection started from the buccal and (d) continuing to the palatal mucosa. Note blanching of the palatal papilla and mucosa in (c).
Topical application of 5% lidocaine solution for mouth washing before taking an impression can be of help for children with pronounced gag reflexes.
Local analgesic solutions
There are a number of local analgesics available for injection, both with and without vasoconstrictors, and with variable duration. The efficiency of the drug is increased and prolonged by the addition of vasoconstrictors, while the toxicity is decreased. Most commonly used in pediatric dental care are lidocaine (20 mg/mL) with adrenaline (12.5 μg/mL) and articaine (40 mg/mL) with adrenaline (5 μg/mL). Both have an intermediate analgesic duration on the pulp of approximately 60 minutes. More rarely, there may be indications for a local analgesic with long duration, e.g., for long surgical procedures, which can be obtained by bupivacaine with adrenaline.
Methods of administration
Infiltration
Infiltration is the application of a local analgesic solution around the nerve ends. The aim is to deposit the solution as close as possible to the apex of the tooth. The maxillary and mandibular bone plate in children is generally less dense than in adults, which permits a more rapid and complete diffusion of the analgesic solution through the bone. Infiltration can therefore be used with great success in the primary as well as the permanent dentition. Buccal infiltration of 0.5–1 mL solutions is sufficient for pulpal analgesia of most teeth in the maxilla of a child. There can be some difficulties anesthetizing the maxillary first permanent molars by the infiltration technique due to the zygomatic process which is closer to the alveolar bone in children than adults. Infiltration close to the apices of the maxillary central incisors should be performed carefully, as it can be very painful. In the mandible, buccal infiltration of 0.5–1 mL will often be sufficient for pain control in the primary dentition, although the effect on the second primary molar will not always be sufficient.
Technique
After penetrating a stretched and topically anesthetized mucosa the needle is directed towards the apex of the tooth (Figure 9.3b). The injection is done very slowly supraperiostally. Deposition under the periost can be very painful and should be avoided; at least before the periost itself has been anesthetized. Applying finger pressure to the area of injection prior to the injection may distract the patient’s attention. Thin (30 G) or standard (27 G) needles are recommended, and the solution should be at room temperature – never used directly from the refrigerator.
In principle, infiltration can be done anywhere in the oral cavity including the palate. However, in order to prevent painful injection in the palate, transpapillary injection is preferred (Figure 9.3c,d). All injections must be done very slowly [7].
Blocks
By far the most used block analgesia in children is the mandibular foramen block (Figure 9.4). Prior to the injection, it is of great importance to prepare the child for the procedure in a way that is adjusted to its age and maturity (see Chapter 6). The patient should open maximally and the needle be introduced just laterally to the pterygomandibular fold at its deepest point (the pterygoid notch). In children, the direct injection from the opposite primary molar region is recommended. This technique requires less needle movement after tissue penetration. To secure a good block, the needle should be introduced into the tissue without any resistance. If resistance is felt, the needle is slightly withdrawn and redirected. The most common reason for resistance is the strong medial tendon from the temporal muscle along the temporal crest.

Figure 9.4 Mandibular block analgesia in a preschool child.
The foramen of the mandibular nerve lies below the occlusal plane on a line where the ramus is narrowest, two‐thirds of the way back from the anterior concavity (Figure 9.5). Palpation of the mandibular ramus will give the point of insertion, the direction in the horizontal and vertical planes, and the depth.

Figure 9.5 The position of the mandibular foramen changes during growth. However, the mandibular foramen is below the occlusive plane in children. The foramen is always situated on the line, where the ramus is narrowest, two‐thirds of the way back from the anterior concavity.
The needle is inserted between the pterygomandibular raphe and the ascending border of the mandibular bone. Inject a small amount of solution before advancing the needle into the deeper tissues. In young children, bone will be reached after about 15 mm and a 25‐mm needle can be used. However, in older children a depth of penetration up to 25 mm may be necessary, thus requiring a 35‐mm needle. When contact with the bone is obtained the needle is slightly withdrawn to avoid periost. Aspiration is performed and 1.2–1.5 mL of the solution is deposited. The lingual nerve is blocked when withdrawing the needle halfway and depositing a little of the remaining solution here. A chair‐side dental assistant is necessary when delivering a mandibular block to prevent sudden movements of hands and head in a comfortable way.
A mental block may be the result of an infiltration close to the primary mandibular first molar (Figure 9.6). Use of the regional maxillary block technique is seldom if ever required in a young child.

Figure 9.6 Pictures of skulls. The mental foramen is located closer to the primary mandibular first molar in children and the permanent second premolar in adults.
Computerized delivery systems
In recent years, computerized delivery systems have become part of the equipment for many dental practitioners treating children (Figure 9.7a,b). These systems deliver the analgesic solution at a constant and very slow speed which minimizes the pressure to the tissue and thereby the pain from the injection [8]. They can be used for all types of injection, both infiltration and blocks [9], but the great advantage is to be found in the palatal block procedures and in the modified periodontal ligament (PDL) injection. When using these techniques, the child does not experience the soft tissue analgesia which often is difficult to accept, especially for young children. A disadvantage is that the analgesic solution may leak into the child’s mouth and produce a bitter taste. Therefore, it is important that the chair‐side assistant is aware of the problem and uses a cotton bud to assemble the solution.
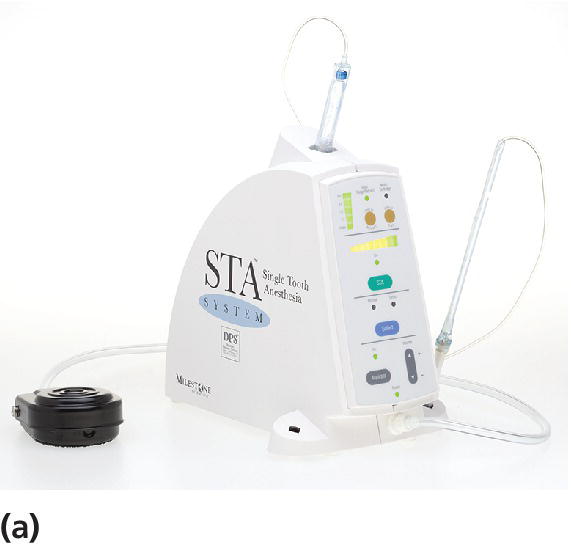
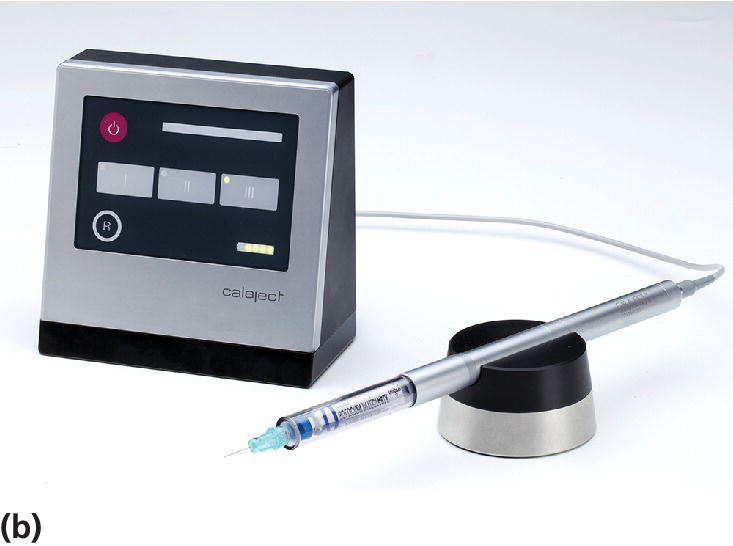

Figure 9.7 (a) The STA™ System and (b) Calaject™ are examples of computerized systems that delivers the analgesic solution with a constant and very slow speed which minimizes the pressure to the tissue and thereby the pain from the injection. (c) The AMSA injection technique.
The lightweight handpiece held like a pen enables the dentist to manipulate the needle placement with finger‐tip accuracy, rotating the needle when penetrating the mucosa, and to deliver the local analgesic with a foot‐activated contact. The flow rate is computer‐controlled and remains constant irrespective of variations in tissue resistance.
By using the computerized delivery systems, two palatal block procedures, plus a modified PDL injection, are possible:
- Anterior middle superior alveolar (AMSA) injection technique. The AMSA provides pulpal analgesia to the upper primary molars and canines, the surrounding palatal tissue and mucoperiosteum without any numbness of the lip, cheek, and muscles. For some children, this gives greater comfort and makes it easier to accept. A 30‐gauge extra‐short needle is oriented midway between the upper primary molars and midway between the free gingival margin and the palatine suture (Figure 9.7c). The suggested dosage should be delivered at a slow flow rate.
- Palatal anterior superior alveolar (P‐ASA) injection technique. This is a modified injection technique for the anterior maxilla and provides bilateral analgesia of the maxillary incisors and partially of the canines with a single‐needle penetration without involving lip and face. A 30‐gauge extra‐short needle is inserted adjacent to the incisive papilla. Topical ointment is applied in advance and it is critical to use only a slow flow rate. Aspiration is required when reaching bone contact in the canal and before delivering the anesthetic.
- PDL injection technique. This is a good alternative to the mandibular block when treating lower primary and first permanent molars. The technique is not recommended for teeth with active periodontal inflammation. The modified PDL technique employs two injection sites (mesial and distal). A 27‐ or 30‐gauge extra‐short needle is oriented with the bevel toward the tooth. The bevel should follow the surface of the tooth. The needle is advanced until resistance while the slow flow rate is activated. Approximately 0.2–0.5 mL is deposited at each side of the tooth.
General techniques
The administration of a pain‐free or an almost pain‐free injection depends primarily on the operator and factors that can be controlled by the operator: equipment, materials, and techniques (see Box 9.5).
Topical ointments with different tastes are available, and some children may find some of them more preferable than others. Only sharp needles, thin (30 G) or standard (27 G), should be used. Some authors mention that aspiration through a 30‐gauge needle can be difficult, although possible. A narrow needle is more likely to penetrate blood vessels than a needle with a wider gauge. When using a 35‐mm long needle, the deflection is larger in narrow‐gauge compared with wide‐gauge needles. It is suggested that the syringe is self‐aspirating. The analgesic solution should be at room temperature. The acidity of the analgesic solution is a factor provoking discomfort during injection, and the pH of solutions with vasoconstrictor is generally lower than those without (approximately 5.5 vs. 4.5).
The dental assistant plays an important role in preventing the child from making sudden movements during the administration of local anesthesia (Figure 9.8).
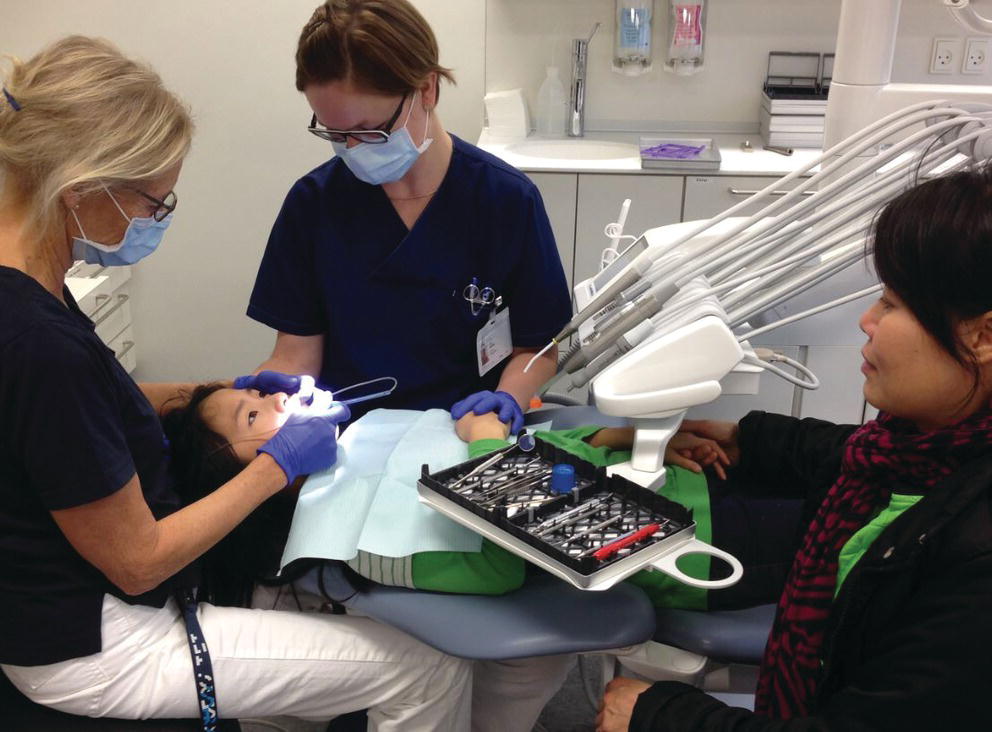
Figure 9.8 During an injection, the assistant can support the child by holding a hand and also keep the other hand on the child’s head to be sure the child does not make a sudden movement.
Contraindications
A child’s resistance, strong aversion or inability to cooperate is one of the few contraindications to local analgesia used alone (e.g., without sedation). Local analgesia is also contraindicated in the case of genuine allergy to the agents, although this is actually very rare. It is also not advisable to inject into deep tissues in patients with bleeding and coagulation disorders due to the risk of uncontrollable hematoma. In such cases, the patient’s physician has to be consulted before local analgesia is given. Medical conditions, such as severe liver disease or poor blood supply to a tissue, may also be contraindications.
Local analgesia with adrenaline is not contraindicated for patients being treated with tricyclic antidepressants if injected slowly, and if the aspiration is performed before injection. Neither is local analgesia with adrenaline contraindicated for patients with hypertension or cardiac arrhythmia, if the same precautions are taken. In these patients, it is actually the amount of endogenously produced adrenaline, due to stress and pain, which is the problem.
Complications
In general, there are few side‐effects and complications following local analgesia in children and adolescents. The most common problem is without doubt that the efficiency of the procedure may be less than desired. Systemic side‐effects such as fainting, a loss of consciousness due to decreased blood supply to the brain, rarely happens. If the patient faints, he or she should be placed in a recumbent position with elevated legs. The airways should be secured by tilting the head backwards and forcing the mandible forward. General allergic reactions to local analgesia solution are very rare and consist of skin eruptions, occasionally bronchial constrictions, and a drop in blood pressure. In the case of proven allergy, another product should be tested under controlled conditions. Most complications or side‐effects are of local origin, such as self‐inflicted bites of anesthetized tissue (Figure 9.9), which are best treated with chlorhexidine gel 1%. Blanching of the cheek after local analgesia injection is a sympaticus reaction (Figure 9.10) and will last in a few patients for up to 10 minutes. Hematoma sometimes arises during injection, and in these cases the patient and the parents should be informed about its nature. The effusion of blood into extravascular spaces can result from inadvertently nicking a blood vessel during the injection of a local analgesic. It should be explained that swelling and discoloration will appear.
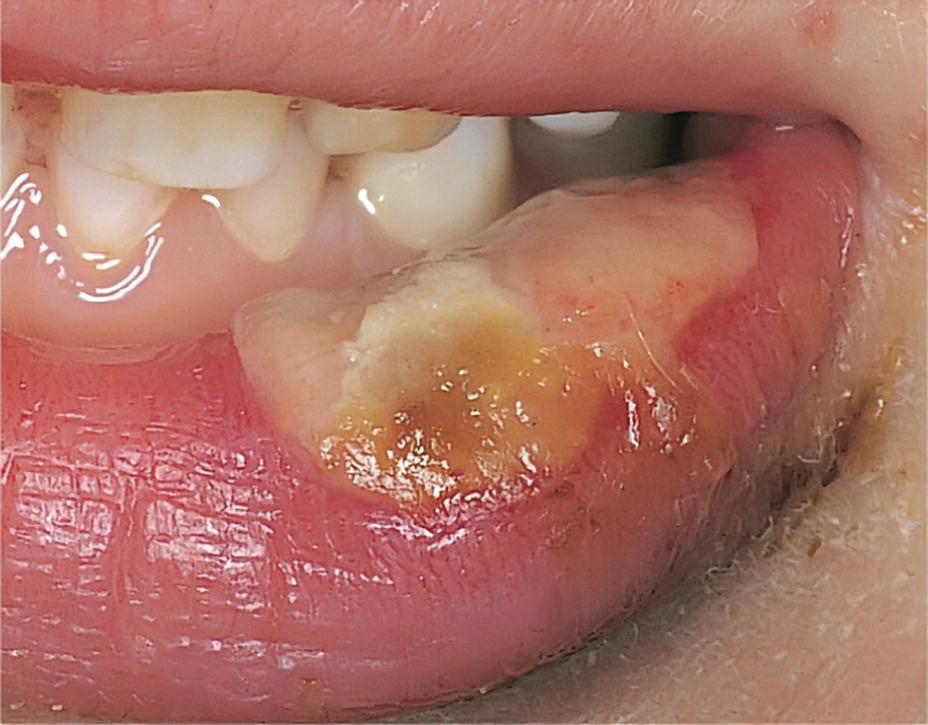
Figure 9.9 Side‐effect of mandibular block analgesia. Bite wound in lower lip.
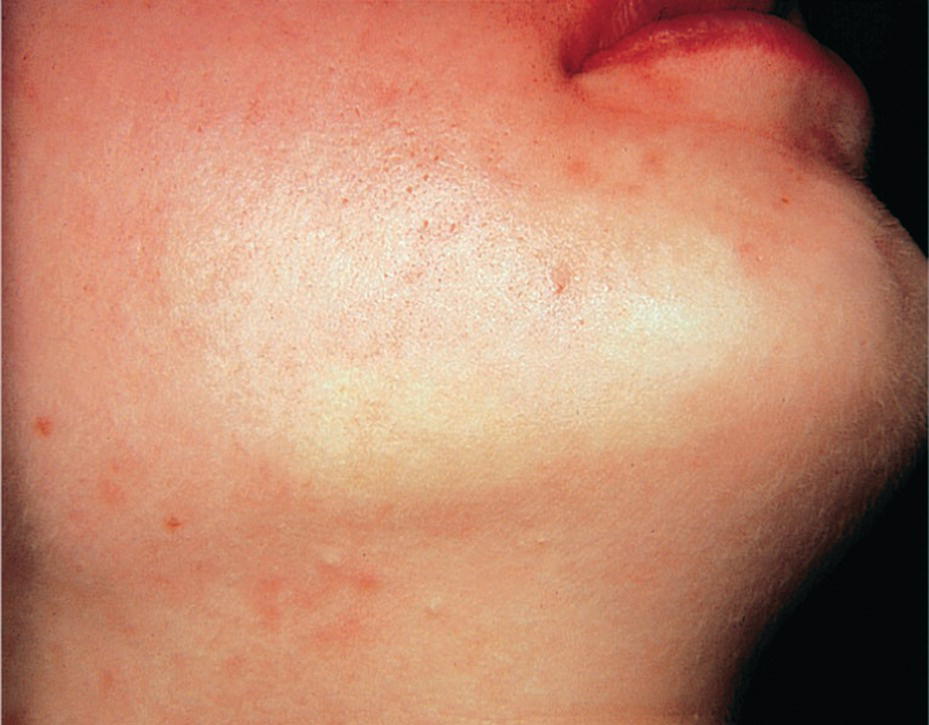
Figure 9.10 Blanching of the cheek (sympaticus reaction) after local analgesia injection in a child.
Toxicity
Toxic reactions from local analgesia can be caused by accidental intravascular injection, excessively rapid injection, or drug overdose. Patients may complain of slight dizziness and visual or hearing disturbances. They may become agitated, confused and have difficulties in breathing. There may be a cardiovascular system reaction with an increased heart rate and blood pressure and – in the second phase of local analgesic toxicity – a drop in blood pressure. Dentists who treat children should always be mindful of local analgesia toxicity, and the recommended maximum safe dose of local anesthetic for each child should be calculated (Box 9.6).
General analgesia
The most commonly used and recommended general analgesics for children are paracetamol and non‐steroidal anti‐inflammatory drugs (NSAIDs). Paracetamol (acetaminophen) is a central‐acting drug, as compared with NSAIDs, which exert their analgesic effect mostly in the peripheral tissue. Both paracetamol and NSAIDs reach their maximal analgesic effect after about 2 hours when administered orally, which is the preferred route. The rectal uptake of paracetamol is more erratic as compared with the oral route and the maximum analgesic effect is reached as late as 3–4 hours after administration. The initial starting dose or single dose of paracetamol should be higher than the maintenance dose used in the postoperative phase [10]. NSAIDs also have an effect on platelets which could have a negative effect on coagulation. An alternative to non‐selective NSAIDs is to use cyclooxygenase (COX)‐2 inhibitors, as celcoxib, if bleeding is a concern during the procedure or in the postoperative phase. A combination of paracetamol and NSAIDs is recommended for optimal pain relief, and they should be administered about 2 hours before the treatment in order to assist in the prevention of procedural pain. If pain is expected during the postoperative phase, it is recommended to give the patient an analgesic on a regular basis, especially during the first 1–2 days [11–13]. Acetylsalicylic acid (aspirin) should be avoided for postoperative pain due to its anticoagulant effect and possible toxicity.
Box 9.7 presents oral doses for short‐time use of some general analgesics for children.
In patients where the use of a sedative drug is indicated, it is a good practice to combine the sedative with an analgesic. The alpha‐2 receptor agonists, clonidine and dexmedetomidine, represent an interesting profile of drugs which both have a sedative and an analgesic effect without the risk of ventilatory depression found with opioid use. Clonidine can be administered orally or rectally. Dexmedetomidine has a limited oral uptake, but transmucosal (buccal and especially nasal) administration has been described as effective. The nasal route is well accepted and does not cause any discomfort as compared to midazolam (see section “Oral and rectal administration of benzodiazepines”). It should be remembered that most sedatives commonly used for various procedures are lacking analgesic effect [14]. The use of analgesics is mandatory in procedures where pain is anticipated.
Minimal sedation (inhalation sedation)
For minimal sedation, nitrous oxide–oxygen is the method most commonly used and its high success rate and safety are well documented [15]. In some countries, a specific authorization to administer nitrous oxide–oxygen sedation is required, while it in other countries is a part of the pre‐graduate dental education of dentists.
Nitrous oxide–oxygen sedation is defined as a state of sedation during which patients respond normally to verbal commands. Although cognitive function and physical coordination may be impaired, airway reflexes, ventilation and cardiovascular function are unaffected (Box 9.8).
Indications for nitrous oxide–oxygen sedation are generally the same as for the benzodiazepines. However, the fact that the gas may have additional analgesic properties and is administered by inhalation makes the method a suitable alternative for a number of specific cases (see Table 9.1). The analgesic effect of nitrous oxide–oxygen sedation has been questionable [14], and nitrous oxide–oxygen inhalation sedation is mainly considered as sedation. Fasting is not required for patients undergoing nitrous oxide–oxygen sedation. A light, non‐fatty meal no later than 2 hours before the procedure is acceptable [15].
Table 9.1 Indications for moderate/conscious sedation and type of sedation used in children (+ + = very good, + = good)
| Benzodiazepines | N2O/O2 | |
| Too immature for treatment, e.g., low age or mental disability (mental age <3–4 years) | ++ | |
| Fear/anxiety | ++ | ++ |
| Medically compromised patients | + | ++ |
| Muscular tone disturbances | ++ | |
| Pronounced gag reflexes | ++ | |
| Treatment stress | + | ++ |
| The nature of the dental treatment (e.g., oral surgery) | + | ++ |
| Facilitates sleep the night before treatment | ++ |
Inhalation sedation is contraindicated for patients belonging to ASA anesthesia risk classes III and IV (Box 9.9), and these patients must eventually be treated only in collaboration with a responsible doctor or anesthesiologist. Only patients in ASA risk groups I and II should be treated in dental clinics. Contraindications specific for nitrous oxide–oxygen sedation are: partial obstruction of the respiratory airways, psychosis, recent otological operation, sinusitis or porphyria.
The administration of nitrous oxide–oxygen sedation requires special equipment. The machine must always supply not less than 30% oxygen (in Scandinavia 40%) in the gas mixtures. The gas flow must be continuous and the apparatus equipped with a failsafe device, i.e., if the oxygen pressure falls, the supply of nitrous oxide automatically stops. If the gas supply is disconnected, the patient must be able to breathe air via an emergency air valve. Excess gas and exhaled gas must be effectively eliminated by scavenging, and leakage from the delivery system should be prevented through proper maintenance and periodic inspection of the equipment. Waste of nitrous oxide can be controlled with an appropriate evacuation system that includes securely fitting masks [15].
The sedation procedure starts by giving the patient 100% oxygen for 2–3 minutes. The nasal mask is adjusted to prevent leakage, and the gas flow is regulated. Nitrous oxide is then administered in increasing concentrations (titration) until a suitable stage of minimal sedation (usually requiring 33% nitrous oxide; max 50%) has been achieved (Figure 9.11). Monitoring of the patient during sedation with nitrous oxide–oxygen is performed by observing their level of consciousness, the patient’s responsiveness, color, and respiratory rate and rhythm. After completion of the dental treatment, 100% oxygen is given for 5 minutes. Although the patient is able to leave the chair after the first 5 minutes, he/she is not ready to leave the surgery before another 30 minutes.
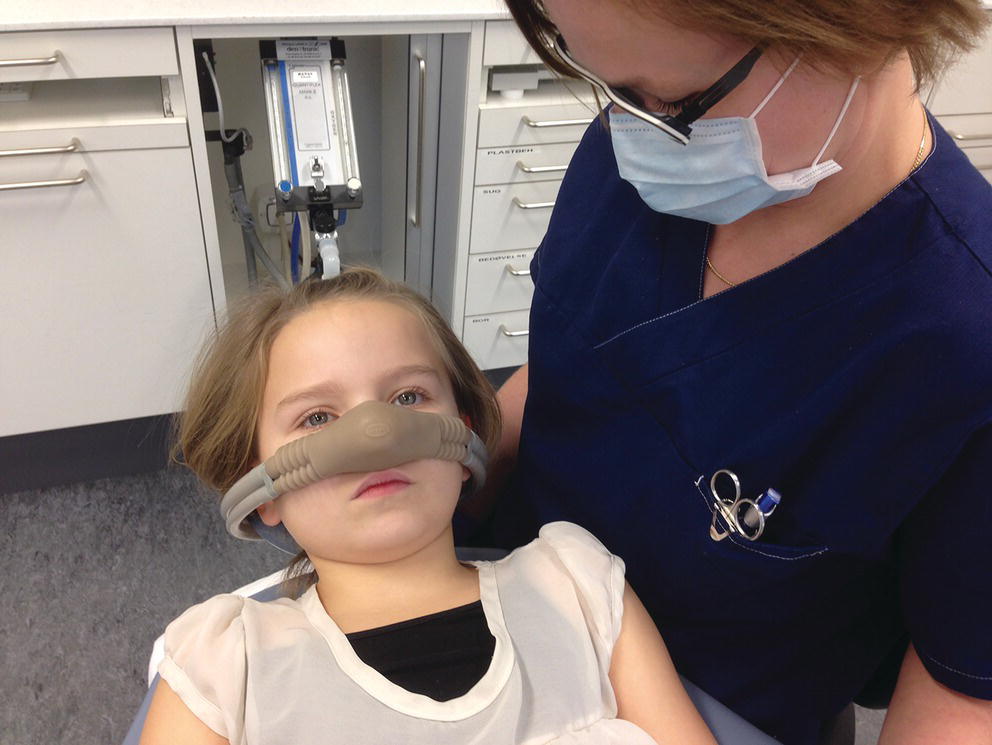
Figure 9.11 Nitrous oxide–oxygen sedation.
After uptake in the lungs, nitrous oxide gas is transported by the blood to the CNS, which is its site of sedative and analgesic actions. The gas does not bind to body fluid or tissue and has little or no effect on respiration, blood circulation, and metabolism. The gas is eliminated quickly after termination of administration. Nitrous oxide has anesthetic, sedative, and analgesic effects, but individual susceptibility varies. Even if the anesthetic effect is rather poor, some patients may lose consciousness breathing a 50% mixture of nitrous oxide. The peak alveolar concentration is attained within a few minutes of inhalation, which implies that the sedative effect of a specific concentration of the gas can be assessed very shortly. Nitrous oxide–oxygen sedation is therefore a method that gives the operator an excellent opportunity to adjust the dose of the medication to a suitable level during the treatment with almost immediate effect, which increases the safety of the procedure. Since the analgesic effect is not fully known and probably is limited, it should be combined with local analgesia during invasive operative procedures.
The most common side‐effects of nitrous oxide are nausea and vomiting. Diffusion hypoxia can occur as a result of rapid release of nitrous oxide from the bloodstream into the alveoli, thereby diluting the concentration of oxygen. This may lead to headache and can, as recommended, be avoided by administering 100% oxygen after nitrous oxide has been discontinued.
Nitrous oxide–oxygen sedation can be used in combination with benzodiazepines, which implies that the two types of sedatives have additive effect. A frequently applied approach is that the patient is given an initial dose of benzodiazepine preoperatively, which is supplemented with nitrous oxide–oxygen if an additional sedative/analgesic effect is needed during the treatment. There is an increased risk of airway and sedative complications when more than one sedative is used [16].
For health‐care personnel, no direct evidence of any causal relationship between chronic low‐level exposure to nitrous oxide and potential biologic effects has been provided. The maximum safe concentration of nitrous oxide has not been determined; however, every attempt should be made to reduce the level of nitrous oxide to exposed health‐care personnel. Practitioners cannot be too cautious with regard to their health as well as health of their coworkers.
Moderate sedation (conscious sedation)
The depth of sedation is a continuum. Depending on the sedation depth, sedation can be divided into minimal, moderate, and deep sedation (Box 9.8).
The term moderate sedation (conscious sedation) is defined as a medically controlled state of depressed consciousness that allows protective reflexes to be maintained, retains the patient’s ability to maintain a patent airway independently and continuously, and permits appropriate response by the patient to physical stimulation or verbal command, e.g., “open your mouth” [17]. The term deep sedation denotes a deeper state of depressed consciousness or unconsciousness from which the patient is not easily aroused. It is acknowledged that deep sedation is accompanied with risks when administered by dentists alone during oral treatment procedures. Only moderate sedation is dealt with in this section, since this may be administered by the operating dentist alone without being assisted by a specialist in anesthesiology [18].
Different guidelines for moderate sedation in dental care have been developed [16–19]. The suggested main goals of using sedation during dental treatment of children and adolescents are listed in Box 9.9. It must be underlined that moderate sedation alone is frequently inadequate to make the very fearful child cope with dental treatment, as deep sedation or general anesthesia will do. Patients are awake and conscious of what is going on, but the anxiolytic and sedative effect makes them less sensitive to unpleasant stimuli and more accessible for behavioral techniques, such as distraction and comforting. Even if the drug itself has no analgesic effect, the pain perception may also be reduced since this is dependent on the anxiety level.
Indications and contraindications
Moderate sedation is indicated when both patient and dental indications are present (Box 9.10). It is the combined assessment of these two types of indications that should be the basis for suggesting whether a certain type of oral examination or treatment should be done conventionally or under some type of sedation or general anesthesia. Children with low coping ability (e.g., immature children) or high dental anxiety, and with extensive or complicated treatment needs, should be treated under deep sedation or general anesthesia, which requires anesthesiology staff. This is probably most appropriate from the perspective of the child, parents, and dental staff, and may also be cost‐effective since the majority of treatment may be done in one session.
For the preoperative assessment of the patient, particularly whether there are any contraindications for use of sedation, the American Society of Anesthesiologists’ (ASA) classification system of the patient’s physical status should be used (Box 9.11). The dentist is expected to take the responsibility for treating patients in Classes I and II under moderate sedation, while treatment of patients in Classes III and IV should be decided on in consultation with a physician/anesthesiologist.
Sedation of children below 2 years of age is connected with increased risks and should therefore be done in collaboration with an anesthesiologist. Sedation of children with any kind of acute general disease should also be avoided. Other contraindications are allergy to any of the drugs, neuromuscular diseases, and if an interaction with other medications is suspected.
Oral and rectal administration of benzodiazepines
Today, the drugs of first choice for sedation of anxious children are the benzodiazepines [16]. The benzodiazepine group contains a variety of chemical variations, where the most frequently used in dentistry in the Nordic countries is midazolam. The clinical effects are anxiolytic, sedative/hypnotic, and muscle relaxing, and the toxicity is low [20]. Side‐effects are few, although anterograde amnesia can be expected and is frequently cited as an advantage. This consideration has, however, been questioned because the amnestic effects mainly affects explicit (conscious) memory, but leaves implicit (unconscious) memory intact [21]. This means that the child remembers a scary experience, but cannot put it into words. For repeated procedures this not uncommonly leads to higher levels of anxiety and an increasingly uncooperative child. The site of action of the benzodiazepines is in the CNS where they increase the effect of the inhibiting neurotransmitter gamma‐aminobutyric acid (GABA) [22]. Other benzodiazepines, such as diazepam and flunitrazepam, have similar pharmacological properties, but different duration time, and the choice between them may therefore be based on the length of time that sedation is needed. Diazepam has a long working time due to long elimination half‐life and active metabolites, and it is therefore most suitable for reducing preoperative anxiety and preventing sleep disturbances prior to treatment. It is available in both tablets and rectal solutions. Midazolam has the shortest working time because of fast elimination and is therefore most suitable for perioperative sedation under treatments of short duration. The drug is available only in ampoules for intravenous injection (tablets available in some countries), but the solution can also be used for rectal and oral administration (i.e., off label). The operator should be aware that using a drug off label demands extended knowledge and responsibility. Since the taste of midazolam is very bitter, it must be mixed with some kind of juice or other additives to taste in order to have children drink it. The working time of flunitrazepam lies between diazepam and midazolam, and it is available in tablets with no particular bad taste. It may be either swallowed whole, placed underneath the tongue (absorption through the mucosa), or crushed and dissolved in some kind of drink.
Routes and doses
Intravenous sedation is the most effective in terms of fast response, effect, and possibility to adjust the dosage to an optimal level of effect, but this is usually not practicable in a dental setting, since it demands assistance of a specialist in anesthesiology. Oral administration is generally preferred from a practical point of view, but some children are unable or unwilling to take an oral medication. The alternative routes are rectal and nasal, but there are drawbacks with both of these. Rectal administration is connected with ethical problems and only applicable in very young children in collaboration with their parents. Nasal administration is effective, but very unpleasant for the child due to irritation (i.e., midazolam) of the nasal and pharyngeal mucosa, and may counteract the goal of establishing a positive relationship between dentist and child. The oral route may, however, be used in children who are unwilling to drink from a cup by placing the solution in the retromolar area from a needleless syringe (Figure 9.12).

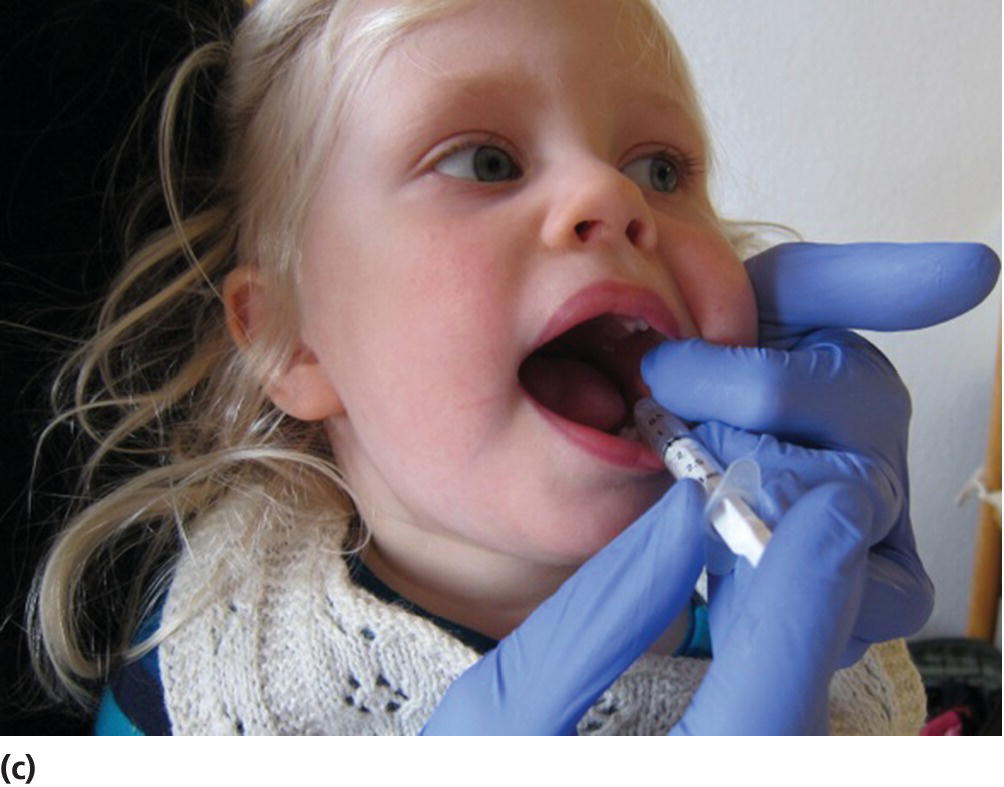
Figure 9.12 (a) Oral administration with midazolam mixed in a juice. (b) Applicator for rectal administration of midazolam. (c) Oral sedation with needleless syringe in a 2‐year‐old child.
The suggested doses of benzodiazepines for conscious sedation are given in Table 9.2. The doses as well as the time to onset of desired effect are dependent on the administration route. The fastest and most effective absorption of the drug is through the nasal mucosa, which means more rapid onset and slightly lower dosage than for the rectal and oral routes. The absorption through the rectal mucosa is also rather fast and more effective than the oral route due to the fact that a portion of the drug is metabolized in the liver after absorption from the ventricle/intestines. However, whatever route, there are extremely large individual variations in the degree of absorption and time until maximum plasma concentration is obtained. Combined with the fact that there are also large individual variations in dose response, there is considerable unpredictability concerning the effect of an individual dosage. Generally, it may be estimated that the time from administration to onset of effect varies between 15 and 30 minutes. Sedation with benzodiazepines should be used with special caution in patients who are using other medications, particularly other substances with depressing effect on the CNS, such as antipsychotics, antidepressants, antiepileptics, and opioids. Interactions may also be important for the dosage, e.g., the absorption of midazolam is enhanced by erythromycin and grapefruit juice. Knowledge on drug interactions may be found in national databases.
Table 9.2 Suggested doses of benzodiazepines for perioperative sedation
Source: Hallorsten et al. 2006 [17]. Reproduced with permission of European Academy of Paediatric Dentistry.
| Drugs and routes | Dosage (mg/kg) | Maximum dose (mg) |
| Midazolam, oral | 0.5 | 12,5 |
| Midazolam, rectal | 0.3 | 10 |
| Flunitrazepam, oral | 0.02–0.025 | 20–40 kg: 0.5>40 kg: 1.0 |
| Diazepam, oral | 4–8 years: 0.5–0.8>8 years: 0.2–0.5 | 1515 |
| Diazepam, rectal | 0.7 | 15 |
Clinical considerations
Since the use of sedation in dentistry is associated with increased risks for the patient and responsibility for the operator, practitioners should estimate their own competency within the framework of their authorization [17]. The competency must include sufficient knowledge about the drugs, their effects, side‐effects, and interactions, as well as the national regulations relating to them. The operator must have knowledge about and be able to practice preoperative considerations based on the ASA classification system, and also be able to handle possible complications. This includes appropriate equipment for airway protection, oxygen delivery, and drugs for emergency use.
Prior to moderate sedation, it is recommended that the child should fast in order to prevent possible aspiration if there is nausea and vomiting. The suggested rules are given in Box 9.12. For the emergency patient, where proper fasting has not been assured, the increased risk of sedation must be weighed against the benefits of the treatment, and the lightest effective sedation should be used. If possible, such patients may benefit from delaying the procedure.
The patient must continuously be monitored during the treatment, both by the operating dentist and by an assistant. Keeping verbal contact with the patient is most important, thereby estimating the depth of the sedation. If the patient is not able to maintain verbal contact, with a tendency to fall asleep, not being able to keep the mouth open, and not react to physical stimulation, the sedation stage may be too deep. This implies reduced reflexes and increased risks for aspiration during oral treatment, and one should aim to cancel such treatment and concentrate on securing the patient’s airways. The use of pulse oximetry in order to be able to disclose possible blood desaturation is recommended to increase safety.
After the treatment, the child must also be monitored and not dismissed until the sedative effect is definitely reduced and the child has regained normal psychomotor abilities. The child must be accompanied back home and be under observation by adults for the rest of the day. Preoperative and postoperative instructions in writing must be given in advance of the procedure to the child and the parent or guardian.
General anesthesia
Some patients lack the physical or mental ability to cooperate during treatment. Dental treatment under general anesthesia may then be the only solution (Box 9.13). Moreover, some surgical procedures are so extended in time and tiring for the patient that no other methods of pain control than general anesthesia can be considered.
The most common reasons for referring a child to dental treatment under general anesthesia are dental caries, molar incisor hypomineralization (MIH) and dental surgery, with dental caries as the most frequent cause. The major age group referred to treatment under general anesthesia is preschool children [23].
When planning the dental treatment carried out under general anesthesia, it is important to be radical to avoid repeated treatments, although the prevalence of serious complications in association with dental treatment under general anesthesia is very low, particularly when performed in hospital settings (Figure 9.13). General anesthesia is probably safer than giving deep sedation to a patient in a normal dental setting. The indication for dental treatment under general anesthesia, however, must be restricted because anesthesia itself can exert physical and mental stress compared with the alternative methods. It should be the last resort when all efforts to treat a child in the conventional manner have failed.
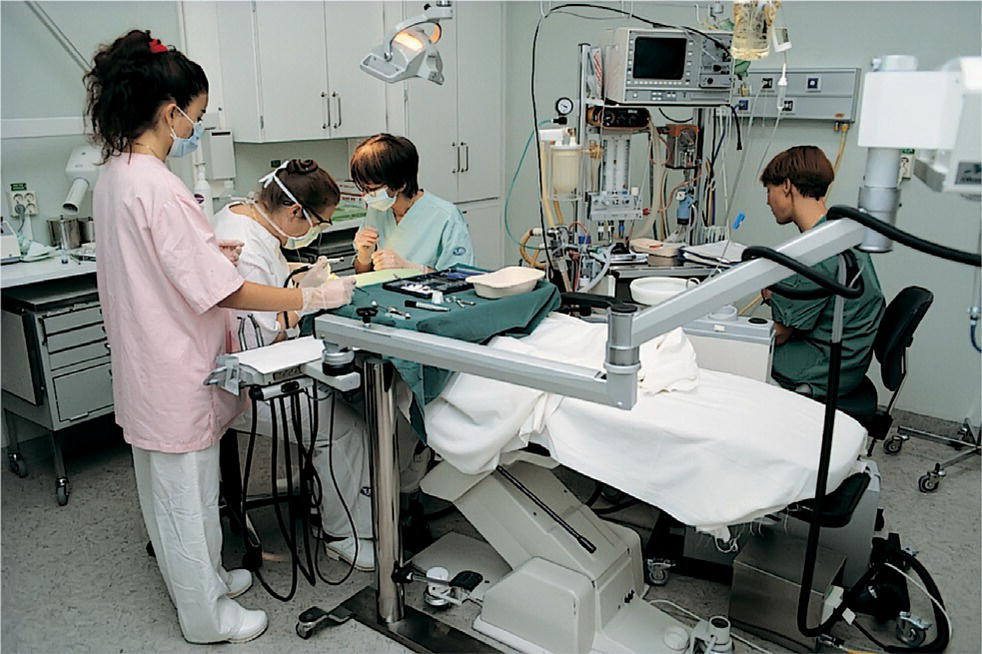
Figure 9.13 Dental treatment under general anesthesia in a hospital setting. Notice the comprehensive equipment and the large amount of personnel.
In the Nordic countries, dental treatment under general anesthesia requires the assistance of a registered anesthetist who selects the method of anesthesia according to the child’s condition and the nature of the treatment to be performed. Postoperative pain should be a concern in the same way as described earlier in this chapter. After major dental surgery more potent analgesics may be required in the first postoperative period. The hospital setting makes this a possibility as compared to outpatient management. In a study from a Swedish county with good access to sedation, the number of children aged 0–19 years in need of dental treatment under general anesthesia was 0.7– 2.2/1000 per year [24].
Every possible effort should be made to return the child to routine therapy in the future. After dental treatment under general anesthesia, adequate prophylactic dental care should start as soon as possible.
References
- 1. International Association for the Study of Pain, IASP Taxonomy, last update May 2012. http://www.iasp‐pain.org.
- 2. Schechter NL, Berde CB, Yaster M. Pain in infants, children, and adolescents. Philadelphia: Lippincott Williams & Wilkins, 2003; 2nd edn.
- 3. Anand KJ, Hickey PR. Pain and its effects in the human neonate and fetus. N Engl J Med 1987;317:1321–9.
- 4. Wall P, Melzack R. Textbook of pain. 6th edition. Eds McMahon, Koltzenburg, Tracey, Turk. Philadelphia: WB Saunders, 2013.
- 5. Rasmussen JK, Frederiksen JA, Hallonsten AL, Poulsen S. Danish dentists’ knowledge, attitudes and management of procedural dental pain in children: association with demographic characteristics, structural factors, perceived stress during the administration of local analgesia and their tolerance towards pain. Int J Paediatr Dent 2005;15:159–68.
- 6. Wondimu B, Dahllof G. Attitudes of Swedish dentists to pain and pain management during dental treatment of children and adolescents. Eur J Paediatr Dent 2005;6:66–72.
- 7. Jones CM, Heidmann J, Gerrish AC. Children’s ratings of dental injection and treatment pain, and the influence of the time taken to administer the injection. Int J Paediatr Dent 1995;5:81–5.
- 8. Primosch RE, Brooks R. Influence of anesthetic flow rate delivered by the Wand local anesthetic system on pain response to palatal injections. Am J Dent 2002;15:15–20.
- 9. Palm AM, Kirkegaard U, Poulsen S. The Wand versus traditional injection for mandibular nerve block in children and adolescents: perceived pain and time of onset. Pediatr Dent 2004;26:481–4.
- 10. Arana A, Morton NS, Hansen TG. Treatment with paracetamol in infants. Acta Anaesthesiol Scand 2001;45:20–9.
- 11. Krauss B, Green SM. Procedural sedation and analgesia in children. Lancet 2006;367:766–80.
- 12. Lönnqvist PA, Morton NS. Postoperative analgesia in infants and children. Br J Anaesth 2005;95:59–68.
- 13. Lundeberg S. Pain in children – are we accomplishing the optimal pain treatment? Paediatr Anaesth 2015;25:83–92.
- 14. Grønbæk AB, Svensson P, Væth M, Hansen I, Poulsen S. A placebo‐controlled, double‐blind, crossover trial on analgesic effect of nitrous oxide‐oxygen inhalation. Int J Paediatr Dent 2014;24:69–75. doi: 10.1111/ipd.12027.
- 15. Clark MS, Brunick AL. Handbook of Nitrous Oxide and Oxygen Sedation. Elsevier, 2015;4th edn.
- 16. Mason K. Pediatric Sedation Outside of the Operating Room: A Multispecialty International Collaboration. Springer, New York 2011.
- 17. Hallonsten AL, Jensen B, Raadal M, Veerkamp J, Hosey MT, Poulsen S. EAPD Guidelines on Sedation in Paediatric Dentistry. European Academy of Paediatric Dentistry, 2006. http://www.eapd.gr/dat/5CF03741/file.pdf (accessed November 2014).
- 18. American Academy of Pediatrics and the American Academy of Pediatric Dentistry. Guideline for Monitoring and Management of Pediatric Patients During and After Sedation for Diagnostic and Therapeutic Procedures. Adopted 2006, Reaffirmed 2011. http://www.aapd.org/media/Policies_Guidelines/G_Sedation.pdf.
- 19. American Academy of Pediatric Dentistry. Clinical guideline on the elective use of minimal, moderate, and deep sedation and general anesthesia in pediatric dental patients. Pediatr Dent 2004;26:95–103.
- 20. Berthold C. Enteral sedation: safety, efficacy, and controversy. Compend Contin Educ Dent 2007;28:264–71; quiz 72, 82.
- 21. Stewart SH, Buffett‐Jerrott SE, Finley GA, Wright KD, Gomez TV. Effects of midazolam on explicit vs implicit memory in a pediatric surgery setting. Psychopharmacology 2006;188:489–97.
- 22. Mohler H, Fritschy JM, Rudolph U. A new benzodiazepine pharmacology. J Pharmacol Exp Ther 2002;300:2–8.
- 23. Haubek D, Fuglsang M, Poulsen S, et al. Dental treatment of children referred to general anaesthesia – association with country of origin and medical status. Int J Paediatr Dent 2006;16:239–46.
- 24. Varpio M, Wellfelt B. Some characteristics of children with dental behaviour problems: Five‐year follow‐up of pedodontic treatment. Swed Dent J 1991;15:85–93.
- 25. Hicks CL, von Baeyer CL, Spafford PA, van Korlaar I, Goodenough B. The Faces Pain Scale–Revised: toward a common metric in pediatric pain measurement. Pain 2001;93:173–83.
- 26. American Society of Anesthesiologists. ASA standards, guidelines and statements, 2006. https://www.asahq.org/For‐Members/Clinical‐Information/ASA‐Physical‐Status‐Classification‐System.aspx (accessed October 2014).