CHAPTER 22
Temporomandibular Disorders
Tomas Magnusson and Martti Helkimo
Definitions
Temporomandibular disorders (TMD) is a collective term embracing a number of clinical problems that involve the masticatory musculature, the temporomandibular joint (TMJ) and associated structures, or both [1]. The term is synonymous with the terms cranio‐mandibular disorders (CMD), functional disorders of the masticatory system, and mandibular dysfunction.
The most frequent reason for seeking treatment at a TMD clinic is pain, usually localized in the muscles of mastication and/or the TMJ. Typically the pain can be provoked and aggravated by chewing or by other jaw functions. TMD have been identified as a major cause of nondental pain in the orofacial region [1].
In addition to pain, limited or asymmetric mandibular movements, and TMJ sounds are commonly found in these patients. Common patient complaints also include jawache, earache, headache, and facial pain. Bruxism (jaw clenching and tooth grinding) and other oral parafunctions (e.g., nail‐biting and gum chewing) may be related problems.
Epidemiology
Signs and symptoms of TMD seem to be relatively common in the general population of adults. In cross‐sectional epidemiologic studies of adult populations, the prevalence rates range from about 40 to 75% having at least one sign and about 35% having at least one symptom of TMD.
It is almost impossible to get a clear picture as to occurrence of TMD in children and adolescents. Estimates of prevalence rates differ markedly from one study to another (Figure 22.1 and Table 22.1). This variation arises from a combination of factors such as inter‐ and intra‐individual variations among examiners, differences in the composition of the study populations, examination methods, choice of variables as well as a multitude of incomparable definitions and criteria for symptoms and signs.
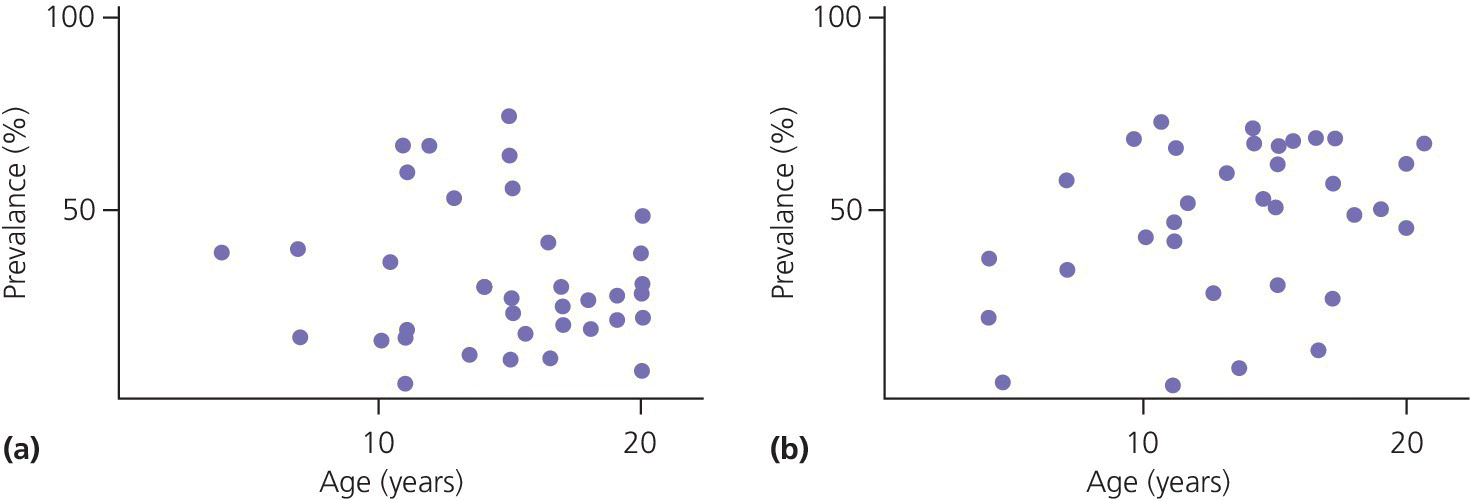
Figure 22.1 Diagrams showing prevalence of one or more (a) subjective symptoms and (b) clinical signs of TMD presented in different epidemiologic studies of children and adolescents. The single dots represent the prevalence figure found in different studies. Note the wide variations within the same age groups, ranging from single percent to more than 70%.
Adapted from Nydell et al. 1994 [2]. Reproduced with permission of the Swedish Dental Journal.
Table 22.1 Range and median values for the prevalences of (a) subjective symptoms and (b) clinical signs of TMD in different epidemiologic investigations
Adapted from Nydell et al. 1994 [2].
| All reported data | ||
| Range (%) | Median (%) | |
| (a) Subjective symptoms | ||
| Tiredness or stiffness in the jaws | 0–59 | 5.0 |
| TMJ sounds | 0–32 | 13.0 |
| Pain on opening the mouth or other jaw movements | 0.4–59 | 4.0 |
| Pain in the face or jaws | 0–19 | 3.0 |
| Headache | 1–88 | 13.2 |
| Difficulties in opening the mouth | 0–9 | 1.8 |
| Locking or luxation | 0.4–7 | 3.0 |
| One or more subjective symptoms | 3–74 | 26.2 |
| (b) Clinical signs | ||
| Impaired opening capacity | 0–29 | 1.8 |
| TMJ sounds | 0–50 | 19.2 |
| Deviation upon opening and closing the jaw | 0–78 | 6.5 |
| Locking or luxation | 0–1 | 0.5 |
| Tenderness on palpation of the TMJ | 0–44 | 4.0 |
| Tenderness on palpation of the masticatory muscles | 1–68 | 21.2 |
| Pain upon movement | 0–18 | 2.5 |
| One or more clinical signs | 2–78 | 51.2 |
Another plausible, and often overlooked, reason for the great variation in the reported frequencies of signs and symptoms of TMD is that examination methods designed for adults have been applied uncritically on children without taking the age and cognitive development of the child into consideration [2].
In spite of the large volume of data from recent literature on the epidemiology of TMD, it is still not possible to make a conclusive statement as to the “true” prevalence of TMD signs and symptoms in children and adolescents, nor can we even say if these phenomena change with age. In recent years, a change has been taking place in how we look upon the condition. In earlier years, research was focused on functional disturbances in the masticatory system, and thereto associated pain. Today, focus has shifted—in many quarters—to research, diagnosis, and treatment of pain occurring in the orofacial area irrespective of its etiology. TMD is, by some authors, regarded as a chronic pain condition. This implies that it is difficult, or even impossible, to compare results from epidemiologic studies from later years with early ones, especially if symptom criteria are ambiguously defined in the studies. Despite these shortcomings, it is obvious from longitudinal studies that symptoms and signs show great fluctuation with time [3,4]. Most signs and symptoms seem to come and go in an unpredictable way [5,6]. In a 20‐year longitudinal study, no significant progression of signs and symptoms of TMD from childhood, through adolescence, to adulthood have been found [7]. In addition, cross‐sectional epidemiologic studies covering a 20‐year period have shown that the estimated TMD treatment need in 10‐ and 15‐year‐olds was 3–4% and 2–5%, respectively [8].
From the many epidemiologic studies performed in this field, it can be concluded that signs and symptoms of TMD in children and adolescents are in most cases occasional and mild, except for some rare conditions and diseases such as juvenile idiopathic arthritis (Still’s disease), which can be progressive and develop into severe functional limitation and pain in some few individuals.
Bruxism
Oral parafunctions include bruxism, lip‐biting, thumb‐sucking, and abnormal posturing of the jaw. In contrast to normal functional behaviors, such as chewing and swallowing, these parafunctional behaviors seem to have no obvious functional purpose.
Parafunctional activities are very common in all age groups, and only occasionally have significant negative effects on the structures of the masticatory system. As in adults, muscular pain, headache, and TMJ overloading may result also in children. In those cases, a protective bite splint may be indicated.
Nail‐biting and excessive chewing on chewing gum are fairly common habits in children and adolescents, and can be as important etiologic factors for TMD as tooth grinding and tooth clenching. Unphysiologic sleeping posture, e.g., belly sleeping, can also result in unfavorable loading of the TMJs and be a contributory factor to dysfunction of the joints.
In most cases, nocturnal bruxism in children does no harm. The patients and parents should be informed that, although very loud sounds may occur, there is little evidence that permanent damage results from bruxism in childhood. On the contrary, it has been shown that tooth wear of primary teeth has a low predictive value for tooth wear of permanent teeth [9]. The child will in most cases outgrow the behavior in time. It has even been suggested that bruxism in small children is a physiologic phenomenon necessary for a normal eruption of the permanent teeth. However, tooth wear at 14 years of age, when the “mature” dentition is established, is a good predictor for further wear [9].
TMD and orthodontics
The possibility that orthodontic treatment in childhood might be a risk factor for the development of TMD later in life has been an issue of great controversy in the dental literature for a long time. Several comprehensive review articles and meta‐analyses have focused on this question [10–13]. The conclusion from these studies is that there is no scientific evidence that orthodontic treatment increases the risk for development of TMD. On the contrary, there is some support for the conclusion that properly performed orthodontic treatment in childhood might have a positive effect on the functional status of the masticatory system later in life [14–16].
Taking the history in children and adolescents with suspected TMD
Taking the history in children entails several difficulties. For instance, many children try to please the questioner by giving the answer they believe is the desired one [17]. Because of this, care must be taken to avoid leading questions as far as possible when reviewing the history [18]. Another problem is that younger children cannot use language to express pain. Not until about the age of 12 years, at the end of the cognitive development and when the ability to think abstractly has developed, are children able to describe their pain verbally [19].
Bearing these limitations in mind, taking the history can be an important key to a correct diagnosis and a subsequent successful treatment. The history should be taken in a calm and confident atmosphere. The questions should be relevant and kept as simple as possible. Some examples of questions that should be included in the history are presented in Box 22.1.
As already stated, pain in the face and jaws, as well as headaches, are fairly common symptoms in children and adolescents. In most cases, however, they occur only occasionally. Only if they are frequent complaints and only if they are associated with clinical signs do they merit TMD treatment. Asymptomatic joint sounds are not, with rare exceptions, an indication for treatment, but observation on recall is the proper course of action. It has even been suggested that joint sounds in newly born and young children are the result of differential growth rates of the different anatomic components of the TMJ [20]. Such sounds will disappear spontaneously when the components mature.
Clinical functional examination of the masticatory system
An important part of the functional examination is registration of pain reaction to palpation. Once again it is important to stress that for a child, pain can be more than the physiologic experience of pain. The discomfort experienced during the examination, e.g., pressure during palpation, can be expressed as pain. Because of this, both reports of palpatory tenderness in masticatory muscles and/or TMJs, as well as reports of pain during mandibular movements should be interpreted with caution in young children.
Measurement of maximal jaw opening capacity is to be included in a functional examination of the masticatory system (Figure 22.2). The individual vertical overbite should be included in the range of movement. The normal variation is large with slightly higher figures for boys, but the individual opening capacity is established in the early teenage years (Table 22.2). Impairment or improvement of the maximal jaw opening capacity is thus a significant clinical parameter of the functional status. If the individual opening capacity at health is unknown, an opening of 36 mm is an accepted minimum limit from the age of 6–7 years [21].
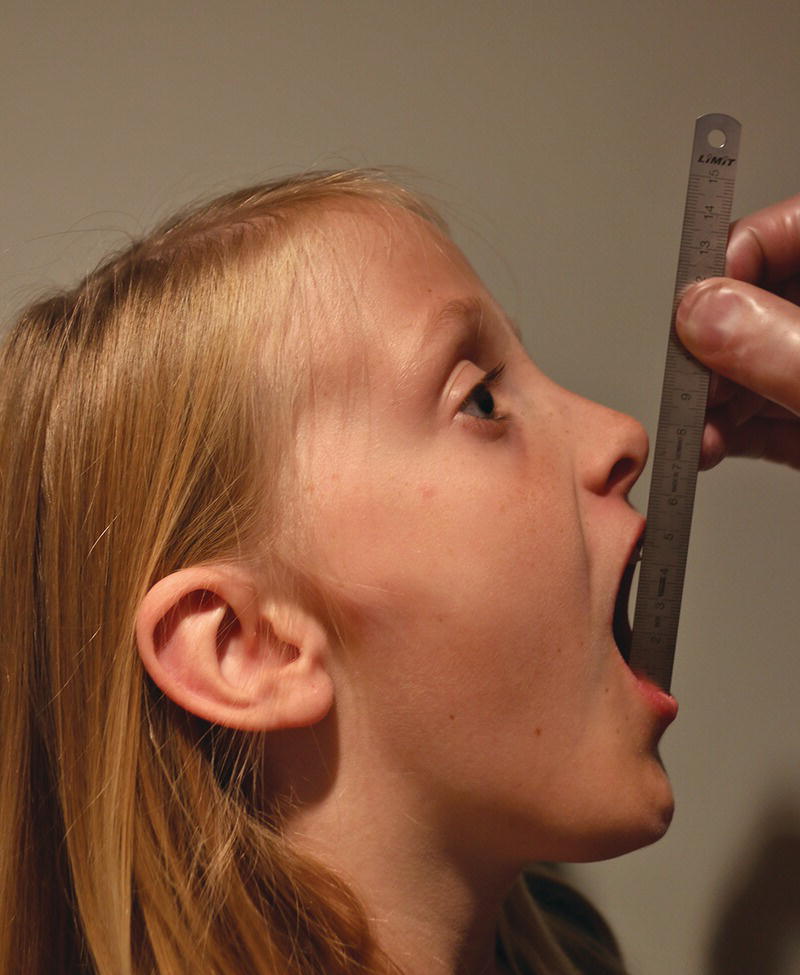
Figure 22.2 Measurement of maximal jaw opening capacity.
Table 22.2 Maximal mouth opening at different ages according to Agerberg [27]
| Age (years) | Mean value (mm) | Range (mm) |
| 1.5 | 38.4 | 32–44 |
| 6 | 44.8 | 33–60 |
| 13 | 53.9 | 41–73 |
| 16 | 56.0 | 39–82 |
| 20 | 56.0 | 42–77 |
Source: Göran Agerberg, University of Umeå, Sweden.
The lateral aspects of the TMJs are palpated bilaterally (Figure 22.3) while the patient performs two or three maximal jaw openings. Pain on palpation, pain during opening, irregular jaw movement as well as TMJ sounds are recorded. The use of a stethoscope to record joint sounds is questionable, since it may reveal insignificant or normal joint sounds.
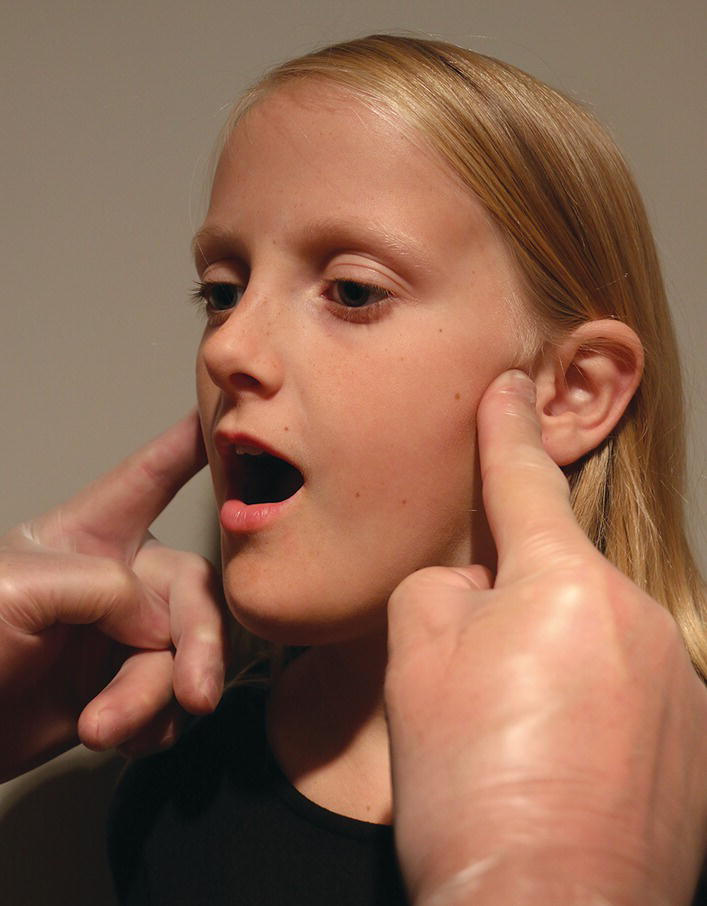
Figure 22.3 Bilateral palpation of the lateral aspects of the TMJs.
Palpation of the jaw muscles should be limited to a few, informative muscles. We recommend that the masseter and temporalis muscles are palpated bilaterally. The anterior part of the superficial portion of the masseter muscle is easy to locate, and is palpated bidigitally from its origin on the zygomatic arch to the insertion on the mandible (Figure 22.4). The large, fan‐shaped attachment of the temporalis muscle can be palpated extraorally on the lateral part of the head, but more informative, and thus more important, is to palpate the insertion of this muscle on the medial part of the coronoid process.
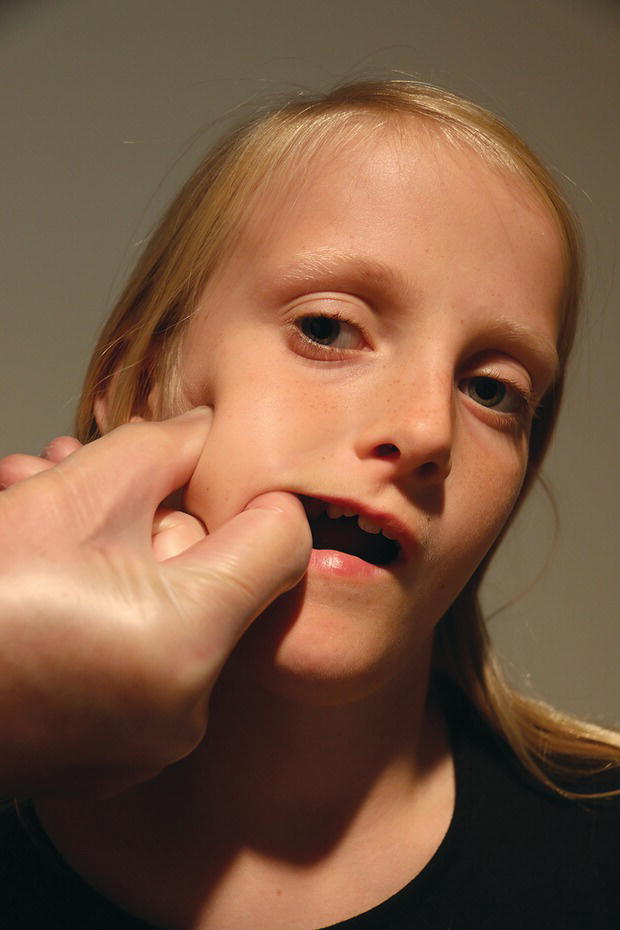
Figure 22.4 Palpation of the anterior part of the superficial portion of the right masseter muscle.
A functional examination should also include an evaluation of the occlusion since it may act as a predisposing factor for TMD in the individual patient. It is, however, important to consider the extensive dynamics of the occlusion in children and adolescents due to exfoliation and eruption of teeth, as well as growth of the jaws. The role of occlusal factors in the etiology of TMD is controversial, but unilaterally forced cross bite has, in several studies, been found to be of importance in this respect [6,14,22].
Radiographic imaging of the TMJs
Imaging is warranted when the clinical examination and/or the history indicate a recent or progressive pathologic joint condition such as trauma, significant dysfunction, or limitation in movement, sensory/motor alteration, or significant change in occlusion [23] as well as in cases with general joint disease with suspected engagement of the TMJs. However, radiographic findings will rarely influence the initial, conservative treatment plan for TMD.
The most common radiographic projections and methods used are the oblique transcranial lateral projection, which may be supplemented by axial and frontal views, and tomography. The latter method has the greatest relevance concerning osseous structure and intra‐articular skeletal relationships [24]. To reveal soft tissue changes as well as inflammation, magnetic resonance imaging is the method of choice.
It is, however, of utmost importance to realize that each period in the growth of the child and adolescent has its own morphological character. The ever‐changing normality criteria of the radiographic image of the TMJ are a complicating factor when information from the images is interpreted [24]. There is a great risk of misinterpretation if criteria from images of adults are adopted without due consideration (Figure 22.5).
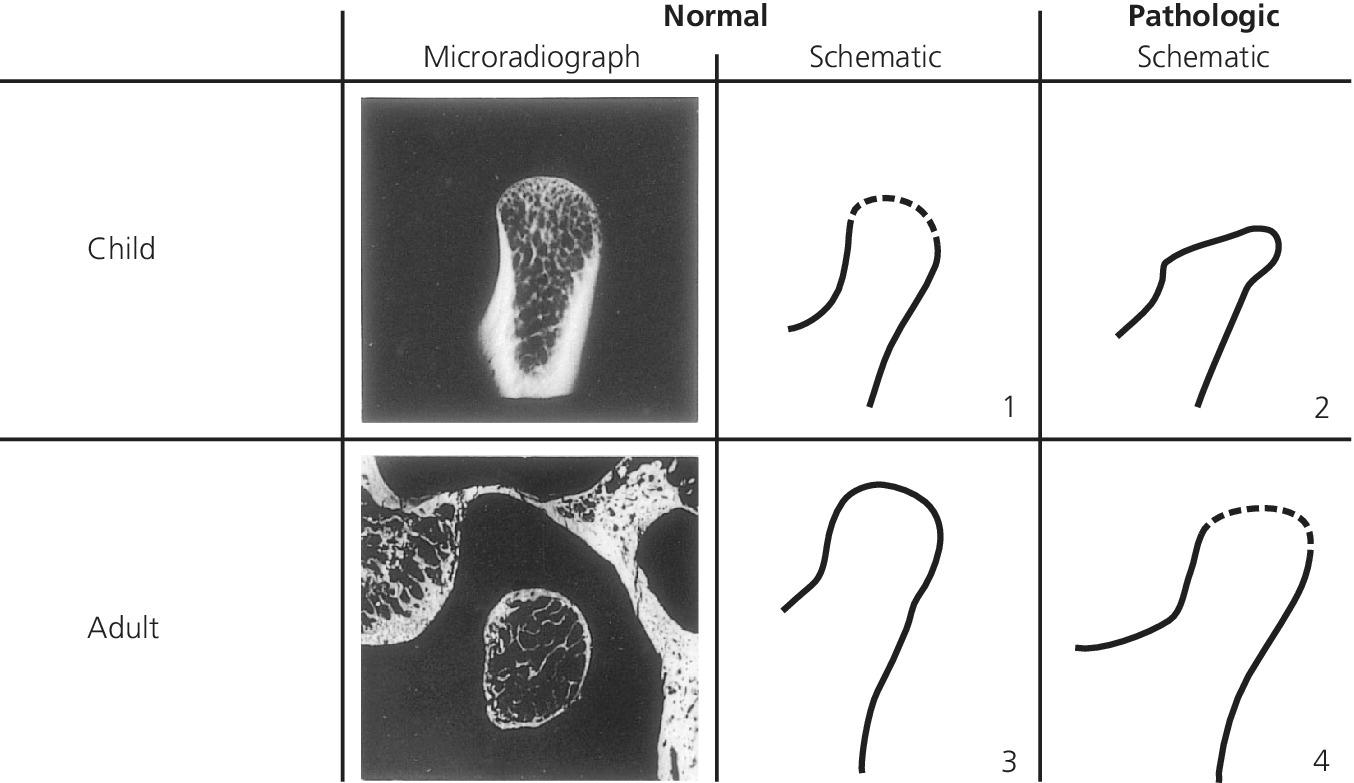
Figure 22.5 Schematic illustration of the apparent paradoxical peripheral delineation of the condylar heads of normal and pathologic joints in the child and the adult. A normal delineation of the condylar head of a child (1) has characteristics in common with that of a pathologic adult TMJ (4) and the pathologic condylar head of a child (2) with that of a normal adult (3).
Source: Eckerdal 1997 [24]. Reproduced with permission of Gothia Fortbildning.
Treatment of TMD in children and adolescents
With some exceptions, the TMD treatment concepts in children and adolescents are the same as for adults. In the following, we will focus mainly on these exceptions, but for details concerning the different treatment modalities, we refer to other current textbooks [25].
There is no scientific support for the opinion that TMD can be prevented with early treatment. Because of this, prophylactic intervention to prevent TMD is not indicated, with the possible exception of orthodontic treatment of laterally forced cross bites.
Only a few scientific data are available that support the need and efficacy of different treatment modalities of TMD in children and adolescents. Because of this, simple, conservative, and reversible treatments are strongly recommended. In the authors’ opinion, such treatments have proved to be effective in reducing most TMD symptoms in children and adolescents.
When the history has been taken and the clinical examination performed, the patient, and even more the parents, should be reassured about the benign character of the condition, and the favorable prognosis of simple, conservative treatments should be stressed. It is also important to educate the patient to avoid unnecessary loading of the masticatory system such as by nail‐biting and excessive use of chewing gum.
When treating TMD in adults, occlusal adjustments are sometimes used as a part of the treatment. Due to the dynamics of the occlusion in children and adolescents, this treatment should be avoided or performed with great caution in these age groups, but minor adjustments can be made as well as smoothing ragged incisal edges.
Exercise therapy is an often effective treatment of TMD [26] that has been used for many years. This treatment also merits its place in the treatment of children and adolescents with TMD. Its mode of action is probably a combination of several muscle physiologic mechanisms, such as proprioceptive neuromuscular facilitation, reciprocal inhibition, and stretching, but it is also a cognitive and behavioral treatment. The success of therapeutic jaw exercises depends on the motivation, cooperation, understanding, and compliance of the patient. Many different programs, as well as how they are to be performed [25], have been presented in the dental literature. To be successful with this treatment modality, it is important that the patient is thoroughly instructed in how the exercises are to be performed (Figure 22.6).

Figure 22.6 Examples of therapeutic jaw exercises. (a) Active jaw opening against a slight resistance. (b) Active lateral excursion to the right against a slight resistance.
Another reversible, conservative, and often effective treatment of TMD is interocclusal appliance therapy. To minimize the risk of negative effects on growth and development, a soft appliance placed in the mandible can be recommended as the appliance of first choice in children and adolescents (Figure 22.7). Most patients in these age groups respond quickly and the appliance therapy can often be interrupted after 6–8 weeks. In single cases, where an appliance is needed for a longer period of time (more than 3 months), or when large forces from parafunction are exerted, appliances of hard acrylic are to be used. In these cases, however, it is important to consider the developmental stage of the dentition and the type of occlusion when the appliance is designed (Figure 22.8), and the patients should be submitted to regular check‐ups as long as the treatment continues. Not until the permanent dentition (excluding third molars) is developed (around the age of 14 years) is the appliance treatment of adolescents similar to the treatment of adults.
Figure 22.7 Soft interocclusal appliance placed in the mandible.

Figure 22.8 (a) A 12‐year‐old girl in whom the canines in the maxilla are not completely erupted. (b) She is provided with a hard acrylic appliance (Shore‐plate), where the acrylic is removed in the region of the canines to allow further eruption. (c) A 7‐year‐old boy with a mixed dentition and a deep bite. Severe bruxism and frequent headaches. (d) He is provided with a hard acrylic bite‐plate to prevent the teeth from further wear, to unload the jaw muscles, and eventually to decrease the deep bite.
Summary
Signs and symptoms of TMD are common in children and adolescents, but are in most cases mild and infrequent. When taking the history, the age and maturity of the patient must be considered. A functional examination of the masticatory system should comprise measurement of maximal mouth opening capacity, palpation of TMJs and a few informative jaw muscles, as well as an examination of the occlusion. Those patients who merit treatment mostly respond quickly to simple, conservative treatment methods such as therapeutic jaw exercises and/or interocclusal appliances.
References
- 1. Okeson JP. In: Okeson JP, ed. Orofacial pain: guidelines for assessment, diagnosis and management. Carol Stream, IL: Quintessence, 1996.
- 2. Nydell A, Helkimo M, Koch G. Craniomandibular disorders in children. A critical review of the literature. Swed Dent J 1994;18:191–5.
- 3. Könönen M, Nyström M. A longitudinal study of cranio‐mandibular disorders in Finnish adolescents. J Orofacial Pain 1993;7:329–6.
- 4. Wänman A, Agerberg G. Two‐year longitudinal study of signs of mandibular dysfunction in adolescents. Acta Odontol Scand 1986;44:333–42.
- 5. Heikinheimo K, Salmi K, Myllärniemi S, Kirveskari P. Symptoms of craniomandibular disorders in a sample of Finnish adolescents at the age of 12 and 15 years. Eur J Orthod 1989;11:325–1.
- 6. Nilner M, Kopp S. Distribution by age and sex of functional disturbances and diseases of the stomatognathic system in 7–18 year olds. Swed Dent J 1983;7:191–8.
- 7. Magnusson T, Egermark I, Carlsson GE. A prospective investigation over two decades on signs and symptoms of temporo‐mandibular disorders and associated variables. A final summary. Acta Odontol Scand 2005;63:99–109.
- 8. Anastassaki Köhler A. On temporomandibular disorders. Time trends, associated factors, treatment need and treatment outcome. PhD Thesis. University of Jönköping, Sweden. Swed Dent J 2012;Suppl. 227:73.
- 9. Nyström M, Könönen M, Alaluusua S, Evälahti M, Vartiovaara J. Development of horizontal tooth wear in maxillary anterior teeth from five to 18 years of age. J Dent Res 1990;69:1765–70.
- 10. Henrikson T, Nilner M. Temporomandibular disorders, occlusion and orthodontic treatment. J Orthod 2003;30:129–37.
- 11. Kim MR, Graber TM, Viana MA. Orthodontics and temporomandibular disorders: a meta‐analysis. Am J Orthod Dentofacial Orthop 2002;121:438–46.
- 12. McNamara JA, Seligman DA, Okeson JP. Occlusion, orthodontic treatment and temporomandibular disorders: a review. J Orofac Pain 1995;9:73–90.
- 13. McNamara JA, Turp JC. Orthodontic treatment and temporomandibular disorders: is there a relationship? Part 1. Clinical studies. J Orofac Orthop 1997;58:74–89.
- 14. Egermark I, Magnusson T, Carlsson GE. A 20‐year follow‐up of signs and symtoms of temporomandibular disorders and malocclusions in subjects with and without orthodontic treatment in childhood. Angle Orthod 2003;73:109–15.
- 15. Okeson JP. Temporomandibular disorders in children. Pediatr Dent 1989;11:325–9.
- 16. Olsson M, Lindqvist B. Mandibular function before and after orthodontic treatment. Eur J Orthod 1995;17:205–14.
- 17. Doverborg‐Österberg E, Pramling I. Att förstå barns tankar. Metodik för barnintervjuer. Stockholm: Liber Utbildningsförlaget, 1985.
- 18. Okeson JP, O’Donell JP. Standards for temporomandibular evaluation in the pediatric patient. Pediatr Dent 1989;11:329–30.
- 19. Jeans ME. The measurement of pain in children. In: Melzack R, ed. Pain measurement and assessment. New York: Raven Press, 1983; 183–9.
- 20. Razook SJ, Gotcher Jr JE, Bays RA. Temporomandibular joint noises in infants: Review of the literature and report of cases. Oral Surg, Oral Med, Oral Pathol 1989;67:658‐64.
- 21. Agerberg G. Maximal mandibular movements in children. Acta Odontol Scand 1974;32:147–59.
- 22. Egermark‐Eriksson I, Carlsson GE, Magnusson T, Thilander B. A longitudinal study on malocclusion in relation to signs and symptoms of cranio‐mandibular disorders in children and adolescents. Eur J Orthod 1990;12:399–407.
- 23. American Academy of Pediatric Dentistry. Treatment of temporomandibular disorders in children: summary statements and recommendations. J Am Dent Assoc 1990;120:265–9.
- 24. Eckerdal O. Radiologic reproduction of healthy and arthritic temporomandibular joints of children and adolescents. Stockholm: Förlagshuset Gothia, 1997.
- 25. Carlsson GE, Magnusson T. Treatment of TMD. In: Carlsson GE, Magnusson T, eds. Management of temporomandibular disorders in the general dental practice. Chicago, IL: Quintessence, 1999; 87–121.
- 26. Magnusson T, Syrén M. Therapeutic jaw exercises and inter‐occlusal appliance therapy. A comparison between two common treatments of temporomandibular disorders. Swed Dent J 1999;23:27–37.
- 27. Agerberg G. On mandibular dysfunction and mobility. PhD Thesis. University of Umeå, Sweden. 1974.