As soon as it became apparent that Steve had responded so profoundly to the use of coconut oil, it became my mission to get this information out to as many people as possible. My primary goal was to bring to the attention of high-profile individuals and groups the research related to ketones and how they can provide an alternative fuel for neurons in certain neurodegenerative diseases, as well as the need for funding for production of Dr. Veech’s ketone ester. My secondary goal was to make them aware of the research related to medium-chain fatty acids, which are converted to ketones by the liver and can provide this alternative fuel, even if on a more limited basis, until the ketone ester is commercially available.
I believed that if I could get this information to the right person, and that person investigated and understood the scientific basis of how ketones work as an alternative fuel, this message would get out to the general public in a big way.
But who was the right person?
My sister Angela suggested that I write a letter to retired Supreme Court Justice Sandra Day O’Connor, whose husband had Alz heimer’s disease. This was widely publicized around the time in March 2008 that she testified on Capitol Hill at the annual hearing of the U.S. Senate Special Committee on Aging related to Alzheimer’s disease. I also learned that she was a member of the Alzheimer’s Study Group (ASG), a government task force created in 2007 that focuses on ways to speed up the development of new medicines and ways to provide better resources and care to families affected by Alzheimer’s. I drafted the following letter to her and mailed it in care of the ASG.
LETTER TO SUPREME COURT JUSTICE SANDRA DAY O’CONNOR
To Honorable Sandra Day O’Connor and Other Members of the Alzheimer’s Study Group:
I want to bring to your attention promising research in the area of Alzheimer’s disease that needs to be made public urgently, since the premise can be instituted now by a simple change in diet.
I am a full-time working physician in the area of neonatology (critical care of sick and premature newborns). My husband is only fifty-eight and has moderately severe Alzheimer’s disease, which became apparent about five years ago. He has gotten considerably worse over the past year but is still able to stay at home. He has been on the usual drugs to slow the decline, and we have been hopeful that, if his progress is slow enough, one of the new drugs will be approved.
Very recently, we learned of two clinical trials recruiting in our area and scheduled him to be screened for both studies. As I researched the two drugs I came upon another study for a therapeutic agent called AC-1202, under development by a company called Accera. In the course of their research, they concluded that persons with Alzheimer’s disease overall improved with a daily dose in as little as forty-five days, with even greater improvement if they were also taking one or more of the Alzheimer’s medications, such as Aricept or Namenda. The results were more striking for persons who do not carry an Apo E4 gene, which constituted about half of their study participants. In their small preliminary study, some people improved after a single dose.
In trying to further research what is in AC-1202, I located their patent application, which can easily be found on the website www.freepatentsonline.com by searching for AC-1202. The application is United States Patent #20080009467, Kind Code: A1. This patent application goes into great detail about Alzheimer’s disease and the science behind their “invention.” Their original application dates back to 2000. In their studies and news releases related to it, their therapeutic agent is called AC-1202. The therapeutic agent involved is actually MCT oil (medium-chain triglycerides), which they state is commonly derived from coconut or palm kernel oil. There is also a small amount of MCT in butter fat.
If they will take the time to read the patent application, the physicians and other scientists in your study group will recognize the biochemical principles involved and why this should help people with Alzheimer’s disease. The application explains their study results using MCT oil. The remainder of the patent application goes into great detail about a vast number of formulations of MCT with other entities, such as vitamins and enzymes that may enhance the performance of the MCT oil. They acknowledge that the exact composition of the MCT oil does not seem to make a difference. The product they used was obtained from Stepan Company, a large supplier for other companies making food and other preparations. I spoke with a sales representative and they sell this to distributors in fifty-five gallon drums, a minimum of sixteen drums per order. It is therefore plentiful.
The references for the other research they have relied upon are included throughout the very lengthy and detailed AC-1202 patent application.
To simply summarize the science, researchers have found that neurons in certain areas of the brain in persons with Alzheimer’s disease do not take up and use glucose normally and eventually die, since this is the primary energy that the neurons use. Some [scientists] are now calling this type 3 diabetes. The only other known substrate that neurons can use are ketones. Normally, humans do not produce ketones unless they are in a condition of starvation or [are] on a ketogenic (very low-carbohydrate) diet, such as Atkins. In contrast to the usual fats that Americans eat, medium-chain triglycerides (as in MCT oil) are easily absorbed when taken orally and are metabolized by the liver directly into ketones. Other fats are not processed this way by the body. A 1996 study referenced in the patent application shows that, when this occurs, blood flow to the brain increases by 39 percent. Ketones are known to pass directly into the circulation in the brain, where the neurons can readily take them up for use as an alternative energy to glucose, thereby preserving the life and integrity of the neuron.
Accera states in its patent application that the defect associated with Alzheimer’s disease they are targeting may begin to occur decades before there are symptoms, therefore use of MCT oil would be a significant benefit, not only for people who are already suffering from Alzheimer’s, but also for prevention for those who are at risk. Most Americans have family members with the disease and may be at risk themselves.
MCT oil is readily available on the open market (I have purchased a one-liter bottle online). It is used for weight loss and to enhance athletic performance due to the type of energy it provides (ketones). In addition, coconut oil contains about 57 percent MCT oil and can be purchased in some grocery stores, Asian markets, and many natural food stores. Coconut milk and many other coconut products now on the market contain a substantial quantity of coconut oil. Larger quantities of coconut oil from 16 ounces to 5 gallons can easily be purchased online. In addition to MCT oil, coconut appears to have other fats that may be very beneficial as well.
The dosage used in the AC-1202 study was a single daily dose of 20 grams of MCT oil, which translates into about four teaspoons of MCT oil or seven teaspoons of coconut oil. This can easily be incorporated into the foods we eat.
My nurse friends from the Philippines have advised me that, in their country of origin (as well as other Asian countries, such as India), coconut and coconut oil are a staple, used on a daily basis, which may explain why there is a much lower incidence of Alzheimer’s disease in that part of the world. I have checked out other studies of coconut oil and learned that it does not raise, in fact lowers, “bad cholesterol.” Also, areas of the world that primarily use coconut oil as their fat have a much lower mortality related to cardiovascular disease, contrary to myths that have been perpetuated for more than fifty years in our country since the development of the hydrogenated vegetable oils, including those containing trans fats.
If you look at the Accera website at www.accerapharma.com, under “Energy Metabolism,” they state that they have developed “novel treatments … designed to increase energy to neuronal cells,” etc. Nowhere in this website do they mention that this is simply MCT oil that anyone can purchase.
I am the caregiver for my husband. About two weeks ago, I purchased coconut oil and started adding seven teaspoons (equivalent to the amount used in the study) to my husband’s oatmeal at breakfast. I have also experimented with a number of recipes that are available in cookbooks and online. Within a few days there was noticeable improvement in his gait, his ability to converse, his sense of humor has returned; he remembers the month and the season immediately, which he could not remember if repeated over and over to him before. He is following through on things that he wants to accomplish during the course of the day. His latest MRI shows extensive atrophy in the amygdala and hippocampus, the areas affected by Alzheimer’s. Realistically speaking, I cannot expect him to fully recover, but to see this much improvement in such a short time is very encouraging for both of us. He is well aware that he is suffering from this disease and fully supports and enjoys our dietary change.
Steve is just one person, but his improvement supports the research done by Accera. We hope this improvement will be sustained.
It is disappointing that the people behind Accera have been aware of this science for at least eight years and have failed to provide this information to the medical community and public at large. How many people have deteriorated unnecessarily due to their negligence in this regard? My husband’s MRI was “normal” and he could work as an accountant just three years ago.
Accera states in the patent application that there are other entities in which glucose is not used by neurons in different areas of the brain, including Parkinson’s, Huntington’s disease, and epilepsy. This has treatment implications well beyond just Alzheimer’s disease.
I implore the members of the ASG to study this research on an urgent basis and make the findings public knowledge so that everyone who suffers from this disease can potentially benefit. I hope that at least one of you will take the time to read through the AC-1202 patent application that is public record and have your medical experts review this.
I am also sending copies of this letter to important government figures and the media with the hope that someone who is in a position of power to make change will pick up on this, research it urgently, and do something about it for all of us and our families.
I would be grateful for any feedback on this issue.
Sincerely yours,
Mary T. Newport, M.D.
Director of Neonatology
Neonatal Intensive Care Unit
Spring Hill Regional Hospital
Spring Hill, Florida 34609
Cc: Chairperson, Alzheimer’s Association (National Office)
While waiting for a response from Justice O’Connor, I developed a list of other high-profile people and organizations and mailed or e-mailed similar letters to them as well. Among them were the Alzheimer’s Association (a leading voluntary health organization in Alzheimer care, support, and research) and the Michael J. Fox Foundation; former Senator Hillary Rodham Clinton, Senator Bill Nelson, Ron Reagan (son of former President Ronald Reagan), and Acting Surgeon General Steven K. Galson; television personalities such as Oprah Winfrey and Mehmet Oz; television and print media like CNN, ABC News, CBS News, Fox News, NBC Studios, the Today Show, as well as the New York Times, Washington Post, News & Observer, and USA Today.
This was just the beginning of my campaign to get the message out, and many more attempts were to follow in the ensuing months. I was very well aware that this was just one case study, one man whose wife believed he responded to coconut oil. Why should anyone believe me? Why shouldn’t they be skeptical? I was careful to mention the studies performed by Accera, and that this case study was merely further confirmation of its results. My point was that the substance studied was available over the counter and others should have access to this information immediately and not have to wait for a prescription product to become available at some nebulous point in the future.
I thought, at the very least, one of these individuals would contact me for more information, but much to my surprise and chagrin, I heard from none of them. I was far from ready to give up. If I couldn’t get the message out by way of the media, it would have to go by way of the grassroots.
A month after Steve’s first breakfast of oatmeal and coconut oil, we visited our families in Cincinnati for the first time in a year. It did not take long for my father and his wife, my sisters, and Steve’s siblings to notice that Steve was very different, much better, than on the previous visit. They remarked that in May 2007 he seemed lost, didn’t remember names of many family members, had no sense of humor, and his responses during conversations didn’t make sense. By contrast, on this visit he immediately said hello to nieces and brothers-in-law by name, laughed and joined in, and even made up some of his own jokes. At one point, my sister Rosemary mentioned that, when texting her daughters, she signs off with the numbers “666” because that happens to correspond to “Mom” on the telephone keypad. Steve laughed out loud, immediately understanding the reference to the devil. With Steve’s permission, I shared the drawings of the clocks with our families. They all agreed that he was markedly improved from the previous year and that I might be on to something. There were a number of coconut oil converts during that visit.
Encouraged by the reaction of our families to Steve’s improvements, and now certain that this was not simply my imagination, it was time to step up my efforts to get the message out to other people who were dealing with Alzheimer’s so they could potentially benefit as well. I saw on a news program that there was a conference scheduled for the end of July in Chicago, the International Conference on Alzheimer’s Disease (ICAD). I thought this might be a golden opportunity to cross paths with physicians and researchers and increase their awareness of ketone’s potential benefits.
The night before we left Cincinnati, I lay awake thinking about what else I could do to get the message out. I could not get the words out of my head, “What if there was a cure for Alzheimer’s disease and no one knew?” I quietly got up and found my way to my father’s office, where I drafted the foundation for my article, using the words that I couldn’t get out of my head for the title. The “cure” I referred to is the ketone ester beta-hydroxybutyrate, derived from coconut oil. Though that is a relatively poor substitute, it’s a viable option that can be used immediately for its ketone-producing effect. As a physician, I am perfectly well aware that it is brazen to announce a “cure” for any disease and that people would be highly skeptical, but on the other hand, I strongly believe that ketone ester will be a true cure, capable of reversing disease for those in the earliest stages. It may even result in some improvement for those in the more advanced stages, bringing back to life neurons that are faltering but not yet dead.
Over the next several weeks, I fine-tuned the article, adding a few more details and references, until I arrived at the version I planned to produce in quantity for the ICAD. With no response to my first letter, I sent a second letter to Justice O’Connor, this time with a copy of my article. I contacted the ASG requesting the status of the investigation, noting that some weeks had passed and I had not received a response.
In early July, just two weeks before the ICAD conference, Dr. Veech approached me with the idea of obtaining levels of the ketones, beta-hydroxybutyrate and acetoacetate (the two types of ketones converted into cellular fuel), on Steve before and at intervals after ingesting coconut oil. We worked it out with the lab director at my hospital to have his personnel draw and prepare the samples for mailing, placing them in an extremely low-temperature freezer, according to a procedure provided by Todd King, who routinely runs this assay in Dr. Veech’s lab. My job was to take the samples for overnight delivery to Dr. Veech’s lab.
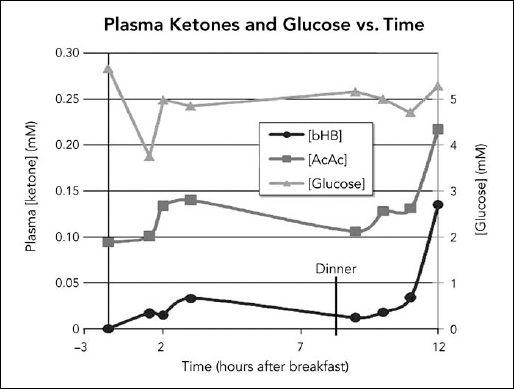
On July 11, we went to the hospital at about 7 A.M. for the first sample before breakfast. We then went to breakfast nearby, and I gave Steve 35 milliliter (ml), the equivalent of seven teaspoons, of coconut oil, noting the exact time. We returned to the hospital and additional blood samples were drawn at timed intervals thereafter up to three hours. Dr. Veech called me and suggested that we repeat the process with the dinner meal. Steve was up for it and so we did, taking samples before and at intervals up to three hours after receiving another 35 ml of the oil with dinner. On that particular day, he did not receive any other coconut products to avoid confounding the results. The next day, I delivered the samples in a special cooler for express shipping, and the day after we received the results from Dr. Veech. Steve’s acetoacetate levels were higher than his beta-hydroxybutyrate levels (Figure 6.1).

Figure 6.1. Measurements of glucose, acetoacetate (AcAc), and beta-hydroxybutyrate (bHB) at timed intervals up to three hours after Steve consumed coconut oil. The ketone levels were relatively low, but peaked at about three hours following the breakfast dose, and were close to baseline before dinner. The levels were still rising and considerably higher three hours after the dinner dose. (Diagram courtesy of Richard L. Veech, NIH.)
Dr. Veech was concerned that the ketone levels were quite low, with the exception that three hours following dinner, the levels were on the rise but had not yet peaked, so we didn’t know how high they might have gotten before falling again. He was also concerned that the acetoacetate levels were higher than the beta-hydroxybutyrate levels. In the Accera trials, only beta-hydroxybutyrate levels were reported. He conjectured that during Steve’s activity in the daytime, his muscles might have used up some of the ketones. In the evening, in a more sedentary state, there might be less use of the ketones by other tissues, and therefore more ketone was available to the brain during sleep, a time when the body is known to repair.
Prior to this small study, Steve received measured amounts of coconut oil for breakfast and dinner, but not for lunch. He sometimes received another coconut product if I happened to make a smoothie. As a result of the test, it was apparent that Steve should receive a measured dose of coconut oil for lunch as well.
Two weeks later, just before the ICAD conference, Dr. Veech suggested that another set of ketone levels be performed, but this time after consuming MCT oil instead of coconut oil. He wanted to run the levels on a second individual as a control. It just so happened that my associate F had started using MCT oil shortly after I told him about Steve and his response to coconut oil. He has type 2 diabetes, lost his father to vascular dementia, and was very concerned about his own future health. He was more than happy to participate, and levels were drawn on him and Steve both before and after consuming 20 grams (21 mL or about 4 teaspoons) of MCT oil (see Figure 6.2 on the following page). This time, Steve’s beta-hydroxybutyrate levels were higher than his acetoacetate levels.
Comparing the two studies showed that ketone levels from coconut oil were lower and peaked later than that from MCT oil, but were still present considerably longer than with MCT oil. The levels with MCT oil were higher and peaked at about ninety minutes for Steve, but were minimal by three hours. Also, the levels of beta-hydroxybutyrate were more elevated than acetoacetate levels after taking MCT oil, whereas the opposite was true after taking coconut oil. F’s dieting and losing a substantial amount of weight weekly at the time of the study may explain why his ketone levels were already elevated before taking MCT oil at breakfast.
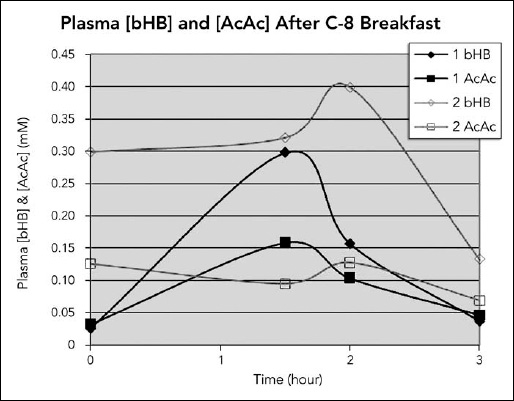
Figure 6.2. Measurements of acetoacetate and beta-hydroxybutyrate at timed intervals after consuming MCT oil. Steve ( ) and my associate F (
) and my associate F ( ) were each given 20 grams of MCT oil with breakfast. AcAc and bHB levels were measured at intervals up to three hours after that. Steve’s levels peaked at about ninety minutes, and F’s levels peaked at two hours and were essentially back to baseline or below at three hours.
) were each given 20 grams of MCT oil with breakfast. AcAc and bHB levels were measured at intervals up to three hours after that. Steve’s levels peaked at about ninety minutes, and F’s levels peaked at two hours and were essentially back to baseline or below at three hours.
(Diagram courtesy of Richard L. Veech, NIH.)
Dr. Veech strongly advised me to include MCT oil in Steve’s diet, pushing the quantity a little at a time until he vomited and had diarrhea, so, with Steve’s approval, I did. However, it made sense to me that, since he had responded initially to coconut oil, there might be something else in it that was not present in the more refined MCT oil that could be helping Steve. So we continued to use coconut oil as well.
I began to experiment with a mixture of coconut and MCT oil, keeping a minimum of one tablespoon of coconut oil with each dose and increasing the amount of MCT oil gradually until we found Steve’s limits of tolerance; then I backed off slightly. My rationale was that we would take advantage of the higher levels from MCT oil and the longer duration of the levels from the coconut oil. By mixing them, Steve should have ketone available to his brain around the clock. Is this necessary? Does the brain take up a volume of ketone and then store it, or is the ketone taken up, immediately processed, and used up? The answers are unknown at this time. To this day, I continue to base the way we are mixing the oils and dosing Steve three times a day on the assumption that it is wise to try to maintain a constant blood level of ketones.
Once we found Steve’s limits of tolerance, we settled on a dose of four teaspoons (20 mL) of MCT oil and three teaspoons (15 mL) of coconut oil. For many months, if I tried to increase the amount of MCT oil beyond 20 mL, Steve had diarrhea. However, after a year, he was able to handle an increase in the mixture from 35 mL (seven teaspoons) per dose to 40 mL (eight teaspoons) and then 45 mL (nine teaspoons) several months later. As of 2011, this is the dose we have settled on three times a day with meals.
There were two occasions when Steve missed getting his dose of coconut oil in the morning. The first was when he needed to fast for blood work, and the lab was so busy that it was nearly noon before he had a bite to eat. We decided to go to a restaurant, and by the time the food arrived, he was very tremorous and had considerable difficulty finding and using utensils. It soon occurred to me that the lack of coconut oil was the cause of this setback, and I pulled out one of the tiny bottles I carried with his premeasured dose. About twenty to thirty minutes after taking the oil, Steve smiled again and the shaking stopped.
The second occasion occurred in October 2008 during a vacation in California, a three-hour difference in time zones from our home in Florida. We slept in the morning after we arrived, and by the time we got to the restaurant to eat breakfast, it was about noon our time. We were sitting in a C-shaped booth with a front-row view of the ocean, and not many other people were there at that point. When the food arrived, once again Steve was very shaky and confused. He had a great deal of difficulty deciding which utensil to use and also had trouble keeping the food on his fork. He said he felt as if he were in a little box, the food was coming in at him, and he had to get out of the booth. I set up a chair for him at a table outside the booth. After he ate and had his oil, once again about twenty to thirty minutes later, he began to smile and joke, and the tremor disappeared. He said when the event was in progress he felt very claustrophobic and he couldn’t taste the food.
The sense of smell is often blunted in the early stages of Alzheimer’s, and some doctors use a test with common odors everyone should recognize in their diagnostic process. After Steve’s experience in California, it occurred to me that perhaps the reason people with Alzheimer’s disease lose interest in eating is that, at some point, not only do they have trouble smelling food but they can no longer taste the food as well, as the neurons that supply the taste buds are no longer functional. Perhaps the neurons involved in hunger and fullness go by the wayside as well.
Since first hearing about the International Conference on Alzheimer’s Disease, I learned that about 5,000 researchers and physicians would be there from all over the world. Beside being the perfect opportunity to get out information about ketones and medium-chain fatty acids to an enormous number of people, I also saw it as an opportunity to learn as much as I could about the disease and the status of various treatments that seemed to be just over the horizon.
Medical conferences usually have an exhibit hall where there are displays by pharmaceutical companies, equipment and supply manufacturers and distributors, hospitals trying to recruit nurses, and medical textbook publishers. This conference was no exception.
I contacted the person in charge of the exhibit hall, whom I will call ST, to advise her of what I wanted to do and learn the logistics of the process. I asked her whether there was any possibility of disseminating an article I had written at the conference, to which she responded in the negative. She said I would have to get a booth for $3,300, and she directed me to an exhibit hall chart on the company’s website so I could pick out a location.
For a day or two, after mulling over the personal cost of the trip to Chicago, the exhibit, and the materials to distribute, I decided it was a small price to pay to get the information out to so many people. I talked with my sister Angela about the logistics of taking Steve with me, and she said that she and her husband John would help us in any way they could, either by staying with Steve at our home or by meeting us in Chicago. Angie researched flights while I looked into hotels.
I decided on the location of a booth and e-mailed ST with my confirmation. That same day I received the following response:
July 16, 2008
Hi Mary,
Glad you found everything. I am copying DB, our exhibit manager, to reserve your space. The conference starts a week from Saturday, so you should make a commitment ASAP. We welcome your participation at ICAD.
Best regards, ST
As I read through the registration information, I learned that, in addition to the $3,300 booths, tables were available for $1,600. This seemed like a more reasonable alternative, given that I was just planning to distribute articles and not have a very fancy exhibit. I wondered why ST did not tell me about the tables and called her back to find out if there were still tables available. She sounded a bit miffed, but advised me that, yes, there were tables available, and she would reserve one for me. I completed the necessary reservation forms, including the payment information. A perk of having an exhibit was one free conference registration. And I could have up to three people assigned to the booth to help me, and Angie and John were more than willing.
Later that day I e-mailed ST again to confirm and pay for the table in the exhibit hall.
The next day she confirmed receipt:
July 17, 2008
Thank you, Mary. I sent it to our exhibit manager at X Exhibitions. They will be in touch. ST
Our daughter Joanna had recently completed her bachelor’s degree in graphic design and happened to be working part-time for a small local graphic design company. Her boss, Dave, who had read the article with interest, was willing to help, even given the relatively short time I had to get an exhibit together. He agreed to let Joanna format the article and then arranged to have 500 color copies made. I also arranged with a copier near our hotel in Chicago to print out 1,500 more copies that we could pick up before the conference started.
After a long weekend of working at the hospital and preparing for the conference exhibit, I received the following e-mail from ST:
July 21, 2008
Dear Mary,
I am very sorry to have to tell you that we have to deny your application and cancel your exhibit space in the exhibit hall for ICAD. The exhibit hall is intended for companies with products and services related to Alzheimer’s disease and dementia. Any funds that you have paid will be refunded. As we previously discussed, we would welcome you to submit an abstract for our next research conference, or consider applying for a research grant. If you have not already done so, go to the website at www.alz.org/icad and enter your name to receive e-mail updates and announcements. Again, I am sorry you cannot exhibit and for your inconvenience.
Best regards, ST
After reading this, I was stunned and immediately called ST to try to convince her otherwise. I suggested that perhaps I could register the table under the name of my company, Spring Hill Neonatology, Inc. She said she would run it by the folks in charge. But next day, unfortunately, she informed me otherwise.
July 22, 2008
Dear Mary,
After additional discussion with our conference leadership, I regret to inform you that we indeed cannot approve your application for exhibit space. All applications for space are approved by the conference leadership to ensure that the exhibit hall space is the proper forum for the applicant according to the Association’s policies. In your case it has been determined that it is not. You are still welcome to come to the conference as an attendee and attend all of the sessions, but you cannot display your material in any way. You may submit your material as a proposal for the next ICAD, or apply for a research grant if you wish. If you have registered for the conference and decide not to attend, I can see that your registration fee is refunded. Let me know if you want to do that.
Again, I am sorry this has not worked out as you wished.
Best regards, ST
I wrote yet another e-mail to her explaining in detail the many reasons why it was so very important for this information to get out as soon as possible to the Alzheimer’s research community as well as the public. I pointed out that Accera, the makers of AC-1202 which had performed the MCT oil clinical trials, were approved for a booth and that the foundation of the dietary intervention discussed in the article I intended to distribute was based on their research. The millions of people with Alzheimer’s should not have to wait for their product to come to market when it was available on the shelf now.
Later that day, ST wrote back.
Hi Mary,
There is no way that you can distribute your material at this conference. It does not qualify for the exhibit hall or any other form of display. Any presentations, either oral or poster, have to be submitted and reviewed by the Scientific Program Committee. You will have to find another way to distribute your material.
Best regards, ST
Still not ready to give up, I pointed out to ST that other groups were approved for exhibits that had no connection with Alzheimer’s, such as a company selling bibles and a tourism company. I also suggested that she take my article to the medical director of the Alzheimer’s Association for review, rather than relying on a nonscientist administrator to make this kind of decision.
July 24, 2008
Dear Mary,
As you have requested, I have passed your comments on to our medical science department. As mentioned in previous e-mails to you, the exhibitors in the exhibit hall represent companies that are promoting products and services, not specific research findings—those are inappropriate for the exhibit hall. The proper forum for research findings at an Alzheimer’s Association conference is through oral and poster presentations selected through the abstract submission process.
We are glad you can attend the conference sessions and visit the exhibit hall as an attendee, but let us be perfectly clear that you cannot distribute your attachment any place at ICAD.
Best regards, ST
I made another attempt to convey to ST that it would be obvious to any reader that my article was simply a case presentation that would support Accera’s AC-1202 research, which the conference had already reviewed and approved, but I made no headway.
July 24, 2008
Mary,
Your document was reviewed by our Medical Science Department and the approval of your application for an exhibit space at ICAD was denied. ST
Obviously, I am not one to give up. I was actually quite taken aback that the people who manage the Alzheimer’s Association would react to this particular information by going out of their way to suppress rather than, at the very least, investigate the concepts involved. Couldn’t they consider the possibility that something in the diet could benefit people with the disease they had charged themselves to find a cure for?
Discouraged, but not ready to give up, I contacted the city government to find out if there were any laws against passing out information on public streets. My Plan B was to hand out copies of the article to people attending the conference as they entered and exited the convention center. I learned this was allowed, but we could not set up a table or stand of any kind, so plans for a poster exhibit were abandoned.
On the evening of July 26, Angie and John met us at the airport in Chicago, and we checked into the hotel. The next morning, we arrived early at the convention center to allow time to distribute copies of the article. To my surprise and dismay, the convention center and the grounds around it are so vast that most people coming and going were already on the private grounds before exiting vehicles. Many others were staying at a hotel with a direct indoor link to the center. Passing out copies to people on public streets was not going to be quite as easy as I had pictured.
We proceeded to the registration area where I checked in and learned that it would not be possible for anyone without a conference badge to sit in on any part of the conference or even enter the exhibit hall. We decided to split up at that point. John and Steve would do some sightseeing; Angie would scout out the area to see if there was a good place just off the grounds to pass out articles; and I would attend lectures. Unfortunately, we followed that plan through much of the four-day conference.
I proceeded to the first plenary session. A plenary session includes all the participants in one room. In this case, the room was massive, far and away the largest hall I have ever seen at a medical conference. The speaker was at the front, and at various intervals there were very large screens, much like you would see at a rock concert, on which the speaker’s audiovisuals were projected. I fully expected that the first plenary sessions would be devoted to an overview of what was known about Alzheimer’s disease, so that attendees would start off the conference on the same page. This is customary at medical conferences. Instead, there were three talks back to back, each lasting forty minutes: the first was about mouse models of Alzheimer’s disease; the second was about imaging, distinguishing diseases, and measuring progress; and the third was about biomarkers.
After a half-hour break, participants were divided into four groups in separate halls with sessions covering different areas of research. Twenty minutes were allotted per talk for two hours. There was a two-and-a-half-hour break in the middle of the day during which lunch was available. The afternoon was structured so there were six groups, with each talk lasting fifteen minutes for two more hours. On each of the four days of the conference, about 500 different poster presentations were set up in the exhibit hall, covering every conceivable niche of Alzheimer’s disease except the topic of ketones that was most important to me and, in my estimation, the most promising one. That added up to an average of seventy-five oral presentations taking place in multiple rooms simultaneously several times a day over four days. During the final afternoon of the conference there was no wrap-up, no summary of what we’d learned, just a continuation of the same fifteen-minute talks in seven rooms over two hours.
My Plan C was to talk with as many attendees as I could about ketones to try to increase awareness and hopefully stimulate some of them to explore this area further. I stopped to talk with anyone presenting on such subjects as insulin, glucose, and nutrition-related research, among many others. I discovered that many of the attendees were physicians involved in treating patients and/or clinical research (testing on people rather than animals), and many more were Ph.D. researchers involved with laboratory and animal research.
With more than 2,300 presentations in four days, only one topic, a promising drug called Rember, made the news. So many tiny niches of research were represented that I could not help but think of them as small pieces of a very large puzzle: in this case, the answer to Alzheimer’s disease, the cause and the cure. With more than 2,000 pieces to this puzzle, someone had taken the box, shaken it, and tossed the pieces in the air so they landed randomly on the floor, scattered in all directions and not even in the same room. I knew that some of the most important pieces of the puzzle were missing.
And who is putting the pieces of this puzzle together? In an ideal world, a group such as the National Institutes of Health or the Alzheimer’s Association, with their vast monetary resources, would create a center, bringing together the top clinical and research physicians and scientists in every related discipline. These experts would not be owned by drug companies and would put aside their personal biases toward their own research to carefully and objectively sift through the pieces to work out this puzzle together. This would be a collaboration, not a competition. They would look beyond the research funded by the Alzheimer’s Association and the drugs companies and beyond the U.S. borders to every part of the world. They would consider every chemical, nutrient, and microorganism that might play a role in the disease process for better or worse. They would use computers to compile and organize the information. A smaller group would oversee and orchestrate the process and keep it moving until all the pieces are put together. After all, this is urgent business for those of us who are losing our loved ones now. There is plenty of precedence for this type of activity to solve a problem. The Human Genome Project is one example in which the human sequence of DNA was completely worked out through collaboration.
One surprising event occurred the first day of the conference. While Angela was scouting out the conference center, she found the pressroom. At the next break, I inquired about leaving copies of my article for members of the press. I was told that only materials that had been previously approved by the Alzheimer’s Association would be accepted for that purpose. In the hallway outside the pressroom, Athena Rebapis, a young reporter for www.intimetv.com, stopped to speak with us. I explained what had happened with Steve, and she asked if I wanted to be interviewed. I said, “Most definitely!” and we set up a time for the taping later that morning. Her director/producer, Troy Ferguson, was extremely interested and taped pictures of Steve’s clocks, which were included in the presentation. An eight-minute segment with this interview appears in their program “Journey through Alz heimer’s” along with other interviews from the Chicago conference, which can be viewed through a link on my website www.coconutketones.com.
Another interesting experience involved an individual who was almost certainly a security guard working for the Alzheimer’s Association. He was a tall man with an Alzheimer’s Association logo pin on the lapel of his navy suit, who seemed to spend an inordinate amount of time very close by. We first noticed him when we met in the lunch area near the exhibit hall on the first day of the conference. He actually sat down at the table without food where the four of us were eating and seemed to be eavesdropping. Thereafter, on several occasions he seemed a little too close when I was in the exhibit hall, particularly when I was speaking with poster presenters. Angie also noticed him when she was hanging out while I attended sessions. Considering how many people were attending the conference, his presence just seemed a little too much of a coincidence.
On my first visit to the exhibit hall, I found what I believed at that time was the reason why my exhibit was denied. I knew that Accera was scheduled to have an exhibit, and as you might expect, I decided to look for that booth right away. It was located directly across the aisle from the table exhibits where I would have been distributing my article. I stopped by the booth several times, hoping to catch their vice president, Dr. Lauren Costantini, an author of their research papers, but we were never in the same place at the same time. Accera had a poster presentation, but no one seemed to be available for discussion during the scheduled time. None of the written materials at their booth mentioned that the active ingredient in AC-1202, by then called Axona, is a medium-chain triglyceride. Instead, the product was presented as a “novel therapy” that increases ketones. I questioned a couple of their sales representatives at the booth, asking what the novel therapy was. For the most part, they either feigned ignorance or provided an evasive answer, such as, “It is a powder.” When pushed, one of the reps finally told me that it was tricaprylic acid, one of the medium-chain triglycerides. I can only speculate why they would not divulge this information at this type of conference. I suspect part of the answer is that, in a conference attended by physicians and scientists, some astute people will know that the active ingredient in their product, MCT oil, is available over the counter. On the other hand, physicians are not likely to prescribe something new for their patients without some basic idea of what the product is.
The exhibit hall was quite a revelation. There were massive booths for the makers of the major Alzheimer’s medications. One passed out expensive-looking engraved pens, and another, a four-port USB hub inscribed with the name of the products. The lines were long at these booths, and by contrast, there was very little activity at the Accera booth. Yet I knew that learning about ketones at their booth was perhaps the single most important piece of information that any attendee could acquire at this conference.
We hoped to distribute copies of my article to 5,000 conference attendees, but at most we were able to leave 300 in the city of Chicago; many of those were distributed at natural food stores and to passersby between Grant Park and the convention hall. Angie, John, and Steve also visited some of the local media and left copies. Overall, with the exception of a brief Internet TV interview, we were quite disappointed with the results of our attempts to get the message out.
We carried more than 1,500 copies of the article back home with us. I thought that since the attempts at getting this information out to the public at large by way of high-profile people, the media, and the Chicago conference were going nowhere, it was time to take a grassroots approach. I kept a box full of the flyers in our car, and we began to make a weekly circuit of the natural food stores in and around Spring Hill, leaving copies of the article for shoppers. We were invited to speak at a natural foods store in Spring Hill after the owner read the article. He posted a notice in his store, but only four people attended this first lecture. Shortly after, I received an invitation from the owners of another natural foods store to give lectures and to be interviewed for a health segment on a local radio program. The lectures were very well publicized, and the radio interview was aired a number of times. The attendance at the lectures increased to capacity, and several more were scheduled for people on the waiting list. There were eighty to ninety people at each talk.
People began sending the article out to other people. I regularly sat down with a list and e-mailed the article to as many on the list as I could during moments of free time, often very late in the evening and early in the morning. After e-mailing a copy to Bruce Fife, N.D., who has written many excellent books about coconut oil, an article appeared in his Healthy Ways newsletter summarizing what had happened with Steve, and he included my entire article in his next newsletter. I was contacted by a magazine distributed in natural food stores called Energy Times, and they likewise ran a story and created a link from which people could download the article.
The biggest break in getting the message out (before publication of this book) occurred several months later on October 28, when the St. Petersburg Times ran a story titled “Sketches in Progress” by Eve Hosley-Moore. The reporter spent considerable time interviewing Steve and me and attending one of my lectures. She also interviewed Dr. Veech and Dr. VanItallie. She eloquently conveyed the story of Steve’s decline and his dramatic improvement after taking coconut oil, along with information about how ketones can provide an alternative fuel to the brain for people with Alzheimer’s and those with certain other diseases. Steve’s clocks were included with the story (Figures 4.1, 5.1, 5.2), and I believe this had more impact than anything else on what happened after the story ran. The article was also published online and can be viewed at www.tampabay.com/news/aging/article879333.ece.
Eve told me a few weeks later that it broke a record for “most e-mailed article,” and it very soon “went viral” on the Internet. I began to receive regular requests to speak, to include my article or summaries of our story in newsletters, and to give radio interviews. Steve comes to all the talks with me and does not hesitate to answer questions and otherwise chime in.
I began to hear regularly from caregivers who were also seeing a positive response in their loved ones and occasionally from people who tried coconut oil and saw no response whatsoever. In Chapter 13, I share some of the many encouraging messages I have received.
There were very many days when I felt excessively stressed by the overload of full-time work, caring for Steve, and spending hours nearly every day trying to get the message out when I should have been relaxing and even sleeping. Then I would receive a phone call or an e-mail telling me about someone who had improved and how grateful the person was that I had written the article, and I knew my efforts were not in vain. This is often the impetus I need to keep going.