In the October 2003 issue of Nutrition Reviews, the lead review article “Ketones: Metabolism’s Ugly Duckling” was written by Theodore B. VanItallie, M.D., and Thomas H. Nufert and included a comprehensive discussion of:
• The discovery of ketones
• The physiology and metabolism of ketones
• The role of ketones in starvation and carbohydrate restriction
• The recognition of ketones as a metabolic fuel
• How ketones are used now to treat disease, such as epilepsy and rare genetic defects
• The potential for treatment of other diseases with ketones
I highly recommend this article for anyone who wishes to read a more technical and detailed discussion of what is currently known about ketones than I’m providing here.
My goal is to provide information about ketones so that most people will be able to understand them. Much of what I discuss is based on that article, as well as information from other articles written by Richard Veech, M.D., George Cahill, Jr., M.D, Oliver Owen, M.D., Sami Hashim, M.D., and their many associates, and a number of others who have researched and written about ketones. Information about the ketogenic diet largely comes from the book entitled The Ketogenic Diet (2007) by John M. Freeman, M.D.
Organic chemistry is the study of compounds that contain hydro-carbons, various combinations of carbon and hydrogen that are the basis of living organisms. A number of other elements may be included in these compounds, such as nitrogen, oxygen, phosphorus, silicon, and sulfur. The various types of compounds are grouped and named according to the way the particular elements are arranged. Some examples of groups of organic compounds are esters, amides, aldehydes, fatty acids, and ketones, to name just a few.
For those who did not take chemistry, very simply, an atom is a single particle of an element, such as hydrogen or carbon, and a bond is a connection between atoms. A molecule is a group of two or more atoms. Each type of atom has a specific number of possible bonds it can make with other atoms. For example, hydrogen can make one possible bond and oxygen two bonds. A water molecule is one oxygen atom that attaches its two bonds to two hydrogen atoms, or H2O (Figure 16.1).

Figure 16.1: A water molecule. Diagram by Joanna Newport.
The carbon atom can have four possible bonds to other atoms. The defining feature of ketones is a single carbonyl group, which is an oxygen atom connected by two bonds to a carbon atom. Also attached to the carbon are two more carbon atoms, and each of these carbon atoms is attached to a variety of atoms or groups of atoms. In the simplest ketone, called acetone, the other two carbons use their three other bonds to attach to hydrogen atoms (Figure 16.2).
Figure 16.2: The ketone body acetone. Diagram by Joanna Newport.
Many different kind of ketones are found in nature, but in the human body there are three ketones, also called ketone bodies, that we will be talking about. These are acetone, acetoacetate, and beta-hydroxybutyrate. Acetone is the simplest ketone, while acetoacetate and beta-hydroxybutyrate are more complex molecules. You should know that acetone made in our bodies is the same acetone found in fingernail polish and paint thinner. Most people are familiar with the odor of acetone. When a person has an excess of ketones in the blood, some of this acetone will be exhaled, so the person’s breath may smell like acetone. Also, some of the acetone is disposed of by the kidneys in the urine.
The process of making ketones is called ketogenesis. This is a complex process (Figure 16.3), but I will attempt to explain it simply.
Ketones are produced when the glucose stored in the body is used up and fat is broken down to be used as energy. Triglycerides, the storage form of fat, are converted to fatty acids in the adipocytes (fat cells) or fat tissue by a process called lipolysis. Fatty acids can be used as a metabolic fuel by a number of tissues such as muscle or the heart. The fatty acids released from fat are transported to these tissues and cross the cell membrane. Fatty acids of more than twelve carbons (long-chain fatty acids) require special membrane transporters to enter the cell, but medium-chain fatty acids, with twelve carbons or less, do not.
Once inside the cell, fatty acids enter the mitochondria, a process that requires special transporters for fatty acids longer than twelve carbons. Inside the mitochondria, the fatty acids are split repeatedly into two carbon fragments called acetyl-CoA by a process known as beta-oxidation. An additional set of reactions occurs when fatty acids are transported to the liver. They are converted in the liver cell mitochondria first to acetyl-CoA as in other tissues, but then the acetyl-CoA is converted through several biochemical reactions to the ketone acetoacetate, which is then converted to the other two ketones, acetone and beta-hydroxybutyrate. So when fatty acids are converted in the liver to ketones, this entire process is called ketogenesis. The ketone bodies then leave the liver and are transported throughout the body, where they can be used as an energy source in the mitochondria of a number of tissues, including the brain.
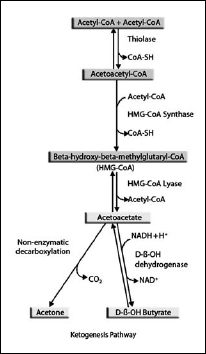
Figure 16.3. Ketogenesis pathway. Diagram by Joanna Newport.
As you know, mitochondria are tiny structures inside of cells in which energy is manufactured. Certain cells, such as heart muscle, neurons, and liver cells, can have a thousand or more mitochondria inside of each cell. Amazing to think a cell that cannot be seen with the naked eye can have a thousand of anything so complex inside of it! When a fuel such as glucose (after being converted to pyruvate) or ketones enters the mitochondria, a chain of events takes place leading to the generation of the basic source of energy, ATP, that drives our cells. The way cells use ATP is much like how a car takes electricity from a battery in order to run. Ketones are a more efficient producer of ATP than pyruvate, improving the hydraulic efficiency of the working heart by over 30 percent (Kashiyawa, 1997).
Fatty acids made from stored fat can be used by the heart for fuel but are too large to cross the protective blood/brain barrier into the brain circulation. But ketones are able to pass into the brain on a special monocarboxylate transporter and can be used by the neurons and other brain cells for fuel.
The fact that our brains can use ketones is very important in our evolution as a species. Compared with other animals, the size of the human brain is very large relative to our total body weight. The considerably larger cerebral cortex, the part of the brain that provides us with higher intelligence, accounts for most of this difference.
The brain in an average adult weighs just over three pounds, just about 2 percent of the total body weight, but it has a very high-energy requirement. When we are at rest, the brain consumes about 20 percent of the calories needed to carry out basic bodily functions. This represents about 100 to 120 grams of glucose per twenty-four hours. In the infant and child, the brain is even larger relative to body weight, and the brain may consume as much as 40 to 50 percent of the basic calorie requirement. The pregnant mother has to provide enough energy for two large brains and may need 200 to 220 grams of glucose every day to provide enough energy for herself and the baby.
For many millennia, our ancestors, and even people in our world today, have endured periods of feast and famine. Our bodies are only able to store enough glucose in the liver (glycogen) to last about forty-eight hours, but then must tap into muscle to make new glucose. This is a very complex process, called gluconeogenesis. During starvation, about 60 percent of this process occurs in the liver and 40 percent in the kidneys. Very simply, protein from muscle is changed into specific amino acids and released into the bloodstream mostly as glutamine and alanine. The glutamine is mainly converted to glucose by the kidneys. The liver uses the alanine, some recycled lactate and pyruvate, which come from red cells and kidney cells, some glycerol from fat, and even some ketone body, to make glucose.
While glycogen stores in the liver are used up within a couple of days, gluconeogenesis can continue for at least forty days. But the calories that we can make from gluconeogenesis alone are not nearly enough to provide the basic calorie requirements of the body and brain for an extended period of time. If glucose were the only fuel our brains could use, a starving human would last only about two weeks before consuming so much muscle that survival would no longer be possible.
When we have an abundance of food, we store extra calories in the form of fat. When our energy needs exceed our calorie intake, we begin to tap into these stores of fat, which are broken down into fatty acids. As we continue to starve, our muscles, including our heart muscle, switch over from using glucose to using primarily fatty acids as fuel. Medium-chain fatty acids can cross the blood/brain barrier but are not made in the body in a form that can be released into circulation to fuel the brain. The only exception to this is that the lactating mother can manufacture medium-chain fatty acids in the breast that become part of the milk for the baby.
Since our brains require so much fuel to survive, when we have used up our stores of glucose, how does the brain survive starvation? As our bodies change over to using stores of fat as the main source of fuel, some of the fatty acids are converted into ketones in the liver. The presence of ketones in general circulation actually increases the blood flow to the brain by as much as 39 percent, at least initially (Hasselbalch, 1996). Ketones cross the blood/brain barrier and are taken up very rapidly by brain cells as fuel. Ketones also perform other important functions, which will be explained shortly.
Ketone bodies do not require insulin to enter the cell, but rather are transported into the cell by a much simpler mechanism than glucose. The ketone body bypasses some of this complex process and enters directly into the Krebs cycle, the chain of chemical reactions that produce acetyl-CoA and ultimately the basic energy molecule ATP. During starvation, about 60 percent of the energy requirement of the brain can be provided by ketones, thus sparing muscle from being broken down to provide energy. Since our brains can use ketones as fuel, we can potentially survive for two months or longer without food, instead of just two weeks. The more fat tissue available, the longer we can survive, since fatty acids can be used by muscle and ketones made from fatty acids can be used by the brain and other tissues.
The average adult will have a low level of ketones after fasting overnight, but these levels drop as soon as we eat our morning meal. We begin to see a significant increase in ketone levels after about two days without food. The levels of the ketone bodies acetone and acetoacetate rise and then begin to level off after about ten days in the 1 to 1.5 micromoles per liter (mmol/l) range, but the levels of beta-hydroxybutyrate continue to increase very dramatically for a time before leveling off in the 6 to 7 mmol/l range. As long as we have fat available, we continue to make ketones.
The infant, child, and pregnant or lactating woman is able to start making ketones considerably sooner than the average adult, reflecting their even greater need for brain fuel during starvation. The newborn uses ketones for as much as 25 percent of the energy requirement during the first days of life (Bourgneres, 1986). Most new mothers learn quickly there is not much breast milk available during the first days after the baby is born. Human babies are quite fat relative to other creatures, and ketones help them get through this period until their mother’s milk is more available.
If our brains did not have the ability to use ketones as fuel, it is unlikely that we would even exist as a species today, or at least not as the very large-brained, intelligent species we are. Our ancient ancestors, and even many people today, have gone without food for extended periods and survived, thanks to the role fat and ketones play in providing fuel.
The fact that the brain can use another fuel was discovered and reported in 1967 in an article entitled “Brain Metabolism during Fasting” in the Journal of Clinical Investigation by Dr. George Cahill and his associates, who included Dr. Oliver E. Owen. In 1965, at about the same time that Dr. Cahill decided that it would be important to reinvestigate the effects of starvation in humans since better methods had become available to measure biologic fuels and hormones, Owen began a fellowship in Cahill’s lab at Harvard. Owen detailed the history of this discovery in his article “Ketone Bodies as a Fuel for the Brain during Starvation” in Biochemistry and Molecular Biology Education (2005).
The first subject was an obese nurse who was highly motivated to participate in the researchers’ study of prolonged fasting. She was referred to their clinic for weight loss due to her concern about having a heart attack. As a nurse she was well aware of the procedures involved. After a few days on a balanced diet, the researchers began a starvation protocol in which she received only water, vitamins, and salt tablets for a period of forty-one days. They chose forty-one days because that was the period of time that Jesus fasted according to the Bible. They took blood samples from catheters placed in arteries and veins around her brain and liver to measure various metabolites. Much to their delight they found that her brain had survived this lengthy period of starvation by using ketones and greatly reducing the use of glucose. About two-thirds of the fuel used by her brain was provided by beta-hydroxybutyrate and acetoacetate.
Dr. Owen reported, “The fact that the brain could derive energy from substrates other than glucose was of monumental importance for understanding human survival during starvation. Our findings explained why normal size humans can survive sixty days or more without food; the brain obtained most of its energy requirements from ketone bodies, a fuel derived from fatty acids. This finding resulted in a total reappraisal of the hierarchy of fuels used by different tissues of humans” (see Figures 16.4 and 16.5).
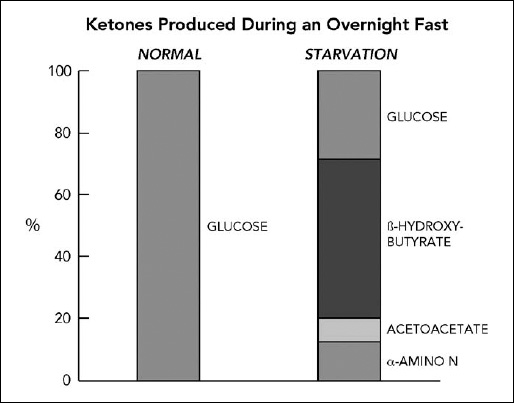
Figure 16.4. Ketones produced during an overnight fast. After an overnight fast in normal individuals, virtually 100 percent of the fuel used by the brain comes from glucose. During starvation, ketones supply two-thirds and glucose just one-third of the fuel for the brain. Data from Owens, 2005.
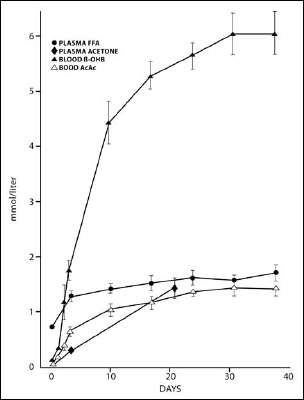
Figure 16.5. Levels of ketones are nearly zero when we eat regularly. While fasting, the levels begin to rise after one or two days, and beta-hydroxybutyrate in particular increases dramatically if starvation continues for more than five days. Data from Owens, 2005.
Dr. Cahill’s group studied two more obese patients in the same manner and confirmed that “during prolonged starvation, the brain extracts significant quantities of acetoacetate and beta-hydroxybutyrate from the blood, thus sparing the metabolism of glucose. We grasped immediately that the metabolism of ketone bodies rather than glucose was the predominant source of energy, sparing muscle protein and ensuring survival during starvation.” They also learned that during starvation, glucose levels drop and then level off after about three days, and this is paralleled by a similar drop in insulin levels.
An even bolder experiment followed (Cahill, 1980). Three obese college-age men were starved for several days until their beta-hydroxybutyrate levels increased. They were then given a dose of insulin to drive down blood sugar into the hypoglycemic range. Normally, without the availability of ketones, such low levels of glucose would be expected to cause serious symptoms, including confusion, difficulty thinking and speaking, weakness, poor coordination, paleness, sweating, rapid heartbeat, and even seizures, loss of consciousness or coma. In this study, the high level of ketones protected the subjects from experiencing typical symptoms of hypoglycemia. Other studies have confirmed this protective effect of ketones in the face of low blood sugar (see Chapter 18).
Fast forward to 1991, when Siegfried Hoyer, M.D., reported “Abnormalities of Glucose Metabolism in Alzheimer’s Disease” in the Annals of the NY Academy of Sciences. He found that there is a shift in the types of fuel used by the brain in people as they age that is even more prominent in people with Alzheimer’s disease. Young, normal people use fuel in the cerebrum of the brain in a ratio of 100:1 of glucose to alternative fuels; in elderly people without Alzheimer’s, this ratio is 29:1; and in people in the early stages of Alzheimer’s this ratio is 2:1. This means that someone in the beginning stages of Alzheimer’s requires that about one-third of the fuel for brain cells comes from alternative fuels. Dr. Hoyer speculated that possible alternative fuels could be fatty acids and amino acids present in the brain, such as glutamate, that could be converted to glucose, but he did not specifically mention ketones in this article as a possible alternative fuel.
We know from the work of Drs. Cahill and Owen that ketones could also provide this alternative fuel. Since most of us do not have ketones circulating and readily available to the brain, it stands to reason that brain cells lacking this necessary fuel would malfunction and eventually die. Treatment with foods that contain medium-chain fatty acids, which are converted to ketones in the liver, or, even better, direct treatment with ketone bodies themselves, could provide the brain with this necessary fuel.
The body makes ketones in response to a number of conditions, including fasting. As we fast overnight while sleeping, a relatively tiny amount of ketones are made, but as soon as we eat a typical breakfast, they disappear. If we continue to fast for a period of two or more days, the levels of ketones increase very significantly. As a modern-day example of the biochemical miracle of ketogenesis, one need only recall the images of people emerging from beneath the piles of concrete where they were trapped without food for as long as fourteen days after the disastrous 2010 earthquake in Haiti. Upon being pulled from the rubble, many of these people were alert, smiling, talking and even singing, their cognitive function apparently intact. With their stores of glucose gone in less than a day or two, ketones came to their rescue to preserve their brains and allow them to survive while awaiting release from their concrete prisons.
Helpful levels of ketones are produced by the body through exercise and diet. However, not all production of ketones is healthy. Life-threatening levels of ketones can also be produced in diabetics, called diabetic ketoacidosis (discussed later).
Most of us in the United States do not have to struggle with the problem of feast and famine. The standard American diet (sometimes called “SAD”!) is very high in carbohydrate, or sugar. As a result, there is not much ketone circulating in the bloodstream, since plenty of glucose fuel is available.
One way to produce ketones through diet is to consume an extremely high-fat, low-carbohydrate diet, called the “ketogenic diet.” This diet is sometimes used to treat children, and occasionally adults, who have severe epilepsy. This diet is also relatively low in protein, allowing enough protein for the child to grow or for the adult to maintain lean body mass. The Atkins and South Beach diets are less restrictive forms of the ketogenic diet and result in a milder “ketosis” (more on these diets in Chapter 17).
There is yet another way that ketones can become available to brain cells through diet, and that is the basis of the dietary intervention discussed in Part Three in this book. When a person eats foods that contain medium-chain fatty acids, they are absorbed from the small intestine and transported directly to the liver, where they are partly converted to ketones. The ketones are then released into the blood circulation. Some of the medium-chain fatty acids are also released into the circulation and are used immediately as energy. Medium-chain fatty acids are known to be used directly as fuel by muscle mitochondria (Turner, 2009); are small enough to cross the blood/brain barrier; are not stored as fat but rather used immediately as fuel by tissues; and may be used as fuel by the mitochondria in the brain. Animal data shows that medium-chain fatty acids are used in the brain (Johnson, 1990). Further studies need to be done to determine what specific tissues, in addition to muscle, are able to use medium-chain fatty acids directly.
The most concentrated food sources of medium-chain fatty acids are coconut oil and palm kernel oil, which contain about 55 to 60 percent medium-chain fatty acids. These are saturated fats, but do not behave the same way in the body as long-chain saturated fats that doctors worry about (see Chapter 21). Oils normally consumed in this country, such as soybean, olive, canola, corn, peanut, safflower, and sesame, do not contain any medium-chain fatty acids. Some foods that do contain medium-chain fatty acids are goat’s milk, whole cow’s milk, butter, heavy cream, and goat and feta cheese, although not nearly in the concentrations found in coconut and palm kernel oils.
We can take advantage of the biochemical process that naturally occurs in our bodies when we consume fats with medium-chain fatty acids. The more medium-chain fatty acids we consume, the more ketones we produce. If you do not eat medium-chain fats on a given day, you are not likely to produce a significant amount of ketones, unless you are starving for a couple of days or longer or you are on a ketogenic diet.
Medium-chain fatty acids cross the blood/brain barrier, where they are used by brain cells for fuel. Considering the process that occurs in Alzheimer’s and certain other neurodegenerative diseases, it is very conceivable that medium-chain fatty acids are an important source of fuel for the brain. They might even be considered essential fatty acids for some people: the brain needs medium-chain fatty acids, but we cannot make them; therefore, we need to consume them. So there appears to be a great advantage to including foods with medium-chain fatty acids in the diet on a daily basis. Not only will that provide the brain with ketones, but also with medium-chain fatty acids that can be used directly as fuel by certain brain cells.
More extensive research is needed to learn about the various functions of medium-chain fatty acids in the brain and other organs in addition to their function as fuel.
We produce ketones when we exercise. This was first described in 1909 by G. Forssner, who noticed that the levels of ketones always increased in his urine after a brisk 4-kilometer walk lasting over thirty-six minutes. In 1911, L. Preti reported the same phenomenon in a patient who had a minor stomach complaint after climbing up and down stairs until exhausted. In 1936, F. C. Courtice and C. G. Douglas reported that following moderate early morning exercise after fasting overnight, ketones begin to rise upon completion of exercise and continue to rise for at least nine hours. They hypothesized that the muscles, which were glucose-deprived due to lack of food overnight, use the circulating ketones while the exercise occurs. When the exercise ceases, the liver continues to make ketones for a short period, which then accumulate in the circulation. This phenomenon was thereafter called the “Courtice-Douglas effect” and is also known as “post-exercise ketosis.” In all three of these studies, the subjects were on some kind of a restricted diet, reported as either low carbohydrate or high protein, for a period of time before the experiments. Later researchers noted that if a high-carbohydrate meal was taken before the exercise, there was no increase in ketones.
While research on post-exercise ketosis continues to confirm early findings (Passmore, 1958; Johnson, 1974; Koeslag, 1979), let’s now fast forward to the new millennium. A protein found in the brain has become a focus of interest in the fight against Alzheimer’s and other neurodegenerative diseases. Brain-derived neurotrophic factor (BDNF) is undergoing intense study due to the role it plays in the areas of the brain that are especially susceptible to degeneration in Alzheimer’s disease, the hippocampus and dentate gyrus, part of the hippocampus that helps form new memories. BDNF has widespread functions throughout the brain, such as promoting the growth and survival of neurons and strengthening of synapses (Blurton-Jones, 2009). Synapses, you may recall, are the connections between neurons in the brain that are so important to learning and the formation of memories. Therefore, BDNF is important to normal cognitive functioning. Levels of BDNF are reduced in the brains of people with Alzheimer’s. When levels of BDNF are below normal, neurons are more susceptible to damage and degeneration.
Within just the past few years, researchers have learned that BDNF increases in the brain in response to learning and exercise. This increase in BDNF appears to be the mechanism by which learning and exercise improve cognitive function and stave off memory decline in the hippocampus (Vaynman, 2004; Yamada, 2002).
But what substance specifically stimulates the brain to make BDNF when we exercise? Is it possible that the increase in ketones related to exercise stimulates the brain to make BDNF? The answer to this question is not known for certain at this time.
When doctors worry about the possible dangers of raising ketone levels, they are thinking about diabetic ketoacidosis. When a person with type 1 diabetes does not have an adequate supply of insulin to allow sugar to enter the cells, and blood glucose levels become extremely high, the person may develop a condition called diabetic ketoacidosis and may even go into a coma. Among other metabolic events, large amounts of ketones are suddenly released into the circulation. The levels of ketones become dangerously high, as much as five to ten times higher than in the situation of starvation or on a classic ketogenic diet. If the person is not given insulin in short order, this condition may be fatal. Diabetic ketoacidosis is a common presentation for children and adults signaling the beginning of type 1 diabetes.
The levels of ketones from consuming very large amounts of medium-chain fatty acids would never remotely come close to reaching the levels of diabetic ketoacidosis. The levels of ketones are fifty times higher in diabetic ketoacidosis than after consuming a large quantity (20 grams) of MCT oil (see Table 16.1).
TABLE 16.1. COMPARISON OF BETA-HYDROXYBUTYRATE LEVELS UNDER VARIOUS CONDITIONS
| CONDITION | BETA-HYDROXYBUTYRATE (MMOL/LITER) |
| Exercise1 | 0.25 |
| Coconut oil and/or MCT oil2 | 0.25 to 0.5 |
| Classic ketogenic diet3 | 2 to 5 |
| Starvation4 | 2 to 7 |
| Beta-OH Butyrate Ester5 | 2 to 7 (proposed levels) |
| Diabetic ketoacidosis6 | 25 |
Sources:
1. Levels of ketones after running for 90 minutes (Koeslag, 1979).
2. Levels of ketones based on Accera Studies (Henderson, 2009) and levels at NIH (Tables 6.1 and 6.2).
3. Levels of ketones on a classic ketogenic diet (Gilbert, 2000).
4. Levels increase between two and ten days’ duration of starvation (Owens, 2005).
5. Proposed levels (Veech, 2001).
6. Levels can reach as high as 25 mmol/l (Veech, 2001).