3
The Nutritional Benefits of Mushrooms
Carolina Barroetaveña1,2,3 and Carolina V. Toledo1,3
1 Centro de Investigación y Extensión Forestal Andino Patagónico CIEFAP, Argentina
2 Universidad Nacional de la Patagonia San Juan Bosco, Facultad de Ingeniería, Ruta 259 Km 4, Esquel (9200), Chubut, Argentina
3 Consejo Nacional de Investigaciones Científicas y Tecnológicas (CONICET), Avenida Rivadavia 1917, Ciudad Autónoma de Buenos Aires Argentina (1033), Buenos Aires, Argentina
3.1 Introduction
Wild edible mushrooms are appreciated and consumed in different parts of the world, not only for their delicate organoleptic qualities, but also for their chemical and nutritional characteristics (Maga 1981; Manzi et al. 2001). The culinary value of mushrooms is due mainly to their organoleptic properties such as odor, flavor, and texture (Guedes de Pinho et al. 2008; Maga 1981). Regarding nutritional qualities, mushrooms stand out due to their amino acid composition (Chang & Miles 2004; Crisan & Sands 1978; Kalač 2009), which is considered of high biological value and can be correlated to animal proteins (Gruen & Wong 1982). This consideration is relatively important due to increasing disease prevalence all over the world associated with high meat consumption. However, the potential nutritional value and the implication of a gradual substitution of meat with mushrooms requires careful examination involving detailed chemical and biological studies (Aletor 1995). Mushrooms’ chemical characteristics associated with pharmacological uses have also been widely studied (Bobek & Galbavy 1999; Bobek et al. 1991, 1995). All these special features, in addition to their variable colors, particular shapes, and rarity of several wild species only present in specific geographical areas, make mushrooms a very valuable resource, with importance for gourmet cooking in many parts of the world, where dishes prepared from wild mushrooms can achieve high prices on the market (Hall et al. 2003).
Consumption of wild edible mushrooms goes back to the beginnings of civilization and has been developed in many countries around the world, especially in China, Japan, United States, Spain, and Italy (Boa 2004; Wang 1987). In Latin America, Mexico, and, to a lesser extent, in Guatemala and Honduras, people have a deeply rooted mycological knowledge (Estrada‐Martínez et al. 2009; Ruan‐Soto et al. 2004), and the diversity of species present in these countries has been incorporated into several activities such as cooking, traditional medicine, and especially religious rituals (Villarreal & Pérez‐Moreno 1989). Mushrooms constitute an important source of food and monetary income in developing as well as developed countries, especially those having important forest resources (Boa 2004; Hosford et al. 1997; Wong et al. 2001). For small rural communities, selling wild edible mushrooms allows families to work toether, generating complementary incomes in a diversified economic strategy, or being the main income during the rainy season (Martínez‐Carrera 2010).
This chapter presents information on the nutritional composition of different wild edible mushroom species taken from reports from different authors, and also the potential benefits they can provide as a source for the human diet.
3.2 Nutritional Properties of Mushrooms
Scientific studies indicate that mushrooms are a healthy food source, having low calorie and fat content. They have a high protein content with an important ratio of essential amino acids, dietary fibers, carbohydrates, vitamins, and minerals (Agrahar‐Murugkar & Subbulakshmi 2005; Barros et al. 2008; Heleno et al. 2009; Kalač 2009; Ouzouni & Riganakos 2007; Reis et al. 2012). Investigation of nutritional composition includes determination of macronutrients such as proteins, amino acids, dietary fibers, lipids, carbohydrates, ash, as well as micronutrients, namely vitamins and minerals, among others, which are determined and analyzed following the methods suggested by the AOAC (2005).
Chemical composition of mushroom species may be affected by several variables such as genetic structure, strains, maturation stage, environmental conditions, such as soil composition, as well as the specific part of the mushroom, postharvest preservation method (dry or fresh procedures), and cooking process (Barros et al. 2007b; Chang & Miles 2004; Crisan & Sands 1978; Manzi et al. 2001, 2004).
Dry weight (dw) content of fresh mushrooms is relatively low, around 10%, and mainly consists of carbohydrates, proteins, dietary fibers, and minerals (Wang et al. 2014). Besides, since fresh fructifications provide about 90% of moisture content, data on chemical composition of mushrooms, usually need to be normalized according to dry matter content (Chudzyński & Falandysz 2008).
3.2.1 Proteins and Amino Acids
The nutritional value of mushrooms is directly related to their protein content. According to a report published by the Food and Agriculture Organization (FAO 1991), mushroom protein has better nutritive quality than that from vegetables. Crisan and Sands (1978) proposed a “nutritional index” to determine food nutritional values based on the amount and quality of amino acid fraction (EAA Index), as a way of solving difficulties related to comparing mushrooms with low amounts of proteins with high nutritional value with those having high amounts of low nutritional value proteins. Most of the edible mushrooms with a high EAA Index could be placed near to meat and milk, while those having a low EEA Index value can be placed between vegetables and legumes (Chang & Miles 2004).
Protein determination could represent a problem since different conversion factors have been used calculated on the base of nitrogen content. The crude protein content of most foods is currently calculated from the nitrogen content adjusted by a conversion factor (N × 6.25) assuming that proteins contain 16% of digestible nitrogen with insignificant amounts of nonprotein nitrogen. However, the cell walls of fungi contain an important amount of nonprotein nitrogen in the form of chitin. Therefore, another conversion factor applied to mushroom is N × 4.38, based on the presence of 70% of digestible protein (0.7 × 6.25 = 4.38) (Barros et al. 2007a, 2008; Breene 1990). Furthermore, Bauer‐Petrovska (2001) recommended another factor (N × 4.16), which was proposed by observing a mean proportion of 33.4% of nonprotein nitrogen (from total nitrogen) in numerous samples. In this way, in some articles, crude protein is thus overestimated. Crude protein values for wild mushrooms, depending on the species analyzed, range between 12.0 and 59.4 g/100 g dw, as in the case of Sarcodon aspratus (Berk.) S. Ito (Zhang & Chen 2011) and Lepista nuda (Bull.) Cooke (Barros et al. 2008), respectively.
Examples of crude proteins for four different mushroom species are presented in Table 3.1, displaying variations in this macronutrient value calculated for one species by different authors, depending on the protein conversion factor used. Kalač (2009) warns that when this value is overestimated in some studies, it mainly affects the carbohydrate value if calculated by difference through the following equation: 100% – (% moisture + % crude protein + % lipids + % ash).
Table 3.1 Crude protein content using different conversion factors for four species of wild edible mushroom species (g/100 g dry weight).
| Species | Crude protein (N factor used) | Reference |
| Amanita rubescens Pers. | 31.9 (N × 6.25) | Colak et al. 2007 |
| 26.0 (N × 4.38) | Ouzouni & Riganakos 2007 | |
| 17.4 (N × 4.38) | León‐Guzmán et al. 1997 | |
| Cantharellus cibarius Fr. | 53.7 (N × 4.38) | Barros et al. 2008 |
| 34.1 (N × 6.25) | Colak et al. 2009 | |
| Lepista nuda (Bull.) Cooke | 19.8 (N × 4.38) | Ouzouni & Riganakos 2007 |
| 44.2 (N × 6.25) | Colak et al. 2007 | |
| 59.4 (N × 4.38) | Barros et al. 2008 | |
| Lycoperdon perlatum Pers. | 17.2 (N × 4.38) | Barros et al. 2008 |
| 44.9 (N × 6.25) | Colak et al. 2009 |
The distribution of proteins within a fruiting body and changes in protein content during the development of the fruiting body remain mostly unclear. Vetter and Rimóczi (1993) reported the highest crude protein content, together with the highest digestibility (92%), in cultivated Pleurotus ostreatus (Jacq.) P. Kumm. (oyster mushroom) at a cap diameter of 5–8 cm. At that stage of development, crude protein contents were 36.4 and 11.8 g/100 g dw in cap and stipe, respectively. Thereafter, both crude protein and its digestibility decreased.
Proteins consist of over 20 amino acids in variable amounts. Humans can convert some of these amino acids into others but nine of them are considered as essential amino acids (lysine, methionine, tryptophan, threonine, valine, leucine, isoleucine, histidine, and phenylalanine). Free amino acid content is relatively low in mushrooms, only representing about 1% dry matter, and for this reason, their nutritional contribution for the human diet is limited (Kalač 2009). However, some authors point out that mushrooms are a good source of these compounds (Cheung 2010; Heleno et al. 2009), probably because the amount of free amino acids in wild mushrooms is highly affected by environmental factors.
Moreover, the amount and type of amino acids vary according to fungal species. For example, Mdachi et al. (2004) indicate that leucine was abundantly found, between 32% and 28% of the total essential amino acid content, in Boletus pruinatus Fr. & Hök and Boletinus cavipes (Opat.) Kalchbr, and the second most abundant essential amino acid was valine, recorded between 23% and 21%. Moreover, Ayaz et al. (2011) found that among the essential amino acids, leucine was the most abundant (48%) in Agaricus arvensis Schaeff.
On the other hand, other studies indicate that proteins in wild edible mushrooms contain considerable amounts of nonessential amino acids, such as in Amanita rubescens Pers. (73.16%), Boletus frostii J.L. Russell (81.83%), and Ramaria flava (Schaeff.) Quél. (81.86%) (León‐Guzmán et al. 1997). Data on essential and nonessential amino acids for some wild edible mushrooms are given in Tables 3.2 and 3.3, respectively.
Table 3.2 Essential free amino acid content (g/100 g dry weight) in some edible wild mushroom species.
| Species | Val | Leu | Thr | Ile | Lys | Trp | Met | Phe | References | |
| Boletinus cavipes (Opat.) Kalchbr. | 7.96 | 10.6 | 7.79 | – | 3.43 | 2.23 | 2.31 | 3.32 | Mdachi et al. 2004 | |
| Boletus pruinatus Fr. & Hök | 6.04 | 8.40 | 5.02 | – | 2.59 | 2.94 | 1.53 | nd | Mdachi et al. 2004 | |
| Clitocybe maxima (P. Gaertn., G. Mey. & Scherb.) P. Kumm.) | 2.74 | 0.44 | 7.24 | Nd | 0.79 | 8.37 | nd | 6.91 | Liu et al. 2012 | |
| Craterellus cornucopioides (L.) Pers. | 0.41 | 17.51 | 6.37 | 0.08 | 8.09 | nd | 12.74 | nd | Liu et al. 2012 | |
| Laccaria amethystea (Bull.) Murrill | 7.99 | 16.83 | 12.82 | Nd | 11.97 | 0.73 | 2.59 | 7.31 | Liu et al. 2012 | |
| Pleurotus sajor‐caju (Fr.) Singer | 7.81 | 0.43 | 8.56 | – | 6.33 | 0.41 | nd | nd | Mdachi et al. 2004 |
Ile, isoleucine; Leu, leucine; Lys, lysine; Met, methionine; nd, not detected; Phe, phenylalanine; Thr, threonine; Trp, tryptophan; Val, valine.
Table 3.3 Nonessential free amino acid content (g/100 g dry weight) in some wild edible mushroom species.
| Species | Ala | Arg | Asp | Glu | Gly | Ser | Tyr | Cys | Reference |
| Clitocybe maxima (P. Gaertn., G. Mey. & Scherb.) P. Kumm. | 18.62 | 0.08 | 2.72 | 0.90 | 11.69 | nd | 9.42 | 2.74 | Liu et al. 2012 |
| Craterellus cornucopioides (L.) Pers. | 0.86 | nd | 0.54 | 7.67 | 5.71 | 16.79 | 0.82 | 0.34 | Liu et al. 2012 |
| Boletinus cavipes (Opat.) Kalchbr. | – | 1.49 | 10.0 | – | 8.37 | 8.93 | 4.78 | – | Mdachi et al. 2004 |
| Boletus pruinatus Fr. & Hök | 11.5 | 0.03 | 8.36 | 15.4 | 6.14 | 7.42 | 3.42 | – | Mdachi et al. 2004 |
| Laccaria amethystea (Bull.) Murrill | 10.32 | nd | 0.49 | 13.12 | 2.20 | 10.34 | nd | 2.57 | Liu et al. 2012 |
Ala, alanine; Arg, arginine; Asp, aspartic acid; Cys, cystine; Glu, glutamic acid; Gly, glycine; nd, not detected; Ser, serine; Tyr, tyrosine.
Other amino acids that affect the flavor of mushrooms are glutamic and aspartic acids (Maga 1981; Mau et al. 2001). Glutamic acid and alanine were reported as the prevailing free amino acids in Tricholoma portentosum (Fr.) Quél. and T. terreum (Schaeff.) P. Kumm. (Díez & Alvarez 2001).
3.2.2 Carbohydrates: Available Carbohydrates and Dietary Fiber
Nutritionally, it is important to differentiate between two broad categories of carbohydrates: the “available carbohydrates”, which are digested and absorbed by the small intestine and provide energy to the human body cells, and “dietary fiber,” considered as nondigested carbohydrates that pass through to the large intestine, forming the substrate for colonic microflora fermentation. Available carbohydrates include monosaccharides, disaccharides, trisaccharides, starch, and some maltooligosaccharides. Dietary fiber includes nonstarch polysaccharides such as cellulose, hemicellulose, pectins, gums, mucilages, β‐glucans, oligosaccharides, and chitin (EFSA 2010).
Carbohydrates represent about 50% of mushroom dry weight (Kalač 2013). They play a major role in mushroom cell energetic metabolism, and can also be used for storage and structural polysaccharide synthesis (Lehninger et al. 2008). Data on carbohydrates for some wild edible mushrooms are given in Table 3.4. Grangeia et al. (2011) have reported that the Mycorrhizal species they studied presented a higher total sugar content (16–42 g/100 g dw) than saprotrophic mushrooms (0.4–15 g/100 g dw). Available carbohydrates include mannitol (0.3–5.5 g/100 g dw as reported by Vaz et al. 2011) and trehalose (9.3–42.8 g/100 g dw as reported by Vaz et al. 2011) as the main representatives of polyols and oligosaccharides, respectively (Kalač 2013), and also, glucose (0.5–3.6 g/100 g dw as reported by Kim et al. 2009), and glycogen (5–10 g/100 g dw as reported by Kalač 2013). Anyway, different authors present divergent values for the same or different especies, as shown in Table 3.5.
Table 3.4 Recent data on approximate composition (g/100 g dry weight) and energy value (kcal/100 g dry weight) for some wild edible mushroom species.
| Species | Proteins | Lipids | Ash | Carbohydrates | Energy value | References |
| Agaricus campestris L. | 18.57 | 0.11 | 23.16 | 58.16 | _ | Pereira et al. 2012 |
| Armillaria mellea (Vahl) P. Kumm. | 16.38 | 5.56 | 6.78 | 71.28 | 400. 68 | Vaz et al. 2011 |
| Boletus aereus Bull. | 17.86 | 0.44 | 8.87 | 72.83 | 366.69 | Heleno et al. 2011 |
| Boletus edulis Bull. | 21.07 | 2.45 | 5.53 | 70.96 | 390.11 | Heleno et al. 2011 |
| Calvatia utriformis (Bull.) Jaap | 20.37 | 1.90 | 17.81 | 59.91 | 338.26 | Grangeia et al. 2011 |
| Coprinus comatus (O.F. Müll.) Pers. | 15.67 | 1.13 | 12.85 | 70.36 | 354.27 | Vaz et al. 2011 |
| Flammulina velutipes (Curtis) Singer | 17.89 | 1.84 | 9.42 | 70.85 | _ | Pereira et al. 2012 |
| Lactarius deliciosus (L.) Gray | 20.20 | 8.02 | 7.15 | 64.63 | _ | Akata et al. 2012 |
| Russula olivacea (Schaeff.) Fr. | 16.84 | 1.99 | 37.78 | 43.38 | 258.84 | Grangeia et al. 2011 |
Table 3.5 Soluble sugars content (g/100 g dry weight) in different wild edible mushroom species.
| Species | Trehalose | Mannitol | Arabinose | Fructose | References |
| Agaricus campestris L. | 3.62 | 16.94 | _ | nd | Pereira et al. 2012 |
| Armillaria mellea (Vahl) P. Kumm. | 9.33 | 5.45 | 0.78 | _ | Vaz et al. 2011 |
| Boletus aereus Bull. | 1.34 | 4.65 | nd | _ | Heleno et al. 2011 |
| Boletus edulis Bull. | 2.45 | 12.40 | nd | _ | Heleno et al. 2011 |
| Calvatia utriformis (Bull.) Jaap | 0.40 | nd | _ | nd | Grangeia et al. 2011 |
| Coprinus comatus (O.F. Müll.) Pers. | 42.82 | 0.40 | nd | _ | Vaz et al. 2011 |
| Flammulina velutipes (Curtis) Singer | 15.08 | 5.98 | _ | nd | Pereira et al. 2012 |
| Russula olivacea (Schaeff.) Fr. | 0.71 | 15.25 | _ | 0.23 | Grangeia et al. 2011 |
nd, not detected.
Polyols, mainly mannitol, which are responsible for the development and growth of the fruiting bodies (Barros et al. 2008), have half of the calories of common soluble sugars; since they are poorly absorbed by the human body, they do not raise insulin levels in blood and do not promote tooth decay (Dikeman et al. 2005). Glycogen is the reserve polysaccharide of mushrooms but, as it is widely consumed, mainly in meat, its low intake from mushrooms seems to be nutritionally negligible. Other sugars such as fructose and arabinose have been detected in different species of edible mushrooms, generally in lower amounts than mannitol and trehalose (see Table 3.5).
The total dietary fiber (TDF) is the sum of intrinsic nondigestible carbohydrates, soluble and insoluble fractions. The terms “soluble” and “insoluble” have been used in the literature to classify dietary fiber as viscous soluble in water (e.g. pectins) or as water insoluble (e.g. cellulose). In this way, mushrooms include soluble dietary fibers (SDF), such as oligosaccharides (mainly trehalose), β‐glucans and manans, and insoluble dietary fibers (IDF), mainly chitin. The proportion of each dietary fraction varies according to species, but in general terms, IDF shows higher levels than SDF (Manzi et al. 2001). The study of Sanmee et al. (2003) involving 13 species of wild edible mushrooms reported TDF values between 8.3 g/100 g dw for Craterellus odoratus (Schwein.) Fr. and 16.8 g/100 g dw for Heimiella retispora (Pat. & C.F. Baker) Boedijn. Nile and Park (2014), analyzing 20 species of wild growing edible mushrooms in India, reported a TDF range between 24 and 37 g/100 g dw corresponding to Lactarius sanguifluus (Paulet) Fr. and Pleurotus djamor (Rumph. ex Fr.) Boedijn, respectively, an IDF range of 12–21 g/100 g dw and an SDF range of 2–4 g/100 g dw. The composition of TDF in electron beam‐irradiated samples of Macrolepiota procera (Scop.) Singer and Boletus edulis Bull. ranged between 29.1–33.9 g/100 g dw and 26.7–30.8 g/100 g dw, respectively (Fernandes et al. 2015). Wild species of the genus Boletus when raw (dehydrated and rehydrated) showed higher levels of IDF (2.28–8.99 g/100 g edible weight) and SDF (0.32–2.20 g/100 g edible weight) compared with other fresh cultivated species; the effect of cooking on their chitin content was not significant (Manzi et al. 2004).
The fairly high detected levels of dietary fiber in these mushrooms might be considered as a desirable characteristic, since fiber plays an important role in the human diet (EFSA 2010). Insoluble dietary fiber improves the functioning of the digestive tract, by cleaning waste stuck to the intestine walls and increasing fecal volume. Soluble dietary fiber, besides capturing water, diminishes and slows fat and sugar absorption from food, which helps to regulate cholesterol and glucose levels in the blood (Cho 2001).
Regarding water‐insoluble fiber, chitin is a structural N‐containing polysaccharide that accounts for up to 80–90 g/100 g dw in mushroom cell walls (Kalač 2013). Trehalose, as part of SDF, is common to most immature fructification, being a reserve sugar that is metabolized as fructifications mature (see Table 3.5).
3.2.3 Lipids
The content of total lipids (crude fat) is low in mushrooms compared with the other macronutrients (see Table 3.4), and ranges from 0.11 to 8.02 g/100 g dw in wild Agaricus campestris L. (Pereira et al. 2012) and Lactarius deliciosus (L.) Gray (Akata et al. 2012) as reviewed by Kalač (2013). Lipids play a fundamental role in the human body; they act as hormones or as their precursors, helping the digestion process, and constitute a source of metabolic energy (Burtis et al. 2008). In general, crude fat content is represented by all sorts of lipidic compounds, including free fatty acids, monoglycerides, diglycerides, triglycerides, sterols, and phospholipids.
Fatty acids are the basic components of most lipids, and in mushrooms, polyunsaturated linoleic acid (C18:2, ω6), monounsaturated oleic acid (C18:1, ω9) and nutritionally undesirable saturated palmitic acid (C16:0) prevail (Kalač 2009). Many authors report that unsaturated fatty acids predominate over saturated (Barros et al 2008; Ribeiro et al. 2009 Yilmaz et al. 2006). Linoleic (ω6) and α‐linoleic (ω3) acids are essential polyunsaturated fatty acids (PUFA) since they cannot be synthesized by humans and must be ingested with food. Both compounds are highly correlated with metabolic functions, lowering the risk of cardiovascular diseases, triglyceride level, hypertension, and arthritis (Voet & Voet 2004; Wang et al 2003). Though linoleic acid level is generally low in mushrooms (Yilmaz et al. 2006), it greatly contributes to mushroom flavor on account of its role as a precursor of 1‐octen‐3‐ol, which is the main aromatic compound of most mushrooms (Guedes de Pinho et al. 2008; Maga 1981).
Table 3.6 shows some of the main saturated and unsaturated fatty acids present in different wild edible mushroom species. The high proportion of unsaturated fatty acids in Coprinus comatus (O.F. Müll.) Pers. (74.86%) (Vaz et al. 2011), Calvatia utriformis (Bull.) Jaap Pers. (70.29%) (Grangeia et al. 2011), and Agaricus campestris (68.97%) (Pereira et al. 2012) is mainly due to the presence of linoleic acid.
Table 3.6 Total fatty acids composition (relative percentage, %) for some wild edible mushroom species.
| Species | Fatty acids | References | ||||
| C16:0SFA | C16:1MUFA | C18:0SFA | C18:1MUFA | C18:2PUFA | ||
| Agaricus campestris L. | 12.48 | _ | 2.73 | 6.09 | 68.97 | Pereira et al. 2012 |
| Armillaria mellea (Vahl) P. Kumm. | 11.04 | 6.36 | 3.53 | 47.74 | 27.71 | Vaz et al. 2011 |
| Boletus aereus Bull. | 12.47 | 0.58 | 3.80 | 36.72 | 43.83 | Heleno et al. 2011 |
| Boletus edulis Bull. | 9.57 | 0.55 | 3.11 | 42.05 | 41.32 | Heleno et al. 2011 |
| Calvatia utriformis (Bull.) Jaap | 13.54 | 0.22 | 2.43 | 6.00 | 70.29 | Grangeia et al. 2011 |
| Coprinus comatus (O.F. Müll.) Pers. | 10.56 | 0.59 | 1.90 | 6.27 | 74.86 | Vaz et al. 2011 |
| Flammulina velutipes (Curtis) Singer | 10.31 | _ | 1.38 | 15.08 | 56.33 | Pereira et al. 2012 |
| Russula olivacea (Schaeff.) Fr. | 16.13 | 1.31 | 2.78 | 25.99 | 50.20 | Grangeia et al. 2011 |
C16:0, palmitic acid; C16:1, palmitoleic acid; C18:0, stearic acid; C18:1, oleic acid; C18:2, linoleic acid; MUFA, monounsaturated fatty acid; PUFA, polyunsaturated fatty acid; SFA, saturated fatty acids.
3.2.4 Energetic Value/Caloric Content
Mushrooms are appreciated because of their low caloric content, usually 350–400 kcal/100 g dw (Kalač 2013). According to what is shown in Table 3.4, the range of energetic contribution varies from 258.84 to 400.68 kcal/100 g dw for Russula olivacea (Schaeff.) Fr. and Armillaria mellea (Vahl) P. Kumm species, respectively. Considerable differences in the nutritional composition have been reported, not only among species but also within the same species from different origins. The differences found could be partly due to different stages of fruit body development (Kalač 2013) as well as environmental factors that could affect the abundance of certain compounds, but the reason(s) for the variations in the composition of mushroom species collected from background areas remains unclear (Falandysz et al. 2007). Total energetic value has been calculated according to Regulation (EC) No. 1169/2011 of the European Parliament on the provision of food information to consumers:
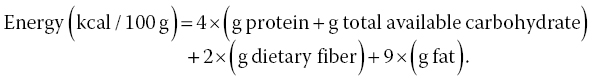
3.2.5 Ash and Mineral Elements
Ash content in wild species is more variable than in cultivated ones, probably due to the diversity of substrates. However, this variability seems to be lower than for proteins, carbohydrates, and lipid content (Kalač 2013).
The amount of ash in wild mushrooms can vary between 5.53 and 37.78 g/100 g dw according to what was recorded in Boletus edulis Bull. and Russula olivacea (Schaeff.) Fr. by Heleno et al. (2011) and Grangeia et al. (2011), respectively (see Table 3.4).
Wild edible mushrooms can accumulate high quantities of macro‐ as well as microelements, which are essential for mushroom development and also for human health. Potassium and phosphorus are usually the predominant elements, followed by calcium, magnesium, sodium, and iron (Okoro & Achuba 2012). Potassium is unevenly distributed within the fructiferous bodies, being more abundant in the cap and less abundant in the spores (Kalač 2013). Usually ash and particularly phosphorus and potassium content is somewhat higher than in most vegetables (Kalač 2013). Table 3.7 shows macro‐ and microelement content for different wild edible mushroom species.
Table 3.7 Composition of macro‐ and microelements (mg/kg dry weight) in different wild edible mushroom species.
| Species | Macroelements | Microelements | References | |||||||
| Na | K | P | Ca | Mg | Fe | Mn | Zn | Cu | ||
| Coprinus comatus (O.F. Müll.) Pers. | 0.8736 | 6.7556 | 4.9412 | 0.0962 | 1.7738 | 0.6889 | 0.02290 | 0.05101 | 0.00456 | Tel et al. 2014 |
| Lepista nuda (Bull.) Cooke | 0.620 | 21.2 | 2.780 | 8.800 | 3.410 | 0.842 | 0.0589 | 0.0755 | 0.0266 | Gençcelep et al. 2009 |
| Morchella esculenta (L.) Pers. | 0.180 | 23.5 | 3.49 | 0.85 | 1.81 | 0.195 | 0.0626 | 0.0989 | 0.0547 | Gençcelep et al. 2009 |
| Pleurotus tuber‐regium (Fr.) | 0.005 | 0.00746 | 0.00446 | 0.00034 | 0.00090 | 0.00240 | 0.00006 | 0.00013 | 0.00004 | Obodai et al. 2014 |
| Suillus granulatus (L.) Roussel | 0.3447 | 2.2503 | 3.0913 | 0.1208 | 1.1326 | 6.028 | 0.2282 | 0.3547 | 0.00213 | Tel et al. 2014 |
| Suillus granulatus (L.) Roussel | 0.15 | 29.1 | 4.49 | 0.46 | 2.74 | 0.458 | 0.0303 | 0.169 | 0.107 | Gençcelep et al. 2009 |
| Termitomyces robustus (Beeli) R. Heim | 0.0029 | 0.02030 | 0.00405 | 0.00040 | 0.00072 | 0.00224 | 0.00007 | 0.00015 | 0.00005 | Obodai et al. 2014 |
| Tricholoma terreum (Schaeff.) P. Kumm. | 1.0612 | 3.3335 | 2.6557 | 0.2109 | 0.9106 | 0.1109 | 0.03094 | 0.04278 | 0.00205 | Tel et al. 2014 |
Different authors such as Ayodele and Odogbili (2010), Aloupi et al. (2012), and Kalač (2010) point out the presence of heavy metals such as lead, cadmium, mercury, nickel, and chrome, whose consumption may produce toxicological effects in humans. Mushrooms can accumulate heavy metals whose levels will depend on species, substrate composition, and environmental factors (Kalač & Svoboda 2000). However, details on toxicological risk and nutritional evaluation of such substances are limited in mushrooms.
3.3 Vitamins
Mushrooms contain different B‐complex vitamins, such as thiamine (B1), riboflavin (B2), and niacin (B3). They also contain chemical compounds with antioxidant properties such as ergosterol (vitamin D precursor), β‐carotene (provitamin A precursor), tocopherols (vitamin E), and ascorbic acid (vitamin C) (Cheung 2010; Heleno et al. 2012; Kalač 2013). For several species, the content range of thiamine was 0.02–1.6 mg/100 g dw, riboflavin 0.3–4.5 mg/100 g dw, niacin 1.2–6.6 mg/100 g dw, and ascorbic acid 1.3–2.7 mg/100 g dw (Quan et al. 2007; Wu et al. 2005; Yin & Zhou 2008; Zhou & Yin 2008).
Ergosterol turns into viosterol under ultraviolet light, and then into ergocalciferol, which is a form of vitamin D. Ergosterol is a cell membrane component in mushrooms, and fulfills the same function as cholesterol in animal cells. A relatively high content of ergosterol could be important for people who have a limited intake of cholecalciferol or vitamin D, for example vegans and vegetarians (Kalač 2013). Ergosterol was the most abundant sterol found in wild Cantharellus cibarius Fr. and Boletus edulis Bull., with 0.17–0.35 g/100 g dw (Teichmann et al. 2007). Mattila et al. (2002) found that ergosterol content was higher in cultivated mushrooms (0.60–0.68 g/100 g dw) than in wild Cantharellus cibarius, Cantharellus tubaeformis Fr., Boletus edulis, and Lactarius trivialis (Fr.) Fr. (0.29–0.49 g/100 g dw) and was similar to levels reported in Huang et al. (1985) and Koyama et al. (1984).
β‐Carotene is a provitamin A precursor with antioxidant properties, which participate in free radical inhibition, thus preventing cell aging. This compound has been detected in variable amounts in wild mushrooms, Agaricus campestris and A. comtulus Fries presenting 0.6 and 0.7 mg/100 g dw, respectively, while Clitocybe costata Kühner & Romagn. yielded 0.07 mg/100 g dw, according to Pereira et al. (2012).
Tocopherols are one of the most widely studied vitamin groups; they protect the human body from effects related to oxidative stress such as cardiovascular diseases and cancer, due to their capacity to eliminate free radicals (Ferreira et al. 2010). Cultivated species generally present lower total tocopherol content than wild species (Kalač 2013). Moreover, total tocopherol content varies with each wild fungi species. High levels of total tocopherols have been detected in Suillus luteus (O.F. Müll.) Pers. (0.45 mg/100 g dw), Cortinarius violaceus (L.) Gray (0.35 mg/100 g dw), and Coprinus comatus (O.F. Müll.) Pers. (0.30 mg/100 g dw) (Reis et al. 2011; Vaz et al. 2011), while Lepista sordida (Schumach.) Singer had a very low total tocopherol content (0.002 mg/100 g dw; Heleno et al. 2010).
Low levels of ascorbic acid are present in different species. Values ranging between 0.66 and 33.16 mg/100 g dw in Hygrophorus chrysodon (Batsch) Fr. and Ramaria aurea (Schaeff.) Quél. have been reported (Pereira et al. 2012).
3.4 Conclusion
Fungi species described in this chapter are, in some cases, widely used and consumed by people from different regions of the world.
The available data summarized in this chapter indicate that wild edible mushrooms constitute an excellent nutrient source for humans, especially in low‐caloric diets due to their low fat content and energetic value, and suitable for people with high cholesterol levels. This is thought to be due to the diversity of unsaturated fatty acids, relevant for metabolic pathways and human health. In addition to this, mushrooms are rich in proteins, amino acids, carbohydrates, dietary fiber, minerals, and vitamins. According to the Dietary Guidelines Advisory Committee on the Dietary Guidelines for Americans (USDA 2010), a 2000 calorie diet should contain 90 g of crude protein daily, and a 100 g portion of dry wild mushrooms could provide between 13.33% and 66% that (Barros et al. 2008). The recommended carbohydrate amount is 260 g daily, and a 100 g portion of dry mycorrhiza mushrooms contributes around 6.15–16.15% of the daily requirement, while 100 g of dry saprotrophic mushrooms contributes 0.15–5.76% of the daily requirement (Grangeia et al. 2011). The recommended dietary fiber amount is 30 g, and a 100 g portion of dry wild mushrooms provides 27.6–123.3% of the daily requirement (Nile & Park 2014; Sanmee et al. 2003). The recommended amount of lipids is 71 g, and 100 g of dry wild mushrooms provides 0.15–11.29% of the daily requirement (Akata et al. 2012; Pereira et al. 2012). Regarding vitamins, the daily recommended amounts are thiamine 1.8 g, riboflavin 2.2 mg, niacin 23 mg, and ascorbic acid 126 mg. A 100 g portion of dried wild mushrooms can provide 1.11–88.88% of the daily thiamine required (Quan et al. 2007), 13.63–204.4% of the daily riboflavin requirement (Wu et al. 2005), 5.21–28.69% of the daily niacin requirement (Yin & Zhou 2008), and 1.03–2.14% of the daily ascorbic acid requirement (Zhou & Yin 2008).
There is great variability in the intraspecific nutritional values reported for wild mushroom species compared to cultivated ones. This is related to the possibility of manipulating and standardizing different stages during production processes. The possibility of genetically selecting particular strains, using different additives in growth substrates, which allow for improving and homogenizing nutrient content, as well as manipulating certain environmental conditions such as light, humidity, and temperature allow for decreasing variability and manipulating concentrations. Even though several studies on the effects of using irradiation techniques on nutrient composition have been recently published (Fernandes et al. 2012, 2013), future research should elucidate aspects such as processing effects on nutrient contents, as well as nutrient bioavailability.
References
- Agrahar‐Murugkar, D. & Subbulakshmi, G. (2005) Nutritional value of edible wild mushrooms collected from the Khasi hills of Meghalaya. Food Chemistry 89, 599–603.
- Akata, I., Ergonul, B. & Kalyoncu, F. (2012) Chemical compositions and antioxidant activities of 16 wild edible mushroom species grown in Anatolia. International Journal of Pharmacology 8, 134–138.
- Aletor, V. A. (1995) Compositional studies on edible tropical species of mushrooms. Food Chemistry 54, 265–268.
- Aloupi, M., Koutrotsios, G., Koulousaris, M. & Kalogeropoulos, N. (2012) Trace metal contents in wild edible mushrooms growing on serpentine and volcanic soils on the island of Lesvos, Greece. Ecotoxicology and Environmental Safety 78, 184–194.
- AOAC (2005) Official Methods of Analysis, 16th edn. Arlington: Association of Official Analytical Chemists.
- Ayaz, F. K. A., Chuang, L. T., Torun, H., Colak, A., Sesli, E., Presley, J., Smith, B. R. & Glew, R. H. (2011) Fatty acid and amino acid compositions of selected wild‐edible mushrooms consumed in Turkey. International Journal of Food Sciences and Nutrition 62, 328–335.
- Ayodele, S. M. & Odogbili, O. D. (2010) Metal impurities in three edible mushrooms collected in Abraka, Delta State, Nigeria. Micología Aplicada International 22, 27–30.
- Barros, L., Baptista, P., Correira, D. M., Casa, S., Oliveira, B. & Ferreira, I. C. F. R. (2007a) Fatty acid and sugar compositions, and nutritional value of five wild edible mushrooms from Northeast Portugal. Food Chemistry 105, 140–145.
- Barros, L., Baptista, P., Correia, D. M., Morais, J. S. & Ferreira, I. C. F. R. (2007b) Effects of conservation treatment and cooking on the chemical composition and antioxidant activity of Portuguese wild edible mushrooms. Journal of Agricultural and Food Chemistry 55, 4781–4788.
- Barros, L., Venturini, B. A., Baptista, P., Estevinho, L. M. & Ferreira, I. C. F. R. (2008) Chemical composition and biological properties of Portuguese wild mushrooms: a comprehensive study. Journal of Agricultural and Food Chemistry 56, 38563862.
- Bauer‐Petrovska, B. (2001) Protein fractions in edible Macedonian mushrooms. European Food Research and Technology 212, 469–472.
- Boa, E. (2004) Wild Edible Fungi. A Global Overview of Their Use and Importance to People. Nonwood Forest Products, vol. 17. Rome: FAO.
- Bobek, P. & Galbavy, S. (1999). Hypercholesterolemic and anti‐atherogenic effect of oyster mushroom (Pleurotus ostreatus) in rabbit. Nahrung 45, 339–342.
- Bobek, P., Ginter, E., Jurcovicova, M. & Kunia, K. (1991) Cholesterol lowering effect of the mushroom Pleurotus ostreatus in hereditary hypercholesterolemic rats. Annals of Nutritional Metabolism 35, 191–195.
- Bobek, P., Ozdyn, L. & Kuniak, L. (1995) The effect of oyster mushroom (Pleurotus ostreatus), its ethanolic extract and extraction residues on cholesterol levels in serum lipoproteins and liver of rat. Nahrung 39, 98–99.
- Breene, W. M. (1990) Nutritional and medicinal value of specialty mushrooms. Journal of Food Protection 53, 883–894.
- Burtis, C. A., Ashwood, E. R. & Bruns, D. E. (2008) Tietz Fundamentals of Clinical Chemistry, 4th edn. Philadelphia: W. B. Saunders Company.
- Chang, S. T. & Miles, P. G. (2004) The nutritional attributes of edible mushrooms. In: Mushrooms: Cultivation, Nutritional Value, Medicinal Effect, and Environmental Impact, 2nd edn. Boca Raton: CRC press, pp 27–37.
- Cheung, P. C. K. (2010) The nutritional and health benefits of mushrooms. Nutrition Bulletin 35, 292–299.
- Cho, S. S., ed. (2001) Handbook of Dietary Fiber, vol. 113. Boca Raton: CRC Press.
- Chudzyński, K. & Falandysz, J. (2008) Multivariate analysis of elements content of Larch Bolete (Suillus grevillei) mushroom. Chemosphere 73, 1230–1239.
- Colak, A., Kolcuoglu, Y., Sesli, E. & Dalman, O. (2007) Biochemical composition of some Turkish fungi. Asian Journal of Chemistry 19, 2193–2199.
- Colak, A., Faiz, O. & Sesli, E. (2009) Nutritional composition of some wild edible mushrooms. Turkish Journal of Biochemistry‐Turk Biyokimya Dergisi 34, 25–31.
- Crisan, E. V. & Sands, A. (1978) Nutritional value. In: S. T. Chang & W. A. Hayes, eds. The Biology and Cultivation of Edible Mushrooms. New York: Academic Press, pp 137–168.
- Díez, V. A. & Alvarez, A. (2001) Compositional and nutritional studies on two wild edible mushrooms from northwest Spain. Food Chemistry 75, 417–422.
- Dikeman, C. L., Bauer, L. L., Flickinger, E. A. & Fahey, G. C., Jr (2005) Effects of stage of maturity and cooking on the chemical composition of select mushroom varieties. Journal of Agricultural and Food Chemistry 53, 1130–1138.
- EFSA Panel on Dietetic Products, Nutrition, and Allergies (NDA) (2010) Scientific opinion on dietary reference values for carbohydrates and dietary fibre. EFSA Journal 8 (3), 1462.
- Estrada‐Martínez, E., Guzmán, G., Cibrián Tovar, D. & Ortega Paczka, R. (2009) Contribución al conocimiento etnomicológico de los hongos comestibles silvestres de mercados regionales y comunidades de La Sierra Nevada (México). Interciencia 34, 25–33.
- FAO/WHO (1991) Protein Quality Evaluation in Human Diets. Report of a Joint FAO/WHO Expert Consultation. Food and Nutrition, vol. 51. Rome: Food and Agricultural Organization and the World Health Organization.
- Falandysz, J., Kunito, T., Kubota, R., Bielawski, L., Mazur, A., Falandysz, J. J. & Tanabe, S. (2007) Selected elements in Brown Birch Scaber Stalk Leccinum scabrum. Journal of Environmental Sciences and Health A 42, 2081–2088.
- Fernandes, Â., Barreira, J., Antonio, A. L., Oliveira, M. B. P. P., Martins, A. & Ferreira, I. C. F. R. (2012) Combined effects of γ‐irradiation and storage times on sugars composition of Lactarius deliciosus: comparison through linear discrimant analysis. Presented at the 7th International Conference on Simulation and Modelling in the Food and Bio‐Industry. Freising, Alemanha.
- Fernandes, Â., Antonio, A. L., Barreira, J. C., Botelho, M. L., Oliveira, M. B. P., Martins, A. & Ferreira, I. C. F. R. (2013) Effects of gamma irradiation on the chemical composition and antioxidant activity of Lactarius deliciosus L. wild edible mushroom. Food and Bioprocess Technology 6, 2895–2903.
- Fernandes, Â., Barreira, J. C., Antonio, A. L., Morales, P., Férnandez‐Ruiz, V., Martins, A., Oliveira, M. B. P. P. & Ferreira, I. C. F. R. (2015) Exquisite wild mushrooms as a source of dietary fiber: analysis in electron‐beam irradiated samples. LWT‐Food Science and Technology 60, 855–859.
- Ferreira, I. C. F. R., Vaz, A., Vasconcelos, J. M. H. & Martins, A. (2010) Compounds from wild mushrooms with antitumor potential. Anti‐Cancer Agents in Medicinal Chemistry 10, 424–436.
- Gençcelep, H., Uzun, Y., Tunçtürk, Y. & Demirel, K. (2009) Determination of mineral contents of wild‐grown edible mushrooms. Food Chemistry 113, 1033–1036.
- Grangeia, C., Heleno, S. A., Barros, L., Martins, A. & Ferreira, I. C. F. R. (2011) Effects of trophism on nutritional and nutraceutical potential of wild edible mushrooms. Food Research International 44, 1029–1035.
- Gruen, E. H. & Wong, M. W. (1982) Distribution of celular amino acids, protein and total inorganic nitrogen during fruit body development in Flammulina veluptipes. Canadian Journal of Botany 60, 1330–1341.
- Guedes de Pinho, P., Ribeiro, B., Gonçalves, R. F., Baptista, P., Valentão, P., Seabra, R.m. & Andrade P. B. (2008) Correlation between the pattern of volatiles and the overall aroma of wild edible mushrooms. Journal of Agriculture and Food Chemistry 56, 1704–1712.
- Hall, I. R., Yun, W. & Amicucci, A. (2003) Cultivation of edible ectomycorrhizal mushrooms. Trends in Biotechnology 21, 433–438.
- Heleno, S. A., Barros, L., Sousa, M. J., Martins, A. & Ferreira, I. C. F. R. (2009) Study and characterization of selected nutrients in wild mushrooms from Portugal by gas chromatography and high performance liquid chromatography. Microchemical Journal 93, 195–199.
- Heleno, S.A., Barros, L., Sousa, M. J., Martins, A. & Ferreira, I. C. F. R. (2010) Tocopherols composition of Portuguese wild mushrooms with antioxidant capacity. Food Chemistry 119, 1443–1450.
- Heleno, S. A., Barros, L., Sousa, M. J., Martins, A., Santos‐Buelga, C. & Ferreira, I. C. F. R. (2011) Targeted metabolites analysis in wild Boletus species. LWT Food Science and Technology 44, 1343–1348.
- Heleno, S. A., Barros, L., Martins, A., Queiroz, M. J. R., Santos‐Buelga, C. & Ferreira, I. C. F. R. (2012) Phenolic, polysaccharidic, and lipidic fractions of mushrooms from northeastern Portugal: chemical compounds with antioxidant properties. Journal of Agricultural and Food Chemistry 60, 4634–4640.
- Hosford, D., Pilz, D., Molina, M. & Amaranthus, M. (1997) Ecology and Management of the Commercially Harvested American Matsutake Mushroom. USDA General Technical Report. Portland: Department of Agriculture, Forest Service.
- Huang, B.H., Yung, K.H. & Chang, S.T. (1985) The sterol composition of Volvariella volvacea and other edible mushrooms. Mycologia 77, 959–963.
- Kalač, P. (2009) Chemical composition and nutritional value of European species of wild growing mushrooms: a review. Food Chemistry 113, 9–16.
- Kalač, P. (2010) Trace element contents in European species of wild growing edible mushrooms: a review for the period 2000–2009. Food Chemistry 122, 2–15.
- Kalač, P. (2013) A review of chemical composition and nutritional value of wild‐growing and cultivated mushrooms. Journal of the Science of Food and Agriculture 93, 209–218.
- Kalač, P. & Svoboda, L. (2000) A review of trace element concentrations in edible mushrooms. Food Chemistry 69, 273–281.
- Kim, M. Y., Chung, L. M., Lee, S. J., Ahn, J. K., Kim, E. H., Kim, M. J., Kim, S. L., Moon, H. I., Ro, H. M., Kang, E. Y., Seo, S. H. & Song, H. K. (2009) Comparison of free amino acid, carbohydrates concentrations in Korean edible and medicinal mushrooms. Food Chemistry 113, 386–393.
- Koyama, N., Aoyagi, Y. & Sugahara, T. (1984) Fatty acid composition and ergosterol contents of edible mushrooms. Nippon Shokuhin Kogyo Gakkaishi 31, 732–738.
- Lehninger, A. L., Nelson, D. L. & Cox, M. M. (2008) Principles of Biochemistry, 5th edn. New York: W.H. Freeman.
- León‐Guzmán, M. F., Silva, I. & López, M. G. (1997) Proximate chemical composition, free amino acid contents, and free fatty acid contents of some wild edible mushrooms from Querétaro, México. Journal of Agricultural and Food Chemistry 45, 4329–4332.
- Liu, Y. T., Sun, J., Luo, Z. Y., Rao, S. Q., Su, Y. J., Xu, R. R. & Yang, Y. J. (2012) Chemical composition of five wild edible mushrooms collected from Southwest China and their antihyperglycemic and antioxidant activity. Food and Chemical Toxicology 50, 1238–1244.
- Maga, J. (1981) Mushroom flavor. Journal of Agriculture and Food Chemistry 29, 1–4.
- Manzi, P., Aguzzi A. & Pizzoferrato, L. (2001) Nutritional value of mushrooms widely consumed in Italy. Food Chemistry 73, 321–325.
- Manzi, P., Marconi, S., Aguzzi, A. & Pizzoferrato, L. (2004) Commercial mushrooms: nutritional quality and effect of cooking. Food Chemistry 84, 201–206.
- Martínez‐Carrera, D. (2010) Hacia un Desarrollo Sostenible del Sistema de Producción‐Consumo de los Hongos Comestibles y Medicinales en Latinoamérica: Avances y Perspectivas en el Siglo XXI. Red Latinoamericana de Hongos Comestibles y Medicinales. Puebla: COLPOS‐UNS‐CONACYTAMCUAEM‐UPAEP‐IMINAP.
- Mau, J. L., Lin, H. C. & Chen, C. C. (2001) Non‐volatile components of several medicinal mushrooms. Food Research International 34, 521–526.
- Mattila, P., Lampi, A.M., Ronkainen, R., Toivo, J. & Piironen, V. (2002) Sterol and vitamin D2 contents in some wild and cultivated mushrooms. Food Chemistry 76, 293–298.
- Mdachi, S. J., Nkunya, M. H., Nyigo, V. A. & Urasa, I. T. (2004) Amino acid composition of some Tanzanian wild mushrooms. Food Chemistry 86, 179–182.
- Nile, S. H. & Park, S. W. (2014) Total, soluble, and insoluble dietary fibre contents of wild growing edible mushrooms. Czech Journal of Food Sciences 32, 302–307.
- Obodai, M., Ferreira, I. C. F. R., Fernandes, Â., Barros, L., Narh Mensah, D. L., Dzomeku, M., Urben, A. F., Prempeh, J. & Takli, R. K. (2014) Evaluation of the chemical and antioxidant properties of wild and cultivated mushrooms of Ghana. Molecules 19, 19532–19548.
- Okoro, I. O. & Achuba, F. I. (2012) Proximate and mineral analysis of some wild edible mushrooms. African Journal of Biotechnology 11, 7720–7724.
- Ouzouni, P. K. & Riganakos, K. A. (2007) Nutritional value and metal content profile of Greek wild edible fungi. Acta Alimentaria 36, 99–110.
- Pereira, E., Barros, L., Martins, A. & Ferreira, I. C. F. R. (2012) Towards chemical and nutritional inventory of Portuguese wild edible mushrooms in different habitats. Food Chemistry 130, 394–403.
- Quan, X. L., Wang, H. J., Shi, T. Y. & Zhang, M. S. (2007) Nutritive components comparison between Tricholoma matsutake and Tricholoma bakamatsutake. Edible Fungi 2, 54–55.
- Regulation (EC) No. 1169/2011 of the European Parliament and of the Council of 25 October 2011 on the provision of food information to consumers. Official Journal of the European Union, 304, 18e63 (L 304/18‐63).
- Reis, F. S., Heleno, S. A., Barros, L., Sousa, M. J., Martins, A., Santos‐Buelga, C. & Ferreira, I. C. F. R. (2011) Toward the antioxidant and chemical characterization of mycorrhizal mushrooms from Northeast Portugal. Journal of Food Science 76, 824–830.
- Reis, F. S., Barros, L., Martins, A. & Ferreira, I. C. F. R. (2012) Chemical composition and nutritional value of the most widely appreciated cultivated mushrooms: an inter‐species comparative study. Food and Chemical Toxicology 50, 191–197.
- Ribeiro, B., Guedes de Pinho, P., Andrade, P. B., Baptista, P. & Valentão, P. (2009) Fatty acid composition of wild edible mushrooms species: a comparative study. Microchemical Journal 93, 29–35.
- Ruan‐Soto, F., Garibay‐Orijel, R. & Cifuentes J. (2004) Conocimiento micológico tradicional en la planicie costera del Golfo de México. Revista Mexicana de Micología 19, 57–70.
- Sanmee, R., Dell, B., Lumyong, P., Izumori, K. & Lumyong, S. (2003) Nutritive value of popular wild edible mushrooms from northern Thailand. Food Chemistry 82, 527–532.
- Teichmann, A., Dutta, P. C., Staffas, A. & Jägerstad, M. (2007) Sterol and vitamin D2 concentrations in cultivated and wild grown mushrooms: effects of UV irradiation. LWT‐Food Science Technology 40, 815–822.
- Tel, G., Çavdar, H., Deveci, E., Öztürk, M., Duru, M. E. & Turkoğlu, A. (2014) Minerals and metals in mushroom species in Anatolia. Food Additives and Contaminants 7, 226–231.
- USDA Center for Nutrition Policy and Promotion (2010) Report of the Dietary Guidelines Advisory Committee on the Dietary Guidelines for Americans, 2010. Alexandria: USDA Center for Nutrition Policy and Promotion. Available at: www. cnppusdagov/DGAs2010DGACReport.htm (accessed 15 June 2016).
- Vaz, J. A., Barros, L., Martins, A., Santos‐Buelga, C., Vasconcelos, M. H. & Ferreira, I. C. F. R. (2011) Chemical composition of wild edible mushrooms and antioxidant properties of their water soluble polysaccharidic and ethanolic fractions. Food Chemistry 126, 610–616.
- Vetter, J. & Rimóczi, I. (1993) Crude, digestible and indigestible protein in fruiting bodies of Pleurotus ostreatus. Zeitschrift für Lebensmittel Untersuchung und Forschung 197, 427–428.
- Villarreal, L. & Pérez‐Moreno, J. (1989) Los hongos comestibles silvestres de México, un enfoque integral. Micología Neotropical Aplicada 2, 77–114.
- Voet, D. & Voet, J. G. (2004) Biochemistry, 3rd edn. New York: Wiley & Sons.
- Wang, Y. C. (1987) Mycology in ancient China. Mycologist 1, 59–61.
- Wang, L., Folsom, A. R. & Eckfeldt, J. H. (2003) Plasma fatty acid composition and incidence of coronary heart disease in middle aged adults: the Atherosclerosis Risk in Communities (ARIC) Study. Nutrition, Metabolism and Cardiovascular Diseases 13, 256–266.
- Wang, X. M., Zhang, J., Wu, L. H., Wanga, X. M., Zhang, J., Wub, L. H., Zhaob, Y.L. Lic, T., Lia, J. Q., Wang, Y. Z. & Liu, H. G. (2014) A mini‐review of chemical composition and nutritional value of edible wild‐grown mushroom from China. Food Chemistry 151, 279–285.
- Wong, J. L. G., Thornber K. & Baker, N. (2001) Resource Assessment of Non‐wood Forest Products: Experience and Biometric Principles. Non‐wood Forest Products Vol. 13. Rome: Food and Agriculture Organization.
- Wu, S. X., Wang, B. X., Guo, S. Y., Li, L. & Yin, J. Z. (2005) Yunnan wild edible Thelehhora ganhajun Zang nutrients analysis. Modem Preventive Medicine 32, 1548–1549.
- Yilmaz, N., Solmaz, M., Türkedul, I. & Elmastaş, M. (2006) Fatty acid composition in some wild edible mushrooms growing in the middle Black Sea region of Turkey. Food Chemistry 99, 168–174.
- Yin, J. Z. & Zhou, L. X. (2008) Analysis of nutritional components of 4 kinds of wild edible fungi in Yunnan. Food Research and Development 29, 133–136.
- Zhang, B. Q. & Chen, J. (2011) Determination and analysis of nutrition components in Sarcodon aspratus. Food Science 32, 299–302.
- Zhou, L. X. & Yin, J. Z. (2008) Yunnan wild edible Boletus nutrition analysis and evaluation. Edible Fungi 4, 61–62.