12
Nuts as Sources of Nutrients
João C. M. Barreira1,2, M. Beatriz P. P. Oliveira2, and Isabel C. F. R. Ferreira1
1 Mountain Research Centre (CIMO), School of Agriculture, Polytechnic Institute of Bragança, Portugal
2 REQUIMTE/LAQV, Faculty of Pharmacy, University of Porto, Portugal
As an introductory note, it should be highlighted that the chemical composition of nuts depends greatly on genotypic and environmental factors, such as growing region, cultivation methods, climatic conditions, harvesting years, and kernel ripeness (Muhammad et al. 2015; Yada et al. 2011). Therefore, some of the indicated values for typical parameters are characterized by having a wide variation range, due to the great variability of results among research groups.
12.1 Prunus dulcis (Miller) D. A. Webb (almond)
12.1.1 Botanical Aspects and Geographical Distribution
Almond, which belongs to the genus Prunus and the subgenus Amygdalus (Rosaceae), is a nutritionally important crop grown in many temperate and subtropical regions in the world. The cultivated almond is designated as Prunus dulcis (Miller) D. A. Webb, despite being also known as Prunus amygdalus Batsch and Prunus communis L., or less commonly as Amygdalus communis L. (USDA 2010).
Almond is one of the oldest cultivated nut trees in the world and a major nut tree crop in hot arid countries of the Mediterranean basin, although it spreads around the area included between the 36th and 45th parallels (Nanos et al. 2002).
12.1.2 Main Applications and Nutritional Overview
Almond seeds are classified as nuts, and are widely used, especially for direct consumption after toasting and for the confectionery industry and the production of sweets, cakes, and sugar‐coated almonds (Nanos et al. 2002). Almonds are eaten raw, roasted, and fried but they can also be used as ingredients in products such as sauces and snacks and marzipan and almond crunch. More recently, almonds are also being processed to make nutritional products such as almond milk used as a substitute for cow’s milk (Aranceta et al. 2006). In each case, the chemical composition is of great importance to establish nutritive value and quality to satisfy the concerns of consumers wanting a healthy lifestyle. The quality of nuts is defined in particular by moisture level, lipid content, oil composition, and oil ultraviolet absorption coefficients (Nanos et al. 2002).
Almonds are one of the most popular nut crops, being significant for human nutrition and health. These nuts are a nutrient‐rich source of lipids, protein, dietary fiber, minerals, vitamins, and polyphenols. The high nutritive value of almond kernels arises mainly from their high lipid content, which constitutes an important caloric source, without contributing to cholesterol formation in humans. Almond oil has been reported to be very rich in monounsaturated fatty acids, especially oleic acid, whereas saturated fatty acids are very low (Kodad et al. 2014).
Considering studies performed in Portuguese (Barreira et al. 2012a), American (Ahrens et al. 2005; Chen et al. 2006; López‐Ortiz et al. 2008; Martín‐Carratalá et al. 1998; Venkatachalam & Sathe 2006), Irish (Maguire et al. 2004), Spanish (Ahrens et al. 2005; Cherif et al. 2009; Piscopo et al. 2010), Italian (Ahrens et al. 2005; Martín‐Carratalá et al. 1998; Piscopo et al. 2010), French (Ahrens et al. 2005; Piscopo et al. 2010), Australian (Ahrens et al. 2005) and Tunisian (Ahrens et al. 2005; Martín‐Carratalá et al. 1998) samples, almonds are characterized by high amounts of fat (42–57%), protein (19–23%), carbohydrates (20–27%), and fiber (11–15%), and low amounts of moisture (3–9%) and ash (2.5–4.5%). Almonds are also acknowledged for their minerals, vitamins, and polyphenols (Yada et al. 2011).
12.1.3 Major Components
The relevant compounds studied in almond are detailed in Table 12.1, as well as the specific methodologies applied in each case.
Table 12.1 Compounds (ordered alphabetically) analyzed in each of the nuts studied in this chapter.
| Source | Compounds | Analysis method | Reference |
| Almond | Dietary fiber | Gravimetry | Yada et al. 2013 |
| Ellagitannins; gallotannins | HPLC‐DAD/FD | Xie et al. 2012 | |
| Fatty acids | GC‐FID | Askin et al. 2007; Barreira et al. 2012a; Cherif et al. 2009; García‐López et al. 1996; Kodad et al. 2014; Madawala et al. 2012; Maguire et al. 2004; Martín‐Carratalá et al. 1998; Matthäus & Özcan 2009; Piscopo et al. 2010; Soler et al. 1988; Venkatachalam & Sathe 2006; Zhu et al. 2015 | |
| GC‐MS | Di Stefano et al. 2014 | ||
| Minerals | ICP‐AES | Prats‐Moya et al. 1997; Yada et al. 2013 | |
| AAS | Saura‐Calixto & Cañellas 1982; Piscopo et al. 2010 | ||
| Phenolic compounds | LC‐DAD/FD MALDI‐TOF‐MS |
Bartolomé et al. 2010 – |
|
| HPLC‐FD/MS | Xie et al. 2012 | ||
| Proteins | Kjeldahl digestion | Ahrens et al. 2005; Askin et al. 2007; Kumar & Sharma 2005; Sathe 1993; Venkatachalam & Sathe 2006 | |
| Sterols | HPLC‐DAD | Maguire et al. 2004 | |
| GC‐FID | Cherif et al. 2009; Yada et al. 2013 | ||
| GC‐MS | Madawala et al. 2012 | ||
| Stilbenes | UHPLC‐MS | Xie & Bolling 2014 | |
| Sugars | Enzymatic kit | Amrein et al. 2005 | |
| Spectrophotometry | Venkatachalam & Sathe 2006 | ||
| HPLC‐RI | Balta et al. 2009; Barreira et al. 2010; Kazantzis et al. 2003 | ||
| Tocopherols | HPLC‐DAD/FD | Barreira et al. 2012a; Kodad et al. 2011, 2014; López‐Ortiz et al. 2008; Maguire et al. 2004; Matthäus & Özcan 2009; Zhu et al. 2015 | |
| RP‐HPLC‐UV | Kornsteiner et al. 2006 | ||
| RP‐HPLC‐FD | Di Stefano et al. 2014 | ||
| Triacylglycerols | HPLC‐ELSD | Barreira et al. 2012a | |
| HPLC‐RI | Martín‐Carratalá et al. 1999; Prats‐Moya et al. 1999 | ||
| Vitamin B complex | Microbiological assay | Daoud et al. 1977; Hoppner et al. 1994; Yada et al. 2013 | |
| HPLC | Rizzolo et al. 1991 | ||
| Fluorometry | Yada et al. 2013 | ||
| RP‐HPLC‐UV | Vasconcelos et al. 2007 | ||
| Chestnut | Ascorbic acid | HPLC‐UV | Pereira‐Lorenzo et al. 2005 |
| Carotenoids | HPLC‐DAD | Pereira‐Lorenzo et al. 2005 | |
| Fatty acids | GC‐FID | Barreira et al. 2009b, 2012b; Borges et al. 2007; Fernandes et al. 2011 | |
| Dietary fiber | Gravimetry | Barreira et al. 2009b, 2012b; Gonçalves et al. 2010; Vasconcelos et al. 2007 | |
| Minerals | AAS | Borges et al. 2008 | |
| ICP‐AES | Pereira‐Lorenzo et al. 2005 | ||
| Organic acids | UFLC‐PDA | Carocho et al. 2013 | |
| Spectrophotometry | Gonçalves et al. 2010 | ||
| HPLC‐UV | Ribeiro et al. 2007 | ||
| Phenolic compounds | RP‐HPLC‐UV | Gonçalves et al. 2010; Vasconcelos et al. 2007 | |
| Protein | Kjeldahl digestion | Barreira et al. 2009b, 2012b; Gonçalves et al. 2010 | |
| Starch | SEM‐LFD | Cruz et al. 2013 | |
| Spectrophotometry | Correia et al. 2012; Demiate et al. 2001; Vasconcelos et al. 2007 | ||
| Sugars | PA | Pereira‐Lorenzo et al. 2005 | |
| HPLC‐RI | Barreira et al. 2010; Fernandes et al. 2011 | ||
| HPLC‐ELSD | Bernárdez et al. 2004 | ||
| Tocopherols | HPLC‐FD | Fernandes et al. 2011; Pereira‐Lorenzo et al. 2005 | |
| HPLC‐DAD/FD | Barreira et al. 2009a,b, 2012b | ||
| Triacylglycerols | HPLC‐ELSD | Barreira et al. 2009b, 2012b, 2013 | |
| Hazelnut | Carotenoids | HPLC‐UV | Kornsteiner et al. 2006 |
| Fatty acids | GC‐FID | Bignami et al. 2005; Botta et al. 1994; Ciemniewska‐Żytkiewicz et al. 2015; Köksal et al. 2006; Madawala et al. 2012; Oliveira et al. 2008; Parcerisa et al. 1998; Seyhan et al. 2007; Venkatachalam & Sathe 2006 | |
| GLC‐FID | Amaral et al. 2006a; Parcerisa et al. 1998 | ||
| Minerals | AAS | Açkurt et al. 1999; Alasalvar et al. 2009; Köksal et al. 2006; Seyhan et al. 2007 | |
| Organic acids | HPLC‐UV | Botta et al. 1994 | |
| Phenolic compounds | HPLC‐DAD‐MS | Jakopic et al. 2011; Slatnar et al. 2014 | |
| RP‐HPLC‐DAD‐MS | Ciemniewska‐Żytkiewicz et al. 2015 | ||
| HPLC‐PDA | Shahidi et al. 2007 | ||
| Proteins | Kjeldahl digestion | Köksal et al. 2006 | |
| Sterols | GLC‐FID | Alasalvar et al. 2009; Amaral et al. 2006a; Parcerisa et al. 1998 | |
| GC‐MS | Ciemniewska‐Żytkiewicz et al. 2015; Madawala et al. 2012 | ||
| Sugars | Spectrophotometry | Venkatachalam & Sathe 2006 | |
| Tocopherols | GLC‐FID | Parcerisa et al. 1998 | |
| RP‐HPLC‐UV | Ciemniewska‐Żytkiewicz et al. 2015; Kornsteiner et al. 2006 | ||
| HPLC‐PDA | Alasalvar et al. 2009; Bignami et al. 2005; Köksal et al. 2006 | ||
| Triacylglycerols | HPLC‐ELSD | Alasalvar et al. 2009; Amaral et al. 2006b | |
| Vitamin B complex | Microbiological assay | Açkurt et al. 1999 | |
| HPLC‐PDA | Köksal et al. 2006 | ||
| Walnut | Carotenoids | HPLC‐DAD | Abdallah et al. 2015 |
| Fatty acids | GC‐FID | Amaral et al. 2003; Bouabdallah et al. 2014; Li et al. 2007; Madawala et al. 2012; Rabrenovic et al. 2008; Tapia et al. 2013; Verardo et al. 2009 | |
| Minerals | AAS | Lavedrine et al. 2000; Tapia et al. 2013 | |
| Phenolic compounds | HPLC‐DAD | Colaric et al. 2005 | |
| UHPLC‐DAD‐MS | Slatnar et al. 2015 | ||
| HPLC‐DAD/LTQ‐MS | Regueiro et al. 2014 | ||
| HSCCC‐ESI‐IT‐TOF‐MS | Grace et al. 2014 | ||
| NMR; ESI‐MS | Zhang et al. 2009 | ||
| CE‐ESI‐TOF‐MS | Gómez‐Caravaca et al. 2008 | ||
| MEKC | Verardo et al. 2009 | ||
| Proteins | SDS‐PAGE | Labuckas et al. 2014 | |
| Sterols | GLC‐FID | Abdallah et al. 2015; Amaral et al. 2003; Schwartz et al. 2008 | |
| GC‐MS | Madawala et al. 2012; Verardo et al. 2009, 2013 | ||
| Tocopherols | HPLC‐FD | Abdallah et al. 2015; Madawala et al. 2012; Schwartz et al. 2008; Verardo et al. 2013 | |
| HPLC/UV‐DAD/MS | Miraliakbari & Shahidi 2008 | ||
| HPLC‐UV | Kornsteiner et al. 2006 | ||
| HPLC‐PDA | Li et al. 2007 | ||
| GC‐FID | Verardo et al. 2009, 2013 | ||
| Triacylglycerols | GC‐FID | Bouabdallah et al. 2014 | |
| Volatile compounds | GC‐MS | Abdallah et al. 2015 |
AAS, atomic absorption spectrometry; AES, atomic emission spectrometry; CE, capillary electrophoresis; DAD, diode array detector; ELSD, evaporative light scattering detector; ESI, electrospray ionization; FD, fluorescence detector; FID, flame ionization detector; GC, gas chromatography; HPLC, high‐performance liquid chromatography; HSCCC, high‐speed counter‐current chromatography; ICP, inductively coupled plasma; IEC, ionic exchange chromatography; IT, ion trap; LC, liquid chromatography; LFD, large field detector; LTQ, linear ion trap; MALDI, matrix‐assisted laser desorption/ionization; MEKC, micellar electrokinetic chromatography; MS, mass spectrometry; NMR, nuclear magnetic resonance; PA, pulse amperometry; PAGE, polyacrylamide gel electrophoresis; PDA, photodiode array detection; RI, refractive index; RP, reverse phase; SDS, sodium dodecylsulfate; SEM, scanning electron microscopy; TOF, time of flight; UFLC, ultra‐fast liquid chromatography; UHPLC, ultra‐high performance liquid chromatography; UV, ultraviolet.
The most abundant fatty acids in almond oil are oleic acid (50.41–81.20%), linoleic acid (6.21–37.13%), palmitic acid (5.46–15.78%), stearic acid (0.80–3.83%), and palmitoleic acid (0.23–2.52%). Linolenic acid and myristic acid were also detected (Askin et al. 2007; Barreira et al. 2012a; Madawala et al. 2012; Zhu et al. 2015). The percentages of fatty acids are also reflected by the triacylglycerol profile, where OOO (30–55%) and OLO (16–3%) were the major molecules, followed by OLL (6–15%), OOP (5–13%), LOP (3–11%), SOO (0.4–4.0%), PLP (0.1–3.2%), LLP (0.4–2.8%), and POP (0.03–0.46%), (L, linoleoyl; O, oleoyl; P, palmitoyl; S, stearoyl) (Barreira et al. 2012a; Martín‐Carratalá et al. 1999; Prats‐Moya et al. 1999).
Regarding the protein content, which is usually calculated from the amount of total nitrogen by applying specific nitrogen‐to‐protein conversion factors, the dominant protein in almonds is a globulin named amandin, which contains 19.3% nitrogen, corresponding to a conversion factor of 5.18. The general factor of 6.25 used in protein calculations is based on a 16% nitrogen content of many common proteins; however, using this factor for almonds would overestimate the protein content (Yada et al. 2011). Concerning the amino acids profile, asparagine (Asn) is by far the most abundant in almond proteins (Amrein et al. 2005).
Among the carbohydrates present in almond, sugars, starch, and some sugar alcohols are the only ones that can be digested, absorbed, and metabolized by humans. Nevertheless, the nonstarch fraction might promote physiological effects that are beneficial for human health. These indigestible polysaccharides (e.g. cellulose, hemicelluloses, oligosaccharides, pectins, gums, waxes) are the main components of the well‐known dietary fiber (Yada et al. 2011). Sucrose was reported as the predominant sugar in almonds, ranging from 11.5 to 22.2 g/100 g dry weight (dw); other individual sugars were detected in minor concentrations: raffinose (0.71–2.11 g/100 g dw), glucose (0.42–1.30 g/100 g dw), maltose (0.29–1.30 g/100 g dw), and fructose (0.11–0.59 g/100 g dw) (Balta et al. 2009; Barreira et al. 2010).
12.1.4 Minor Components
Regarding the vitamins present in almond, most literature is focused on the content of tocopherols in the kernels. Vitamin E (α‐, β‐, γ‐, δ‐tocopherol and α‐, β‐, γ‐, δ‐tocotrienol) is only produced in plants, and is a strong antioxidant with a protective role in biological systems, besides having hypocholesterolemic, anticancer, and neuroprotective properties (Sen et al. 2007). Almonds are considered one of the richest food sources of α‐tocopherol (Barreira et al. 2012a), which is the most biologically active form of vitamin E, utilized in the human body preferentially to the other forms (Brigelius‐Flohé et al. 2002). The content in each of the vitamin E isoforms is highly dependent on the cultivar, maturity stage, and geographical origin, but typically detected vitamers include α‐tocopherol (8.0–38 mg/100 g dw), α‐tocotrienol (0.01–0.30 mg/100 g dw), β‐tocopherol (0.02–0.25 mg/100 g dw), γ‐tocopherol (0.08–2.1 mg/100 g dw), γ‐tocotrienol (0.11–0.24 mg/100 g dw), and δ‐tocotrienol (0.02–0.005 mg/100 g dw). Among the various tree nuts, almonds typically contain the most vitamin E (Barreira et al. 2012a; Kodad et al. 2011; Kornsteiner et al. 2006; Madawala et al. 2012; Maguire et al. 2004; Matthäus & Özcan 2009; Yada et al. 2011; Zhu et al. 2015), with two handfuls of almonds providing the average daily recommended dose (15 mg) (Institute of Medicine 2000).
Studies analyzing the water‐soluble vitamin content of almonds are much scarcer, but these nuts are generally recognized as a good source of riboflavin (vitamin B2) and other complex B vitamins such as thiamine, niacin, pyridoxine, pantothenic acid, folic acid (folate), and biotin (Yada et al. 2011).
Furthermore, almonds are one of the top 40 richest food sources of polyphenols (Pérez‐Jiménez et al. 2010), which are mainly present as proanthocyanidins, followed by hydrolyzable tannins, flavonoids, and phenolic acids (Bolling et al. 2011; Xie et al. 2012). Stilbenes are generally available in lower amounts, but these compounds obtained from the shikimate and phenylalanine/polymalonate pathways may also contribute to the health‐promoting potential of polyphenol‐rich foods, through antioxidant or phytoestrogen activities. In almond, polydatin (Figure 12.1) has been reported as the most abundant stilbene (Xie & Bolling 2014). Furthermore, polyphenols from almond proved to be bioavailable in humans as they were detected as phase II and microbial‐derived metabolites in plasma and urine samples (Bartolomé et al. 2010).
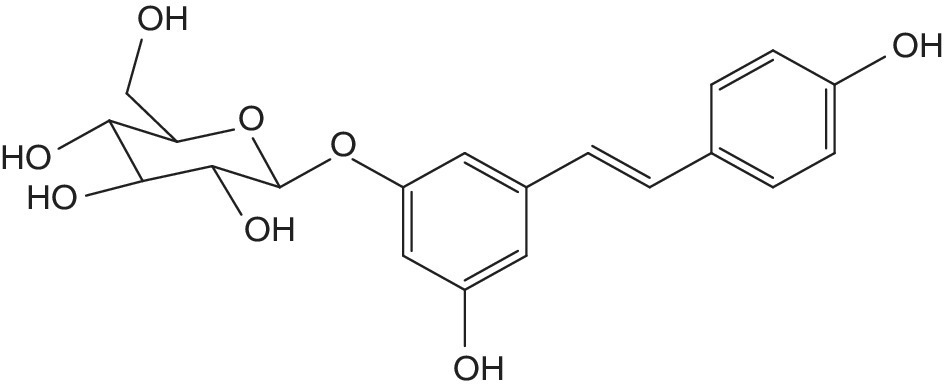
Figure 12.1 Chemical structure of polydatin.
The mineral content of almond, as in other plants, may be affected by many environmental factors and agronomic practices including geographic location, soil composition, water source, irrigation, as well as components of fertilizers and other agronomic production enhancers. Mineral content can also be influenced by the plant species or the botanical component undergoing analysis. In the particular case of almond, the nearly 3% of ash includes mainly potassium (K) > phosphorus (P) > calcium (Ca) magnesium (Mg) >>> iron (Fe) > zinc (Zn) > manganese (Mn), selenium (Se) > sodium (Na) > copper (Cu) (Yada et al. 2011).
12.2 Castanea sativa Miller (Chestnut)
12.2.1 Botanical Aspects and Geographical Distribution
Castanea sativa Miller belongs to the Fagaceae family, which includes several ecologically and economically important species (Manos et al. 2001). Chestnuts are found in three major geographical areas: Asia (with predominance of C. crenata, C. molissima, C. seguinii, C. davidii, and C. henryi), North America (where C. dentata, C. pumila, C. floridana, C. ashei, C. alnifolia, and C. paucispina thrive) and Europe, where C. sativa is predominant (Bounous 2005). According to the Food and Agriculture Organization (FAO), worldwide chestnut production is estimated at about 1.1 million tons. Europe is responsible for about 12% of global production, with relevance for Italy and Portugal, corresponding to 4% and 3%, respectively (Barreira et al. 2010). Chestnut is an important food resource in several countries. In Europe, chestnut is regaining interest, with an increase in production area from 81 511 ha in 2005 to 87 521 ha in 2008 (Fernandes et al. 2011).
12.2.2 Main Applications and Nutritional Overview
Chestnut kernels are a highly appreciated seasonal nut in Mediterranean countries, being consumed fresh or cooked, with roasting, boiling or frying being the most common cooking methods. Although a highly perishable product, nowadays chestnuts can be found on the market all year round due to the availability of frozen and boiled frozen chestnuts. Other important chestnut products are available on the market, such as the highly appreciated “marrons glacés” and chestnut flour obtained by grinding dried chestnuts, used for valorization of small chestnuts or chestnuts with double embryos (Cruz et al. 2013).
Chestnuts have become increasingly important in human nutrition because of their nutrient composition and potential beneficial health effects, for example, as part of a gluten‐free diet in cases of celiac disease (Pazianas et al. 2005) and in reducing coronary heart disease and cancer rates (Sabaté et al. 2000). Chestnuts are rich in carbohydrates and are a good source of essential fatty acids (despite their low fat amounts) and minerals, also providing several vitamins and appreciable levels of fiber (Borges et al. 2008).
12.2.3 Major Components
On a fresh weight basis, the major component in chestnut is water, which generally accounts for more than 50% of its weight (Barreirra et al. 2012b). Starch is the predominant component of dry matter, which makes chestnuts an excellent source of starch, above potatoes or wheat (Borges et al. 2008).
Sugar profiles are typically characterized by three main sugars: fructose, glucose, and sucrose. The concentration of each sugar is highly dependent on the cultivar; sucrose was detected as 3.71–24.17 g/100 g dw, glucose varied between 0.96 and 6.81 g/100 g dw, while fructose was quantified between 0.57 and 5.32 g/100 g dw (Barreira et al. 2010, 2012b; Bernárdez et al. 2004).
Protein content is usually around 3% (2.2–3.1%), depending on cultivar and harvesting year (Barreira et al. 2009b, 2012b), mainly constituted by a total of 17 amino acids: cysteine (Cys), proline (Pro), L‐alanine (Ala), L‐aspartic acid (Asp) (the dominant molecule), glycine (Gly), L‐glutamic acid (Glu), arginine (Arg) and the essential amino acids: isoleucine (Ile), leucine (Leu), lysine (Lys), L‐histidine (His), L‐methionine (Met), L‐threonine (Thr), L‐phenylalanine (Phe), L‐tyrosine (Tyr), L‐serine (Ser), and L‐valine (Val). Asp is the major amino acid (≈1.0 g/100 g dw), closely followed by Glu (≈0.8 g/100 g dw), Leu and Ala (≈0.6 g/100 g dw) and Arg (≈0.5 g/100 g dw). In general, chestnuts are a good source of these compounds; however, amino acid profiles are not well balanced, with certain essential amino acids occurring in limited concentration when compared to FAO recommended levels (Borges et al. 2008).
12.2.4 Minor Components
In chestnuts, the fat content is very low, but the fatty acids profile reveals high predominance of unsaturated molecules: 10–20% saturated fatty acids (mainly palmitic acid, C16:0), 10–30% monounsaturated fatty acids (particularly oleic acid, C18:1), and 50–70% polyunsaturated fatty acids (especially linoleic acid, C18:2, and linolenic acid, C18:3) (Barreirra et al. 2012b; Borges et al. 2007; Fernandes et al. 2011). The composition of fatty acids is, as expected, reflected in the triacylglycerol profile: LLLn, LLL, OLLn, PLLn, OLL, PLL, OOL, POL, PLP, OOO, POO, and PPO (L, linoleoyl; Ln, linolenyl; O, oleoyl; P, palmitoyl) (Barreira et al. 2012b, 2013). Furthermore, chestnuts are cholesterol free and contain a high amount of vitamin C. Some phenolic compounds, particularly gallic acid and ellagic acid (predominant among hydrolyzable and condensed tannins), and organic acids (oxalic, cis‐aconitic, citric, ascorbic, malic, quinic, succinic, shikimic, and fumaric acids) are also noteworthy (Carocho et al. 2013; Gonçalves et al. 2010; Ribeiro et al. 2007; Vasconcelos et al. 2007). Regarding the possible vitamin E isoforms, chestnuts are particularly good sources of γ‐tocopherol (γ‐tocopherol 754–957 μg/100 g dw; γ‐tocotrienol 28–84 μg/100 g dw; δ‐tocopherol 39–66 μg/100 g dw; α‐tocopherol 4–20 μg/100 g dw; α‐tocotrienol 2–8 μg/100 g dw) (Barreira et al. 2009a, 2012b; Fernandes et al. 2011).
The mineral profile in chestnut is characterized by high contents of K (473–974 mg/100 g dw), P (104–148 mg/100 g dw), Mg (63–93 mg/100 g dw) and Ca (41–51 mg/100 g dw), and low amounts of Fe (5.3–10.9 mg/100 g dw), Mn (3.1–8.0 mg/100 g dw), Na (0.9–3.9 mg/100 g dw), Zn (1.4–3.1 mg/100 g dw) and Cu (1.3–2.7 mg/100 g dw) (Borges et al. 2008; Pereira‐Lorenzo et al. 2005). Concerning human nutritional aspects, chestnuts have an important mineral content. K, Mg, Fe, Mn, and Cu have many physiological functions: K is associated with fluid balance and volume, carbohydrate metabolism, protein synthesis, and nerve impulses; P has an important role in mineralization of bones and teeth, energy metabolism, absorption and transport of nutrients; Mg is important in nervous activity and muscle contraction (Diehl 2002).
Additional information regarding the chemical parameters analyzed in chestnuts can be seen in Table 12.1.
12.3 Corylus avellana L. (Hazelnut)
12.3.1 Botanical Aspects and Geographical Distribution
Hazelnut (Corylus avellana L.) belongs to the Betulaceae family and is a popular tree nut worldwide, mainly distributed on the coasts of the Black Sea region of Turkey, southern Europe, and in some areas of the US (Oregon and Washington). Hazelnut is also cultivated in other countries such as New Zealand, China, Azerbaijan, Chile, Iran, and Georgia, among others. Turkey is the world’s largest producer of hazelnuts, contributing ≈74% to total global production, followed by Italy (≈16%), the US (≈4%), and Spain (≈3%) (Seyhan et al. 2007).
Hazelnuts are among the most popular nuts worldwide, with a global production average of nearly 1 million tons (MT) per year (888 328 MT in 2010), on an unshelled basis (Ciemniewska‐Żytkiewicz et al. 2015).
12.3.2 Main Applications and Nutritional Overview
About 90% of the world crop is shelled and sold as kernels with the remaining 10% utilized in shell for fresh consumption. Besides providing desirable flavor and texture to various foods, hazelnuts can play an important role in human nutrition and health due to their high oil, protein, vitamin, and mineral content (Hosseinpour et al. 2013; Tapia et al. 2013).
Hazelnuts play a major role in human health due to their very special nutritional value. One hundred grams of hazelnuts provide 600–650 kcal, mainly due to the fat (43–73%), protein (10–25%), and carbohydrate (10–20%) content. Besides being consumed fresh, hazelnuts are also used as an ingredient in confectionery products and the chocolate industry, as raw materials for pastry, and also add flavor and texture to an increasing variety of sweet and savory food products such as bakery, cereal, and dessert formulations (Amaral et al. 2006a).
The kernels are commercialized mainly after roasting, which gives them a more intense flavor and a crisper texture. Roasted hazelnuts are usually employed for obtaining butter paste or snacks, and also as ingredients for many products (e.g. cookies, ice cream, breakfast cereals, cakes, chocolates, coffee, bread, liqueurs, and spreads) (Jakopic et al. 2011). Eighty percent of the hazelnut kernels are processed in chocolate manufacture, 15% in confectionery, biscuit and pastry manufacture, and 5% is consumed without any further processing (Jakopic et al. 2011).
12.3.3 Major Components
Hazelnuts are particularly valuable for their lipid content (Hosseinpour et al. 2013; Köksal et al. 2006; Parcerisa et al. 1998; Venkatachalam & Sathe 2006), with a recognized prevalence of monounsaturated fatty acids, primarily oleic acid, which may reach 80% of total fatty acids. After oleic acid, linoleic acid (10–20%) and plamitic acid (4–10%) are the most abundant fatty acids in hazelnut kernels (Amaral et al. 2006a; Köksal et al. 2006; Madawala et al. 2012; Oliveira et al. 2008; Parcerisa et al. 1998; Seyhan et al. 2007). The lipid fraction is composed of nonpolar (98.8%) and polar (1.2%) constituents. Triacylglycerols are the major nonpolar lipid class, representing nearly 100% of the total nonpolar lipids in hazelnut oil. The main form in hazelnuts is OOO (71–78%), followed by PLL (10–13%), and POO (7.4–11%); other triacylglycerols detected in minor quantities were LLL, OLL, PLL, POL, PPL, PPO, SOO, and PSO (L, linoleoyl; O, oleoyl; P, palmitoyl; S, steareoyl) (Alasalvar et al. 2003, 2009; Amaral et al. 2006b).
Regarding their protein content, the corresponding amino acids profiles are also noteworthy, with predominance of Glu (2196–3475 mg/100 g) and relevant quantities (400–1000 mg/100 g) of Ala, Asp, Gly, Pro, Ser, and Tyr (Köksal et al. 2006).
12.3.4 Minor Components
Hazelnuts are also recognized for their high content of tocopherols, particularly α‐tocopherol (19–24 mg/100 g dw), and lower levels of the β (0.6–0.9 mg/100 g dw) and γ (1.3–2.3 mg/100 g dw) isoforms; and sterols, with predominance of β‐sitosterol (107–126 mg/100 g dw), high content of campesterol (6.7–8.9 mg/100 g dw), stigmasterol (0.7–0.9 mg/100 g dw) and Δ5‐avenasterol (5.1–6.1 mg/100 g dw) and lower levels of cholesterol (0.3–0.9 mg/100 g dw), chlerosterol (0.7–1.2 mg/100 g dw), β‐sitostanol (4.4–6.2 mg/100 g dw), Δ7‐stigmastanol (0.21–0.35 mg/100 g dw), campestanol (1.4 mg/100 g dw), fucosterol (0.4–0.6 mg/100 g dw), and Δ7‐avenasterol (0.9–1.3 mg/100 g dw) (Alasalvar et al. 2003, 2009; Amaral et al. 2006a; Ciemniewska‐Żytkiewicz et al. 2015; Köksal et al. 2006; Kornsteiner et al. 2006; Madawala et al. 2012; Parcerisa et al. 1998). Hazelnuts also contain dietary fiber as well as other beneficial nutrients, such as plant proteins, essential minerals, B complex vitamins, and phenolic compounds (Bignami et al. 2005; Köksal et al. 2006; Kornsteiner et al. 2006).
Besides their rich mineral content, in which K is prevalent (382–1470 mg/100 g dw), followed by P (202–708 mg/100 g dw), Ca (65–401 mg/100 g dw), Mg (35–310 mg/100 g dw), Mn (2.2–19.0 mg/100 g dw), Fe (3.0–5.0 mg/100 g dw), Zn (1.3–4.4 mg/100 g dw), Na (1.2–3.8 mg/100 g dw), Cu (0.9–3.2 mg/100 g dw), chromium (Cr) (10–18 μg/100 g dw), Se (5.5–8.1 mg/100 g dw), and molybdenum (Mo) (2.1–3.8 mg/100 g dw), hazelnut kernels are a valuable source of essential vitamins, such as vitamins B1, B6 and niacin (Alasalvar et al. 2009; Köksal et al. 2006; Seyhan et al. 2007).
Several studies characterizing phenolic profiles have been performed (see Table 12.1), revealing significant content of phenolic acids and flavonoids. Several compounds, such as gallic, caffeic, p‐coumaric, ferulic, sinapic, caffeoyltartaric and caffeoylquinic acids, procyanidins, catechin, epicatechin, glansreginins (Figure 12.2), and phloretins (Figure 12.3), have been quantified in hazelnut samples by several authors (Ciemniewska‐Żytkiewicz et al. 2015; Jakopic et al. 2011; Shahidi et al. 2007; Slatnar et al. 2014). Phenolics in hazelnut kernels protect the seed against oxidation and are associated with the moderate astringency and characteristic bitter taste of fresh nuts (Slatnar et al. 2014).
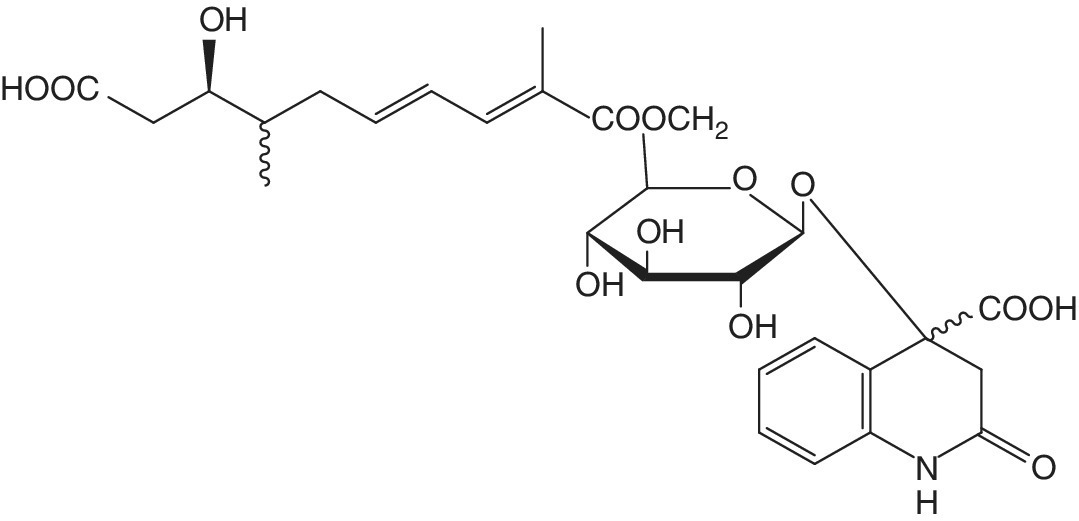
Figure 12.2 Chemical structure of glansreginin A.
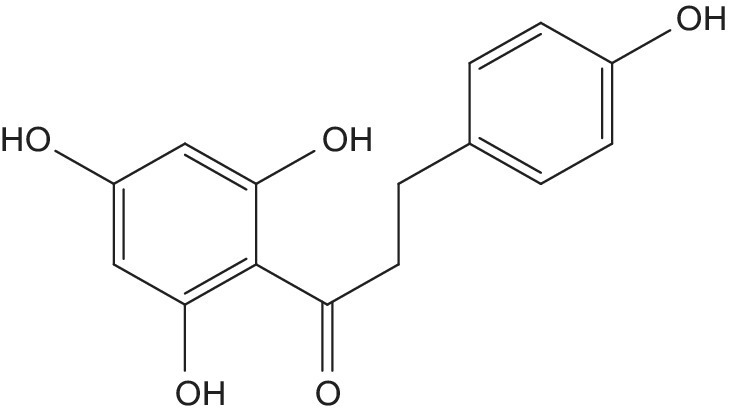
Figure 12.3 Basic structure of phloretin.
Hazelnuts also contain organic acids, but in small quantities, with malic acid as the most abundant compound (Botta et al. 1994).
12.4 Juglans regia L. (Walnut)
12.4.1 Botanical Aspects and Geographical Distribution
Walnut (Juglans regia L.), which belongs to the Juglandaceae family, is a common nut in Mediterranean diets. Originating from Central Asia, the walnut is among the oldest cultivated fruit species. It is commercially planted throughout southern Europe, northern Africa, eastern Asia, the USA, and western South America. The 2012 world production of in‐shell walnut was above 3 400 000 tons (FAO 2013). Recently, walnut has been considered as a natural functional food of high economic interest due to its nutritional and medicinal benefits (Bouabdallah et al. 2014; Martínez et al. 2010).
12.4.2 Main Applications and Nutritional Overview
Walnut is a crop of high economic interest to the food industry. The edible part of the nut (the seed or kernel) is consumed fresh or roasted, alone or in other processed products. It is a nutrient‐rich food mainly due to its high fat and protein content but also contains many vitamins and minerals. The kernel represents between 40% and 60% of the in‐shell nut weight. It contains high levels of oil, 52–72%, up to 24% of proteins (usually 13–17%), 1.5–2% of fiber, and 1.7–2% of ash, depending on the cultivar, geographical location, and irrigation rate (Amaral et al. 2003; Martinez et al. 2006; Prasad 2003).
12.4.3 Major Components
Walnut proteins are highly digestible and have a good balance of essential amino acids. The major protein fraction is glutelins (≈70%), followed by globulins (≈18%), albumins (≈7%), and prolamins (≈5%) (Labuckas et al. 2014; Sze‐Tao & Sathe 2000). The most frequent amino acids in walnut proteins are Arg, Glu, and Ala, but several others are also found, such as Asp, Asn, Ser, glutamine (Gln), Gly, His, Thr, L‐citrulline (Cit), γ‐aminobutyric acid (GABA), Tyr, Val, Met, tryptophan (Trp), Phe, ornithine (Orn), Ile, Lys, Leu, and Pro (Mapelli et al. 2001).
Walnuts contain other beneficial compounds, such as polyunsaturated fatty acids (particularly linoleic acid: 57–66%, oleic acid: 13–24%, linolenic acid: 8–16%, and palmitic acid: 6–11%) and minerals (Bouabdallah et al. 2014; Li et al. 2007; Rabrenovic et al. 2008; Verardo et al. 2009). The triacylglycerol profile is characterized by four major molecules: LLLn, LLL, OLL and PLL, but several others were also detected (LLnLn, OLLn, SLL, OOL, SOL, OOO, SLS, SOO, PLLn, POL, PLS, POO, POS, PLP, and POP) (L, linoleoyl; Ln, linolenyl: O, oleoyl; P, palmitoyl; S, steareoyl) (Bouabdallah et al. 2014).
12.4.4 Minor Components
Tocopherols in walnut kernels are dominated by γ‐tocopherol (12–39 mg/100 g dw), followed by δ‐ (1.1–4.6 mg/100 g), α‐ (0.2–6.6 mg/100 g), and β‐isoforms (0.03–0.32 mg/100 g) (Abdallah et al. 2015; Kornsteiner et al. 2006; Li et al. 2007; Madawala et al. 2012; Miraliakbari & Shahidi 2008; Verardo et al. 2009). The predominant sterol is, by a high margin, β‐sitosterol (97–176 mg/100 g), followed by campesterol (0.5–8.8 mg/100 g), Δ5‐avenasterol (0.5–8.0 mg/100 g), and Δ5,24‐stigmastadienol (0.8–4.6 mg/100 g). Other detected sterols were cholesterol, brassicasterol, β‐sitostanol, Δ7‐campesterol, stigmastanol, Δ5,23‐stigmastadienol, Δ7‐stigmastanol, campestanol, stigmasterol, chlenosterol, and Δ7‐avenasterol (Abdallah et al. 2015; Amaral et al. 2003; Madawala et al. 2012; Schwartz et al. 2008; Verardo et al. 2009).
β‐Carotene is the major carotenoid (0.022–0.062 mg/100 g dw), despite the presence of other compounds such as β‐cryptoxanthin, lutein, zeaxanthin, violaxanthin, and neoxanthin (Abdallah et al. 2015).
The main phenolic compounds in walnut are phenolic acids (chlorogenic, caffeic, ferulic, p‐coumaric, sinapic, ellagic, and syringic acid), syringaldehyde and juglone (Colaric et al. 2005; Zhang et al. 2009), besides several hydrolyzable tannins and different flavonoids (especially vescalagin) (Fukuda et al. 2003; Slatnar et al. 2015; Verardo et al. 2009). Recently, more than 120 phenolic compounds, including hydrolyzable and condensed tannins, flavonoids and phenolic acids, have been identified or tentatively characterized in different walnut cultivars (Grace et al. 2014; Regueiro et al. 2014).
Walnuts are also characterized by high levels of K (300–487 mg/100 g), Mg (129–443 mg/100 g dw), P (308–385 mg/100 g dw), and Ca (58–135 mg/100 g dw) and, in contrast, very low levels of Na (0.3–6.7 mg/100 g dw), Mn (1.1–4.3 mg/100 g dw), Fe (1.5–2.9 mg/100 g dw), Cu (0.7–2.0 mg/100 g dw), Zn (1.2–1.9 mg/100 g dw), and Se (0.7–1.1 μg/100 g dw) (Lavedrine et al. 2000; Tapia et al. 2013).
In all these examples, laboratory determinations were achieved by applying several methodologies (see Table 12.1).
12.5 Conclusion
Nut consumption as part of a balanced diet is recommended. Clinical and preclinical trials have demonstrated that nuts have antioxidant, antidiabetic, and hypocholesterolemic actions (Xie & Bolling 2014). Furthermore, their consumption may improve body weight control and reduce the risk of obesity‐related diseases such as coronary heart disease and type 2 diabetes. In addition to cardiovascular benefits, which are mainly due to the lipids present in many types of nuts, other components might have important protective roles against the onset of several diseases.
Given the described profiles of different phytochemicals, it is also advised to consume a high variability of nuts, since their potential effects are often complementary, in relation to their different compositions of major and minor components.
References
- Abdallah, I. A., Tlili, N., Martinez‐Force, E., et al. (2015) Content of carotenoids, tocopherols, sterols, triterpenic and aliphatic alcohols, and volatile compounds in six walnuts (Juglans regia L.) varieties. Food Chemistry 173, 972–978.
- Açkurt, F., Özdemir, M., Biringen, G. & Löker, M. (1999) Effects of geographical origin and variety on vitamin and mineral composition of hazelnut (Corylus avellana L.) varieties cultivated in Turkey. Food Chemistry 65, 309–313.
- Ahrens, S., Venkatachalam, M., Mistry, A. M., Lapsley, K. & Sathe, S. K. (2005) Almond (Prunus dulcis L.) protein quality. Plant Foods for Human Nutrition 60, 123–128.
- Alasalvar, C., Shahidi, F., Ohshima, T., et al. (2003) Turkish tombul hazelnut (Corylus avellana L.) 2. Lipid characteristics and oxidative stability. Journal of Agricultural and Food Chemistry 51, 3797–3805.
- Alasalvar, C., Amaral, J., Satir, G. & Shahidi, F. (2009) Lipid characteristics and essential minerals of native Turkish hazelnut varieties (Corylus avellana L.). Food Chemistry 113, 919–925.
- Amaral, J. S., Casal, S., Pereira, J. A., Seabra, R. M. & Oliveira, M. B. P. P. (2003) Determination of sterol and fatty acid compositions, oxidative stability, and nutritional value of six walnut (Juglans regia L.) cultivars grown in Portugal. Journal of Agricultural and Food Chemistry 51, 7698–7702.
- Amaral, J. S., Casal, S., Citová, I., Santos, A., Seabra, R. & Oliveira, M. B. P. P. (2006a) Characterization of several hazelnut (Corylus avellana L.) cultivars based in chemical, fatty acid and sterol composition. European Food Research and Technology 222, 274–280.
- Amaral, J. S., Cunha, S. C., Santos, A., Alves, M. R., Seabra, R. M. & Oliveira, M. B. P. P. (2006b) Influence of cultivar and environmental conditions on the triacylglycerol profile of hazelnut (Corylus avellana L.). Journal of Agricultural and Food Chemistry 54, 449–456.
- Amrein, T. M., Lukac, H., Andres, L., Perren, R., Escher, F. & Amadò, R. (2005) Acrylamide in roasted almonds and hazelnuts. Journal of Agricultural and Food Chemistry 53, 7819–7825.
- Aranceta, J., Pérez Rodrigo, C., Naska, A., Vadillo, V. R. & Trichopoulou, A. (2006) Nut consumption in Spain and other countries. British Journal of Nutrition 96, S3–S11.
- Askin, M. A., Balta, M. F., Tekintas, F. E., Kazankaya, A. & Balta, F. (2007) Fatty acid composition affected by kernel weight in almond [Prunus dulcis (Mill.) D.A. Webb.] genetic resources. Journal of Food Composition and Analysis 20, 7–12.
- Balta, F., Battal, P., Balta, M. F. & Yoruk, H. I. (2009) Free sugar compositions based on kernel taste in almond genotypes Prunis dulcis from eastern Turkey. Chemistry of Natural Compounds 45, 221–224.
- Barreira, J. C. M., Alves, R. C., Casal, S., Ferreira, I. C. F. R., Oliveira, M. B. P. P. & Pereira, J. A. (2009a) Vitamin E profile as a reliable authenticity discrimination factor between chestnut (Castanea sativa Mill.) cultivars. Journal of Agricultural and Food Chemistry 57, 5524–5528.
- Barreira, J. C. M., Casal, S., Ferreira, I. C. F. R., Oliveira, M. B. P. P. & Pereira, J. A. (2009b) Nutritional, fatty acid and triacylglycerol profiles of Castanea sativa Mill cultivars: a compositional and chemometric approach. Journal of Agricultural and Food Chemistry 57, 2836–2842.
- Barreira, J. C. M., Pereira, J. A., Oliveira, M. B. P. P. & Ferreira, I. C. F. R. (2010) Sugars profiles of different chestnut (Castanea sativa Mill.) and almond (Prunus dulcis) cultivars by HPLC‐RI. Plant Foods for Human Nutrition 65, 38–43.
- Barreira, J. C. M., Casal, S., Ferreira, I. C. F. R., Peres, A. M., Pereira, J. A. & Oliveira, M. B. P. P. (2012a) Supervised chemical pattern recognition in almond (Prunus dulcis) Portuguese PDO cultivars: PCA‐ and LDA‐based triennial study. Journal of Agricultural and Food Chemistry 60, 9697–9704.
- Barreira, J. C. M., Casal, S., Ferreira, I. C. F. R., Peres, A. M., Pereira, J. A. & Oliveira, M. B. P. P. (2012b) Chemical characterization of chestnut cultivars from three consecutive years: chemometrics and contribution for authentication. Food and Chemical Toxicology 50, 2311–2317.
- Barreira, J. C. M., Carocho, M., Ferreira, I. C. F. R., et al. (2013) Effects of gamma and electron beam irradiations on the triacylglycerol profile of fresh and stored Castanea sativa Miller samples. Postharvest Biology and Technology 81, 1–6.
- Bartolomé, B., Monagas, M., Garrido, I., et al. (2010) Almond (Prunus dulcis (Mill.) D.A. Webb) polyphenols: from chemical characterization to targeted analysis of phenolic metabolites in humans. Archives of Biochemistry and Biophysics 501, 124–133.
- Bernárdez, M. M., Miguélez, J. M. & Queijeiro, J. G. (2004) HPLC determination of sugars in varieties of chestnut fruits from Galicia (Spain). Journal of Food Composition and Analysis 17, 63–67.
- Bignami, C., Bertazza, G., Cristofori, V. & Troso, D. (2005) Kernel quality and composition of hazelnut (Corylus avellana L.) cultivars. Acta Horticulturae 686, 477–484.
- Bolling, B. W., Chen, C. Y., McKay, D. L. & Blumberg, J. B. (2011) Tree nut phytochemicals: composition, antioxidant capacity, bioactivity, impact factors. A systematic review of almonds, Brazils, cashews, hazelnuts, macadamias, pecans, pine nuts, pistachios and walnuts. Nutrition Research Reviews 24, 244–275.
- Borges, O., Carvalho, J. L. S., Correia, P. R. & Silva, A. P. (2007) Lipid and fatty acid profiles of Castanea sativa Mill. Chestnuts of 17 native Portuguese cultivars. Journal of Food Composition and Analysis 20, 80–89.
- Borges, O., Gonçalves, B., Carvalho, J. L. S., Correia, P. R. & Silva, A. P. (2008) Nutritional quality of chestnut (Castanea sativa Mill.) cultivars from Portugal. Food Chemistry 106, 976–984.
- Botta, R., Gianotti, C., Richardson, D., Suwanagul, A. & Sanz, C. L. (1994) Hazelnut variety organic acids sugars and total lipid fatty acids. Acta Horticulturae 351, 693–699.
- Bouabdallah, I., Bouali, I., Martinez‐Force, E., Albouchi, A., Perez Camino, M. C. & Boukhchina, S. (2014) Composition of fatty acids, triacylglycerols and polar compounds of different walnut varieties (Juglans regia L.) from Tunisia. Natural Product Research 28, 1826–1833.
- Bounous, G. (2005) The chestnut: a multipurpose resource for the new millennium. Acta Horticulturae 693, 33–138.
- Brigelius‐Flohé, R., Kelly, F. J., Salonen, J. T., Neuzil, J., Zingg, J. M. & Azzi, A. (2002) The European perspective on vitamin E: current knowledge and future research. American Journal of Clinical Nutrition 76, 703–716.
- Carocho, M., Barros, L., António, A. L., et al. (2013) Analysis of organic acids in electron beam irradiated chestnuts (Castanea sativa Mill.): effects of radiation dose and storage time. Food and Chemical Toxicology 55, 348–352.
- Chen, C. Y., Lapsley, K. & Blumberg, J. (2006) A nutrition and health perspective on almonds. Journal of the Science of Food and Agriculture 86, 2245–2250.
- Cherif, A., Belkacemi, K., Kallel, H., Angers, P., Arul, J. & Boukhchina, S. (2009) Phytosterols, unsaturated fatty acid composition and accumulation in the almond kernel during harvesting period: importance for development regulation. Comptes Rendus Biologies 332, 1069–1077.
- Ciemniewska‐Żytkiewicz, H., Verardo, V., Pasini, F., Bryś, J., Koczoń, P. & Caboni, M. F. (2015) Determination of lipid and phenolic fraction in two hazelnut (Corylus avellana L.) cultivars grown in Poland. Food Chemistry 168, 615–622.
- Colaric, M., Veberic, R., Solar, A., Hudina, M. & Stampar, F. (2005) Phenolic acids, syringaldehyde, and juglone in fruits of different cultivars of Juglans regia L. Journal of Agricultural and Food Chemistry 53, 6390–6396.
- Correia, P. R., Nunes, M. C. & Beirão‐da‐Costa, M. L. (2012) The effect of starch isolation method on physical and functional properties of Portuguese nuts starches. I. Chestnuts (Castanea sativa Mill. var. Martainha and Longal) fruits. Food Hydrocolloids 27, 256–263.
- Cruz, B. R., Abraão, A. S., Lemos, A. M. & Nunes, F. M. (2013) Chemical composition and functional properties of native chestnut starch (Castanea sativa Mill.). Carbohydrate Polymers 94, 594–602.
- Daoud, H. N., Miller, M. W. & Luh, B. S. (1977) Effect of commercial processing on vitamin B6 retention in almonds. Canadian Institute of Food Science and Technology Journal 10, 244–246.
- Demiate, I. M., Oetterer, M. & Wosiacki, G. (2001) Characterization of chestnut (Castanea sativa Mill) starch for industrial utilization. Brazilian Archives of Biology and Technology 44, 69–78.
- Diehl, J. F. (2002) Nuts shown to offer health benefits. International News on Fats, Oils and Related Materials 13, 134–138.
- di Stefano, V., Pitonzo, R., Bartolotta, A., d’Oca, M. C. & Fuochi, P. (2014) Effects of γ‐irradiation on the α‐tocopherol and fatty acids content of raw unpeeled almond kernels (Prunus dulcis). LWT ‐ Food Science and Technology 59, 572–576.
- FAO (2013) FAOSTAT data. Rome: Food and Agriculture Organization.
- Fernandes, Â., Barreira, J. C. M., António, A. L., Bento, A., Botelho, M. L. & Ferreira, I. C. F. R. (2011) Assessing the effects of gamma irradiation and storage time in energetic value and in major individual nutrients of chestnuts. Food and Chemical Toxicology 49, 2429–2432.
- Fukuda, T., Ito, H. & Yoshida, T. (2003) Antioxidative polyphenols from walnuts (Juglans regia L.). Phytochemistry 63, 795–801.
- García‐López, C., Grané‐Teruel, N., Berenguer‐Navarro, V., García‐García, J. E. & Martín‐Carratalá, M. L. (1996) Major fatty acid composition of 19 almond cultivars of different origins: a chemometric approach. Journal of Agricultural Food Chemistry 44, 1751–1755.
- Gómez‐Caravaca, A. M., Verardo, V., Segura‐Carretero, A., Caboni, M. F. & Fernández‐Gutiérrez, A. (2008) Development of a rapid method to determine phenolic and other polar compounds in walnut by capillary electrophoresis electrospray ionization time‐of‐flight mass spectrometry. Journal of Chromatography A 1209, 238–245.
- Gonçalves, B., Borges, O., Costa, H. C., Bennett, R., Santos, M. & Silva, A. P. (2010) Metabolite composition of chestnut (Castanea sativa Mill.) upon cooking: proximate analysis, fibre, organic acids and phenolics. Food Chemistry 122, 154–160.
- Grace, M. H., Warlick, C. W., Neff, S. A. & Lila, M. A. (2014) Efficient preparative isolation and identification of walnut bioactive components using high‐speed counter‐current chromatography and LC‐ESI‐IT‐TOF‐MS. Food Chemistry 158, 229–238.
- Hoppner, K., Lampi, B. & O’Grady, E. (1994) Biotin content in vegetables and nuts available on the Canadian market. Food Research International 27, 495–497.
- Hosseinpour, A., Seifi, E., Javadi, D., Ramezanpour, S. S. & Molnar, T. J. (2013) Nut and kernel characteristics of twelve hazelnut cultivars grown in Iran. Scientia Horticulturae 150, 410–413.
- Institute of Medicine (2000) Vitamin E. In: Dietary Reference Intakes for Vitamin C, Vitamin E, Selenium and Carotenoid. Washington DC: National Academy Press, pp 186–283.
- Jakopic, J., Petkovsek, M. M., Likozar, A., Solar, A., Stampar, F. & Veberic, R. (2011) HPLC‐MS identification of phenols in hazelnut (Corylus avellana L.) kernels. Food Chemistry 124, 1100–1106.
- Kazantzis, I., Nanos, G. D. & Stavroulakis, G. G. (2003) Effect of harvest time and storage conditions on almond kernel oil and sugar composition. Journal of the Science of Food and Agriculture 83, 354–359.
- Kodad, O., Estopañán, G., Juan, T., Mamouni, A. & Socias i Company, R. (2011) Tocopherol concentration in almond oil: genetic variation and environmental effects under warm conditions. Journal of Agricultural Food and Chemistry 59, 6137–6141.
- Kodad, O., Estopañán, G., Juan, T., Alonso, J. M., Espiau, M. T. & Socias i Company, R. (2014) Oil content, fatty acid composition and tocopherol concentration in the Spanish almond genebank collection. Scientia Horticulturae 177, 99–107.
- Kornsteiner, M., Wagner, K. H. & Elmadfa, I. (2006) Tocopherols and total phenolics in 10 different nut types. Food Chemistry 98, 381–387.
- Köksal, A. I., Artik, N., Şimşek, A. & Güneş, N. (2006) Nutrient composition of hazelnut (Corylus avellana L.) varieties cultivated in Turkey. Food Chemistry 99, 509–515.
- Kumar, K. & Sharma, S. D. (2005) Self‐compatible indigenous almond selections: characterizations and assessment. Acta Horticulturae 696, 65–67.
- Labuckas, D., Maestri, D. & Lamarque, A. (2014) Effect of different oil extraction methods on proximate composition and protein characteristics of walnut (Juglans regia L.) flour. LWT ‐ Food Science and Technology 59, 794–799.
- Lavedrine, F., Ravel, A., Villet, A., Ducros, V. & Alary, J. (2000) Mineral composition of two walnut cultivars originating in France and California. Journal of Food Chemistry 68, 347–351.
- Li, L., Tsao, R., Yang, R., Karmer, J. K. & Hernandez, M. (2007) Fatty acid profiles, tocopherol contents, and antioxidant activities of heartnut (Juglans ailanthifolia Var. cordiformis) and Persian Walnut (Juglans regia L.). Journal of Agricultural and Food Chemistry 55, 1164–1169.
- López‐Ortiz, C. M., Prats‐Moya, S., Beltrán Sanahuja, A., Maestre‐Pérez, S. E., Grané‐Teruel, N. & Martín‐Carratalá, M. L. (2008) Comparative study of tocopherol homologue content in four almond oil cultivars during two consecutive years. Journal of Food Composition and Analysis 21, 144–151.
- Madawala, S. R. P., Kochhar, S. P. & Dutta, P. C. (2012) Lipid components and oxidative status of selected specialty oils. Grasas y Aceites 63, 143–151.
- Maguire, L. S., O’Sullivan, S. M., Galvin, K., O’Connor, T. P. & O’Brien, N. M. (2004) Fatty acid profile, tocopherol, squalene and phytosterol content of walnuts, almonds, peanuts, hazelnuts and the macadamia nut. International Journal of Food Science and Nutrition 55, 171–178.
- Manos, P. S., Zhou, Z. K. & Cannon, C. H. (2001) Systematics of Fagaceae: phylogenetic tests of reproductive trait evolution. International Journal of Plant Sciences 162, 1361–1379.
- Mapelli, S., Brambilla, I. & Bertani, A. (2001) Free amino acids in walnut kernels and young seedlings. Tree Physiology 21, 1299–1302.
- Martín‐Carratalá, M. L., García‐López, C., Berenguer‐Navarro, V. & Grané‐Teruel, N. (1998) New contribution to the chemometric characterization of almond cultivars on the basis of their fatty acid profiles. Journal of Agricultural Food Chemistry 46, 963–967.
- Martín‐Carratalá, M. L., Llorens‐Jordá, C., Berenguer‐Navarro, V. & Grané‐Teruel, N. (1999) Comparative study on the triglyceride composition of almond kernel oil. A new basis for cultivar chemometric characterization. Journal of Agricultural and Food Chemistry 47, 3688–3692.
- Martinez, M. L., Mattea, M. A. & Maestri, D. M. (2006) Varietal and crop year effects on lipid composition of walnut (Juglans regia) genotypes. Journal of the American Oil Chemists’ Society 83, 791–796.
- Martínez, M. L., Labuckas, D. O., Lamarque, A. L. & Maestri, D. M. (2010) Walnut (Juglans regia L.): genetic resources, chemistry, by‐products. Journal of the Science of Food and Agriculture 12, 1959–1967.
- Matthäus, B. & Özcan, M. M. (2009) Fatty acids and tocopherol contents of some Prunus spp. kernel oils. Journal of Food Lipids 16, 187–199.
- Miraliakbari, H. & Shahidi, F. (2008) Antioxidant activity of minor components of tree nuts oil. Food Chemistry 111, 421–427.
- Muhammad, S., Sanden, B. L., Lampinen, B. D., et al. (2015) Seasonal changes in nutrient content and concentrations in a mature deciduous tree species: studies in almond (Prunus dulcis (Mill.) D. A. Webb). European Journal of Agronomy 65, 52–68.
- Nanos, G. D., Kazantzis, I., Kefalas, P., Petrakis, C. & Stavroulakis, G. G. (2002) Irrigation and harvest time affect almond kernel quality and composition. Scientia Horticulturae 96, 249–256.
- Oliveira, I., Sousa, A., Morais, J. S., et al. (2008) Chemical composition, and antioxidant and antimicrobial activities of three hazelnut (Corylus avellana L.) cultivars. Food and Chemical Toxicology 46, 1801–1807.
- Parcerisa, J., Richardson, D. G., Rafecas, M., Codony, R. & Boatella, J. (1998) Fatty acid, tocopherol and sterol content of some hazelnut varieties (Corylus avellana L.) harvested in Oregon (USA) Journal of Chromatography A 805, 259–268.
- Pazianas, M., Butcher, G. P., Subhani, J. M., et al. (2005) Calcium absorption and bone mineral density in celiacs after long term treatment with gluten‐free diet and adequate calcium intake. Osteoporosis International 16, 56–63.
- Pereira‐Lorenzo, S., Ramos‐Cabrer, A. M., Díaz‐Hernández, M. B., Ciordia‐Ara, M. & Rios‐Mesa, D. (2005) Chemical composition of chestnut cultivars from Spain. Scientia Horticulturae 107, 306–314.
- Pérez‐Jiménez, J., Neveu, V., Vos, F. & Scalbert, A. (2010) Identification of the 100 richest dietary sources of polyphenols: an application of the Phenol‐Explorer database. European Journal of Clinical Nutrition 64, S112–S120.
- Piscopo, A., Romeo, F. V., Petrovicova, B. & Poiana, M. (2010) Effect of the harvest time on kernel quality of several almond varieties (Prunus dulcis (Mill.) D.A. Webb). Scientia Horticulturae 125, 41–46.
- Prasad, R. B. N. (2003) Walnuts and pecans. In: B. Caballero, ed. Encyclopedia of Food Sciences and Nutrition, 2nd edn. Oxford: Academic Press, pp 6071–6079.
- Prats‐Moya, S., Grané‐Teruel, N., Berenguer‐Navarro, V. & Martín‐Carratalá, M. L. (1997) Inductively coupled plasma application for the classification of 19 almond cultivars using inorganic element composition. Journal of Agricultural and Food Chemistry 45, 2093–2097.
- Prats‐Moya, M. S., Grané‐Teruel, N., Berenguer‐Navarro, V. & Martín‐Carratalá, M. L. (1999) A chemometric study of genotypic variation in triacylglycerol composition among selected almond cultivars. Journal of the American Oil Chemists’ Society 76, 267–272.
- Rabrenovic, B., Picuric‐Jovanovic, K. & Sobajic, S. (2008) Physicochemical properties and fatty acid composition of Juglans regia cultivars grown in Serbia. Chemistry of Natural Compounds 44, 151–154.
- Regueiro, J., Sánchez‐González, C., Vallverdú‐Queralt, A., Simal‐Gándara, J., Lamuela‐Raventós, R. & Izquierdo‐Pulido, M. (2014) Comprehensive identification of walnut polyphenols by liquid chromatography coupled to linear ion trap‐Orbitrap mass spectrometry. Food Chemistry 152, 340–348.
- Ribeiro, B., Rangel, J., Valentão, P., et al. (2007) Organic acids in two Portuguese chestnut (Castanea sativa Miller) varieties. Food Chemistry 100, 504–508.
- Rizzolo, A., Baldo, C. & Polesello, A. (1991) Application of high‐performance liquid chromatography to the analysis of niacin and biotin in Italian almond cultivars. Journal of Chromatography A 553, 187–192.
- Sabaté, J., Radak, T. & Brown, J. Jr. (2000) The role of nuts in cardiovascular disease prevention. In: R. Wildman, ed. Handbook of Nutraceuticals and Functional Foods. London: CRC Press, pp 261–267.
- Sathe, S. K. (1993) Solubilization, electrophoretic characterization and in vitro digestibility of almond (Prunus amygdalus) proteins. Journal of Food Biochemistry 16, 249–264.
- Saura Calixto, F. & Cañellas, J. (1982) Chemical composition of hulls of the sweet almond (Prunus amygdalus). Journal of the Science of Food and Agriculture 33, 336–339.
- Schwartz, H., Ollilainen, V., Piironen, V. & Lampi, A. M. (2008) Tocopherol, tocotrienol and plant sterol contents of vegetable oils and industrial fats. Journal of Food Composition and Analysis 21, 152–161.
- Sen, C. K., Khanna, S. & Roy, S. (2007) Tocotrienols in health and disease: the other half of the natural vitamin E family. Molecular Aspects of Medicine 28, 692–728.
- Seyhan, F., Ozay, G., Saklar, S., Ertaş, E., Satır, G. & Alasalvar, C. (2007) Chemical changes of three native Turkish hazelnut varieties (Corylus avellana L.) during fruit development. Food Chemistry 105, 590–596.
- Shahidi, F., Alasalvar, C. & Liyana‐Pathirana, C. M. (2007) Antioxidant phytochemicals in hazelnut kernel (Corylus avellana L.) and hazelnut byproducts. Journal of Agricultural and Food Chemistry 55, 1212–1220.
- Slatnar, A., Mikulic‐Petkovsek, M., Stampar, F., Veberic, R. & Solar, A. (2014) HPLC‐MSn identification and quantification of phenolic compounds in hazelnut kernels, oil and bagasse pellets. Food Research International 64, 783–789.
- Slatnar, A., Mikulic‐Petkovsek, M., Stampar, F., Veberic, R. & Solar, A. (2015) Identification and quantification of phenolic compounds in kernels, oil and bagasse pellets of common walnut (Juglans regia L.). Food Research International 67, 255–263.
- Soler, L., Canellas, J. & Saura‐Calixto, F. (1988) Oil content and fatty acid composition of developing almond seeds. Journal of the Agricultural Food Chemistry 36, 695–697.
- Sze‐Tao, K. W. C. & Sathe, S. K. (2000) Walnut (Juglans regia L.): proximate composition, protein solubility, protein amino acid composition and protein in vitro digestibility. Journal of the Science of Food and Agriculture 80, 1393–1401.
- Tapia, M. I., Sánchez‐Morgado, J. R., García‐Parra, J., Ramírez, R., Hernández, T., & González‐Gómez, D. (2013) Comparative study of the nutritional and bioactive compounds content of four walnut (Juglans regia L.) cultivars. Journal of Food Composition and Analysis 31, 232–237.
- USDA (2010) US Department of Agriculture, Agricultural Research Service, National Genetic Resources Program. Germplasm Resources Information Network (GRIN), National Germplasm Resources Laboratory, Beltsville, MA. Available at: http://www.ars‐grin.gov/cgi‐bin/npgs/html/taxon.pl?29890 (accessed 29 June 2016).
- Vasconcelos, M. C., Bennett, R. B., Rosa, E. & Cardoso, J. (2007) Primary and secondary metabolite composition of kernels from three cultivars of Portuguese chestnut (Castanea sativa Mill.) at different stages of industrial transformation. Journal of Agricultural and Food Chemistry 55, 3508–3516.
- Venkatachalam, M. & Sathe, S. K. (2006) Chemical composition of selected edible nut seeds. Journal of Agricultural and Food Chemistry 54, 4705–4714.
- Verardo, V., Bendini, A., Cerretani, L., Malaguti, D., Cozzolino, E. & Caboni, M. F. (2009) Capillary gas chromatography analysis of lipid composition and evaluation of phenolic compounds by micellar electrokinetic chromatography in Italian walnut (Juglans regia L.): irrigation and fertilization influence. Journal of Food Quality 32, 262–281.
- Xie, L. & Bolling, B. W. (2014) Characterisation of stilbenes in California almonds (Prunus dulcis) by UHPLC‐MS. Food Chemistry, 148, 300–306.
- Xie, L., Roto, A. V. & Bolling, B. W. (2012) Characterization of ellagitannins, gallotannins, and bound proanthocyanidins from California almond (Prunus dulcis) varieties. Journal of Agricultural and Food Chemistry 60, 12151–12156.
- Yada, S., Lapsley, K. & Huang, G. W. (2011) A review of composition studies of cultivated almonds: macronutrients and micronutrients. Journal of Food Chemistry and Analysis 24, 469–480.
- Yada, S., Huang, G. W. & Lapsley, K. (2013) Natural variability in the nutrient composition of California‐grown almonds. Journal of Food Composition and Analysis 30, 80–85.
- Zhang, Z., Liao, L., Moore, J., Wu, T. & Wang, Z. (2009) Antioxidant phenolic compounds from walnut kernels (Juglans regia L.). Food Chemistry 113, 160–165.
- Zhu, Y., Wilkinson, K. L. & Wirthensohn, M. G. (2015) Lipophilic antioxidant content of almonds (Prunus dulcis): a regional and varietal study. Journal of Food Composition and Analysis 39, 120–127.